Abstract
Ca2+-ATPases belonging to the superfamily of P-type pumps play an important role in maintaining low, nanomolar cytoplasmic Ca2+ levels at rest and priming organellar stores, including the endoplasmic reticulum, Golgi and secretory vesicles with high levels of Ca2+ for a wide range of signaling functions. In this review, we introduce the distinct subtypes of Ca2+-ATPases and their isoforms and splice variants, and provide an overview of their specific cellular roles as they relate to genetic disorders and cancer, with a particular emphasis on recent findings on the secretory pathway Ca2+-ATPases (SPCA). Mutations in human ATP2A2, ATP2C1 genes, encoding housekeeping isoforms of the endoplasmic reticulum (SERCA2) and secretory pathway (SPCA1) pumps respectively, confer autosomal dominant disorders of the skin, whereas mutations in other isoforms underlie various muscular, neurological or developmental disorders. Emerging evidence points to an important function of dysregulated Ca2+-ATPase expression in cancers of the breast, colon, lung and breast where they may serve as markers of differentiation or novel targets for therapeutic intervention. We review the mechanisms underlying the link between calcium homeostasis and cancer and discuss the potential clinical relevance of these observations.
1. Introduction to Ca2+ Transporters
Ionic calcium is a ubiquitous second messenger in the activation of signaling cascades [1–3]. Ca2+ signaling regulates a wide range of cellular and physiological processes, which include transcriptional activation, cell cycle control, muscle contraction, and lactation [4]. On the other hand, prolonged cytoplasmic elevation of free Ca2+ is toxic and triggers cell death [5]. Therefore, under normal circumstances, cells must tightly regulate cytoplasmic calcium levels between a resting (~100 nM) and an activated state (~500nM – 1μM). This sensitive balance is maintained by a cadre of membrane transport proteins that work in concert to move Ca2+ across membranes, in and out of the cell or intracellular storage organelles [3]. In terms of energetics and mechanism, Ca2+ transporters fall into the three broad classes of Ca2+ channels, exchangers, and pumps. Ca2+ channels are activated by a variety of chemical, mechanical or electrical signals: membrane voltage, ligand binding, mechanosensation and the endoplasmic reticulum (ER) store. In response to an activating signal, ion channels residing on cell or organellar membranes open, releasing a flood of Ca2+ down electrochemical gradients into the cytoplasm, resulting in signal transduction and amplification. Upon elevation of cytoplasmic Ca2+, energy-dependent active transporters, including pumps and exchangers, work to refill stores, reset calcium levels to the resting state and re-establish transmembrane electrochemical Ca2+ gradients. Secondary active transporters exemplified by the plasma membrane Na+/Ca2+ exchanger, couple to the sodium electrochemical gradient to rapidly expel the bulk of cytoplasmic Ca2+. ATP-dependent Ca2+ pumps, discussed in this review, scavenge the remaining Ca2+ to establish low, nanomolar resting levels of this second messenger by translocating it out of the cell or by sequestering it in the ER, Golgi or secretory vesicles.
2. P-Type Ca2+-ATPases
Calcium pumps belonging to the superfamily of P-Type ATPases (originally called E1E2-type) move ions across membranes, against their electrochemical gradient, by utilizing the energy from ATP hydrolysis [6–8]. Central to their mechanism is the formation of a phosphorylated reaction intermediate (E~P) that separates a series of distinct conformational states: E1 conformations display high Ca2+ affinity that bind the ion from the cytoplasmic side, and E2 conformations in which the affinity for Ca2+ has been reduced by ~1000-fold following ATP hydrolysis, thereby facilitating release of the ion(s) to the lumenal/extracellular side. Although related by sequence similarity, structural homology and common transport mechanism, there are three subtypes of calcium pumps that are phylogenetically distinct and marked according to their subcellular localizations: namely, the plasma membrane (Plasma Membrane Ca2+-ATPase or PMCA), endoplasmic reticulum (Sarco/Endoplasmic Reticulum Ca2+-ATPase or SERCA), and Golgi/Golgi-derived vesicles (Secretory Pathway Ca2+-ATPase or SPCA) [9]. The separation of these subtypes likely predates the emergence of eukaryotes since representative variants are found in archaea and eubacteria [9]. In humans, multiple isoforms and splice variants exist within each subtype, adding flexibility to tissue specific expression, regulation and kinetic characteristics to fine-tune both temporal and spatial Ca2+ signatures [10].
2.1 Sarco/Endoplasmic Reticulum Ca2+-ATPases
The sarco/endoplasmic reticulum Ca2+-ATPases (SERCA) are the best characterized of the three subtypes, being highly expressed in the specialized ER of muscle where they reach abundance of ~50% of membrane protein [11]. They are responsible for sequestering calcium in the ER, which is the most abundant and readily mobilized store for intracellular calcium [12, 13]. There are 3 genes (ATP2A1-3) coding the SERCA1-3 pumps, and all have varying expression levels and tissue distributions in the body [10, 14]. SERCA1 is expressed in skeletal muscle with 2 variants, SERCA1a, the adult form, and SERCA1b the neonatal form produced by alternate splicing of exon 22, which is expressed in the adult form only [15]. SERCA2 is ubiquitously expressed, with the SERCA2b variant serving an essential housekeeping function. SERCA2a is exclusively expressed in muscle and in neuronal cells, whereas SERCA2c and SERCA2d are expressed in the heart [16, 17]. These specific tissues are physiologically demanding for processes that require Ca2+ such as muscle contraction and propagation of action potentials in the nervous system. SERCA3 is the least characterized isoform with high expression in specific hematopoietic-derived cells of the immune system and in other cell types. There are many variants of SERCA3, with 6 in humans (SERCA3a-f), 3 in mice (SERCA3a-c), and 2 in rats (SERCA3a,b/c), suggesting that these pumps may have a more widespread role in cellular Ca2+ homeostasis than has been appreciated thus far [18, 19]. Aided by natural abundance and years of intensive study, skeletal muscle SERCA1a isoform has been crystallized in multiple distinct conformations, revealing unprecedented insight into the transport mechanism [20–22].
2.2 Plasma Membrane Ca2+-ATPases
There are four plasma membrane Ca2+-ATPases [23], which are PMCA1-4 (gene names ATP2B1-4). Whereas PMCA1 and PMCA4 are widely distributed, PMCA2 and PMCA3 show more restricted tissue expression. PMCA1, the most ubiquitously expressed of the four, has 5 variants (PMCA1a-e) where PMCA1b is ubiquitously expressed and the other isoforms are expressed in the brain and in skeletal muscle. PMCA2 has 6 variants and PMCA3 has 3 variants, all of which are expressed in the brain and in tissues intimately connected to the nervous system [24, 25]. PMCA2 is expressed in the apical membranes of mammary gland acinar cells, where it is substantially induced during lactation for calcium secretion into milk. In mice lacking PMCA2, the milk contained 60% lower levels of Ca2+ relative to the control mice [26]. PMCA3 is exclusively found in the brain and in tissues that are closely associated with neuronal signals; interestingly, this isoform was shown to have a pre-synaptic distribution relative to PMCA2, which was found in post-synaptic regions of the cerebellar cortex [27]. Similarly to PMCA1, PMCA4 is ubiquitously expressed and has 8 variants, which are expressed in smooth muscle, the heart, and in the brain. PMCA4b is the variant that is ubiquitous and has been most widely investigated for function and regulation. [24, 25].
2.3 Secretory Pathway Ca2+-ATPases
The most recent of the three subtypes to be discovered, the secretory pathway Ca2+-ATPases (SPCA), comprise calcium pumps residing in the Golgi compartments and post-Golgi vesicles. The prototypic SPCA pump PMR1 (for Plasma Membrane ATPase-Related) was discovered by homology cloning in the yeast Saccharomyces cerevisiae [28] and shown to have unique biochemical properties distinct from the SERCA and PMCA subtypes [29]. For example, PMR1 is insensitive to inhibition by nanomolar concentrations of thapsigargin, a classical hallmark of SERCA pumps. In addition to delivering Ca2+ into the secretory pathway where it is required for cargo sorting and protein processing, PMR1 also transports Mn2+ with very high (~20 nM) affinity into the Golgi lumen, serving a dual role in clearing excess Mn2+ via exocytosis and providing an essential co-factor for mannosyltransferases, required for protein glycosylation [30]. Null mutants (Δpmr1) thus have pleiotropic defects, and require ion-supplemented media for growth [30, 31].
Heterologous expression studies in yeast laid the groundwork for characterization of the mammalian orthologs [32], represented by SPCA1 and SPCA2, which are encoded by ATP2C1 and ATP2C2 respectively. It is interesting that invertebrates and lower vertebrates, including fish, have only one SPCA gene, as in yeast. A second gene appears in tetrapods, including modern amphibians, reptiles, birds and mammals [33]. SPCA1 is ubiquitously expressed in all tissues, whereas SPCA2 is restricted to absorptive (intestinal) and secretory (pancreas, salivary and mammary glands) epithelia [34]. There are four splice variants of SPCA1, differing only at the C-terminus [35], and no known splice variants of SPCA2. SPCA2 has ~65% identity with SPCA1, differing largely at the N-terminus which is significantly longer than that of SPCA1 [33]. Both SPCA pumps share similar kinetic properties, with SPCA2 exhibiting lower apparent affinity for Ca2+ transport [36, 37].
In addition to the full-length ~103 kDa SPCA2 protein, Garside et al. [38] demonstrated the presence of a much smaller transcript that generates a ~20 kDa C-terminal fragment, expressed in pancreatic acinar cells under control of the MIST1 transcription factor (also known as basic helix loop helix a15, Bhlha15). Examination of other MIST1 expressing tissues (salivary, prostate and gastric glands, seminal vesicles) also revealed the presence of this shorter form, predicted to lack the majority of the membrane domains, as well as the essential phosphorylation and nucleotide-binding domains required for Ca2+ transport and ATP hydrolysis. Surprisingly, Mist1−/− mice lacking this fragment display defective Ca2+ signaling and secretion defects that suggest a functional role for the C-terminus, independent of Ca2+ pumping. An explanation for this “moon-lighting” function came from an independent study by Feng et al. [39] showing that the C-terminus of SPCA2 physically interacts with and restores trafficking of the calcium channel, Orai1, and rescues store-operated calcium entry (SOCE) in a SPCA2 knockdown model in mammary epithelial cells. Furthermore, the C-terminal domain is capable of store-independent activation of Ca2+ influx, mediated by Orai1 and possibly other channels. These studies assign a highly unusual and novel functional role of the SPCA2 C-terminus, independent of the Ca2+-ATPase function, in chaperoning and activation of ion channels. Similarly, some ABC-type transporters are famously known to interact with and regulate ion channels [40, 41], and it remains to be seen if this function is shared by other P-type Ca2+-ATPases.
In secretory and absorptive epithelia, Ca2+ transport processes must be coordinated at multiple membranes to affect Ca2+ transcytosis, as exemplified by movement from the blood to the lumen in the lactating mammary gland, resulting in accumulation of high millimolar concentrations of complexed calcium in milk [42]. During lactation, the mammary gland undergoes drastic morphological changes and a coordinated induction of Ca2+ transport proteins (dubbed CALTRANS), which support the increased demand for calcium transport into the milk [43]. Whereas SPCA2 expression is massively induced immediately prior to parturition, SPCA1 expression is relatively moderate and occurs during the mid phase of lactation [44, 45]. Upon involution of the mammary gland and cessation of lactation, both isoforms return to basal levels [46]. Using a three dimensional mammosphere model of lactation induction, SPCA2 was shown to mediate basolateral Ca2+ influx, in conjunction with Orai1 [44]. Taken together, these observations suggest that Ca2+ entering the polarized secretory cells of the mammary gland is pumped into vesicles by SPCA, packaged into casein-containing micelles and released into the lumen by exocytosis at the apical membrane, in addition to being pumped directly across the apical membrane by the PMCA2 isoform of the plasma membrane Ca2+-ATPase [43]. A recent study investigating the role of Orai1 in lactation in both Orai1 null and conditional knockout mouse models in the mammary gland, found that mammary development was undisrupted, but milk delivery was perturbed due to decreased SOCE and alveolar contractions [47]. These contractions, generated by the surrounding myoepithelial cells, require Orai1-mediated Ca2+ transport.
3. Gene Disorders of Ca2+ Pumps
Various genetic disorders have been associated with specific mutations of the Ca2+ pumps that show a wide range of different physiological phenotypes and clinical manifestations. Mouse models of Ca2+-ATPase knockouts have been particularly insightful in modeling human disease phenotypes, although differences exist. Here, we summarize disease phenotypes and physiological consequences of disrupting Ca2+-ATPase gene subtypes.
3.1 Gene disorders of PMCA
Mice homozygous for pmca1−/− null mutations were embryonic lethal whereas the heterozygous mice exhibited no apparent disease phenotype [48]. Since PMCA1 is ubiquitously expressed, it was concluded that it serves as a housekeeping gene in all tissue types. Okunade et al. also found that pmca4−/− null mice had no noticeable phenotype but that sperm motility was impaired, resulting in infertility. Furthermore, this study showed that in heterozygous pmca1+/− mice with homozygous pmca4−/− knockout, there was vascular contraction impairment in the smooth muscle, but only with one copy of the PMCA1 [48]. A genome-wide association study linked ATP2B1, the gene encoding PMCA1, to hypertension, and the conditional knockout of PMCA1 in smooth muscle cells resulted in increased blood pressure [49, 50]. Together, this provides evidence that PMCA1 has an important role in muscle contraction in the vascular system and that its loss may result in having a predisposition for hypertension.
Mutations or deletions resulting in the loss of function of PMCA2 lead to deafness phenotypes in both mice and humans [51–53]. Another interesting disease phenotype that has been associated with mutations in PMCA2 is the impaired ability to balance and walk normally, which was produced in the Wriggle Sagami mouse model [54]. The disorders associated with PMCA2 mutations result in impairment of organs and processes that are intimately connected with the nervous system. Disease phenotypes from PMCA3 mutations have a predominant effect on the nervous system due to its tissue distribution in the neurological organs. A knockout mouse model has not been developed, possibly consistent with an essential role for PMCA3 in early embryonic development.
3.2 Gene disorders of SERCA
Gene knockout studies of SERCA pumps in mouse models have revealed unexpected phenotypes, sometimes dissimilar to the loss of function gene effects in human [55]. There are two well-defined SERCA disorders, Brody and Darier disease, which are phenotypically quite different in their clinical manifestations. Brody disease is a rare autosomal recessive disorder affecting the skeletal muscle during exercise, with symptoms including painless cramps, slow muscle relaxation and stiffness [56]. This disorder was linked to the decreased uptake of Ca2+ in the sarcoplasmic reticulum and was later connected to the recessive mutations in ATP2A1, the gene encoding the SERCA1 isoform in Brody disease patients and in cultured muscle cells. Interestingly, some Brody disease patients do not harbor mutations in ATP2A1, suggesting that the disease may be heterogeneous in origin [57–59]. Null mutants of SERCA1 in mouse are born normal, but develop respiratory insufficiency due to contractile defects in the diaphragm muscle that leads to cyanosis and death [60].
Darier disease is a rare autosomal dominant genetic skin disorder that is characterized by keratotic papules as a result of the loss of desmosomal proteins at the cell-to-cell junctions that bind keratin filaments. Lesions can form resulting in plaques in specific areas of the body such as the nails and the scalp. There have been over 100 mutations in ATP2A2, the gene encoding SERCA2, reported in patients, distributed through the gene. The only mutations that are specific to SERCA2 in Darier disease are those that are common in the SERCA2a and the SERCA2b isoforms [61, 62]. Patient-derived keratinocytes revealed lower Ca2+ concentrations in the ER, but cytosolic Ca2+ levels were compensated by SPCA1 in these patients [63]. Despite the compensated Ca2+ levels, the decreased ER concentrations could impact protein processing and the progression through the secretory pathway. This ER stress gives rise to the impaired sorting and trafficking of desmosomes to the plasma membrane [64]. Although heterozygous SERCA2 knockout mouse models do not recapitulate Darier disease, other phenotypes ranging from a selective predisposition to heart failure [65], to late developing squamous cell carcinoma [55] may be predictive of similar problems in Darier patients. Similarly, mouse models of ATP2A3 knockout suggest subtle defects in vascular and tracheal smooth muscle contraction, as well as altered Ca2+ signaling in pancreatic β cells that potentially correlate with disease phenotypes in human [55]. This is partly corroborated by observations that SERCA3 sequence variants have been associated with genetic susceptibility to Type II diabetes [66].
3.3 Gene disorders of SPCA
Dysregulated expression or inactivating mutations in SPCA have been linked to two distinct disorders. Like SERCA2 (ATP2A2) mutations, heterozygous mutation of the ubiquitous SPCA isoform, ATP2C1 results in a blistering and ulcerative skin disorder reminiscent of Darier disease, known as Hailey-Hailey disease [67, 68]. Haploinsufficiency, resulting from loss of function SPCA1 mutations [69], appears to disrupt the processing of desmosomal and cell-cell adhesion proteins in keratinocytes that may result in the blistering symptoms in these patients. Given the ubiquitous expression and essential role of both SERCA2 and SPCA1, it is unclear why these disorders of calcium pump haploinsufficiency are largely confined to skin. Presumably, there is adequate compensation by other pump isoforms in other tissues or the skin may be exquisitely and uniquely sensitive to perturbations of these intracellular calcium pump isoforms in ways that we do not yet understand. It has been suggested that skin phenotypes arise from lack of expression of the SERCA3 isoform in skin [70]. Consistent with its widespread distribution, SPCA1 has an essential housekeeping function: homozygous atp2c1−/− null mice show embryonic lethality, expansion of the Golgi and altered ER stress response, indicating that SPCA2 cannot adequately compensate for the loss [71]. Interestingly, heterozygous atp2c1+/− mice do not exhibit blistering phenotypes, similar to the atp2a2+/− mouse model. Instead, the mice displayed high propensity to develop squamous cell tumors in epithelia including skin and esophagus, as they matured into adulthood [71].
Genome wide association studies have linked deletions and variants in ATP2C2 with speech language impairment (SLI) disorders [72, 73]. Risk genes in SLI disorders and in dyslexia have also been connected to children with autism spectrum disorders. SPCA2 has been detected in the brain, including hippocampal neurons [37], and the general idea is that risk associated variants elicit neurological disorders although the cellular and physiological basis for etiology of the disorder is unclear. A knockout mouse model for SPCA2 has not yet been reported, but we anticipate that this deletion will not be as detrimental due to its limited tissue expression.
4. Calcium and Cancer
There is increasing evidence for the importance of Ca2+ homeostasis in cancer [74]. A majority of the hallmarks of cancer [75], if not all, involve calcium signaling to mediate critical cellular processes, including transcriptional regulation which underlies the gene expression in a wide range of pathways crucial to tumorigenesis and metastasis, such as proliferation, angiogenesis, migration, cell cycle progression, immune system evasion, and bypass of apoptosis. To acquire tumorigenic potential, a cell must undergo many transformations before becoming malignant. The cellular demands for tumorigenesis make cancer a disease of many mutations and not the result of one specific genetic alteration. Calcium dysregulation is an example of a low input change that results in a large impact, disrupting homeostasis across the entirety of a cell. A cancer cell can hijack and transform calcium transients such that they fulfill the needs of a malignant tumor for a constitutively active and dividing state.
Many calcium transporters have altered expression levels in various cancers, which can accommodate this high demand for calcium movement within a cell. Within a cancer subtype, there are characteristic isoform specific alterations, as depicted in Figures 1–2, that contribute to the unique characteristics of each tumor. Patient derived tumor cell lines offer the opportunity to evaluate the role of individual Ca2+-ATPases using knockdown and overexpression strategies, as has been reported for SPCA2 [39]. An interesting aspect of breast cancer is the development of microcalcifications, which are common radiographic signature that serve as a diagnostic of both benign and malignant tumors [43]. This phenomenon of excess calcium and phosphate depositions is not well understood at the mechanistic level but is a readily observable and common phenotype of calcium dysregulation in cancer [76–78]. Specific findings linking each of the three Ca2+-ATPase subtypes to cancer are summarized below.
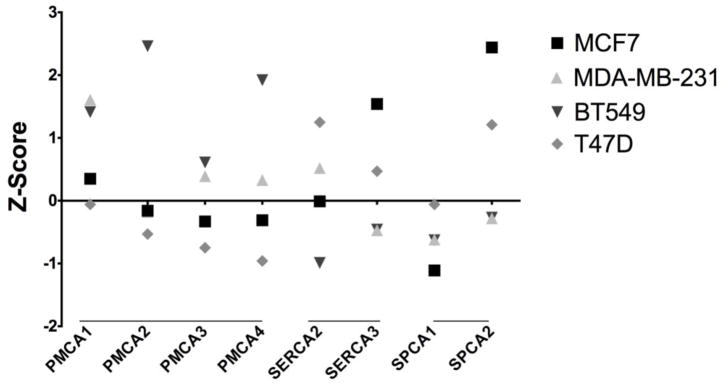
Figure 1.
Breast cancer cell lines from the NCI-60 human tumor cell line database [99] showing expression profiles for each of the Ca2+ pump isoforms depicted as Z score. Z-scores indicate how many standard deviations away a sample is from the mean expression value that was measured in a reference or control sample. Positive values reflect overexpression. The MCF7 and T47D cell lines are representative of the luminal A subtype and MDA-MB-231 and BT549 are representative of the claudin-low subtype. The database includes specific cell lines that are commonly utilized for cancer studies but is not comprehensive of all commercially available cancer cell lines. This database did not contain data for the SERCA1 isoform.
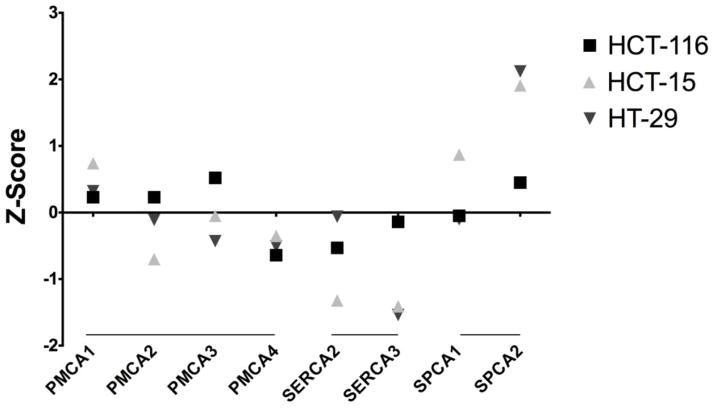
Figure 2.
Colon cancer cell lines from the NCI-60 human tumor cell line database [99] showing the expression profiles for each of the Ca2+ pump isoforms depicted as Z score. HCT-116 is a colorectal carcinoma cell line derived from a late stage primary tumor. HCT-15 and HT-29 are colorectal adenocarcinoma cell lines that have been derived from an advanced stage primary tumors.
4.1 PMCA and Cancer
A common hallmark of cancer, the Warburg effect, is characterized by a preferential and high glycolytic rate relative to mitochondrial respiration. This phenomenon occurs in pancreatic ductal adenocarcinoma (PDAC) where glycolytic ATP fuels Ca2+ efflux via the PMCA pumps. Inhibition of glycolysis in PDAC led to irreversible intracellular Ca2+ overload and cell death, thus exposing a special vulnerability of cancer cells [79]. Altered expression of PMCA isoforms occurs in various types of cancer including lung, colon and breast. Certain breast cancer cell lines exhibit high expression of PMCA2, which is the isoform that is predominantly expressed in mammary epithelia for the apical efflux of Ca2+ during lactation. In parallel with the SPCA2 pump, PMCA2 levels dramatically increase during lactation and return to basal levels upon reaching involution [80]. In these breast cancer cell lines, exemplified by ZR-75-1 where PMCA2 is constitutively expressed at levels as high as 100-fold over non-tumorigenic lines [81], lowered cytosolic Ca2+ levels bypass apoptosis by preventing increased uptake of Ca2+ into mitochondria and activation of cell death cascades. Conversely, in colon cancer PMCA4 is downregulated, increasing cytosolic Ca2+ due to the decreased Ca2+ efflux and increasing cell proliferation [82, 83]. Similarly, in human oral squamous cell carcinoma, epigenetic downregulation of PMCA1 was suggested to be an early event in malignancy that promotes cell proliferation [84]. Thus calcium straddles a fine line between promoting apoptosis in cell death versus cell proliferation through activation of cell cycle. This unique quality of Ca2+ supports the observation that cancer is not a uniform disease and that both up and down regulation of Ca2+-ATPases can bypass normal cellular maintenance to promote tumorigenesis.
4.2 SERCA and Cancer
SERCA pumps have essential roles in calcium transport into the ER for replenishing stored calcium, promoting protein folding and maturation, and the synthesis of lipids and steroids. Within the ER lumen, calcium concentrations are tightly regulated to maintain sufficient stores for signaling, via release by calcium channels, and to activate calcium-dependent proteins such as the protein folding chaperones, calnexin and calreticulin. The importance of luminal Ca2+ is highlighted by the finding that SERCA inhibition preferentially impairs the maturation of leukemia-associated mutant NOTCH receptors, inducing a G0/G1 arrest [85]. Mutations and altered expression levels of SERCA isoforms have been implicated in many cancers, including colon, prostate, and in lung cancers. In colon adenocarcinoma cell lines, SERCA3 expression is induced during differentiation and appears to be progressively lost during multistage process of colon tumorigenesis [86]. Thus, it may be an important marker of colon cancer progression in patients that indicates the remodeling of calcium transients and ensuing morphological changes resulting in cancer differentiation [87]. Papp and colleagues have shown that SERCA3 expression is modulated during differentiation of colon and gastric carcinomas, choroid plexus tumors, and in myeloid leukemias [86–89]. SERCA3 is also downregulated during immortalization of B lymphocytes by the Epstein Barr virus, a human gammaherpesvirus involved in various malignancies including Burkitt’s and other lymphomas, to shape the amplitude, intensity and duration of cytosolic calcium signals, and hence cell activation [90]. Conversely, SERCA2 overexpression in colorectal cancer cells drives proliferation and migration and is reversed by treatment with the curcumin analog, F36 [91]. Interestingly, although homozygous knockout of the SERCA2 gene in mouse is lethal, the heterozygous mutant develops high incidence of squamous cell tumors after a long latency, exemplifying the link between dysregulation of calcium homeostasis and cancer [92].
4.3 SPCA and Cancer
Of the two Secretory Pathway Ca2+-ATPases, SPCA2 is frequently elevated in cancers of breast, prostate and colon, as exemplified in Figures 1–2. Patient data also showed that SPCA1 is highly expressed in basal-like breast cancers and has a low expression in the luminal subtypes [93]. A good in vitro model cell line for basal cancers is MDA-MB-231, a claudin-low subtype, where ATP2C1 knockdown slowed proliferation and had a modest effect in reducing thrombin/trypsin (PAR)-mediated cytosolic calcium transients. Knockdown of SPCA1 had the interesting phenotype of halting the proteolytic processing of the pro-IGF1R protein (insulin growth factor 1 receptor) [93]. The expression of pro-IGF1R has been correlated to poor prognosis in breast cancer suggesting that SPCA1 overexpression is a potential marker for basal-like breast cancers. Conversely, SPCA2 is significantly overexpressed in ERBB2/HER2-positive and luminal breast cancer cell lines. MCF7 cells had a significant overexpression of SPCA2 with low expression of SPCA1 (Figure 1), which served as a good model for Feng et al. [39] to probe the role of SPCA2 in breast cancer. Knockdown of SPCA2 resulted in attenuated growth as well as decreased colony formation of MCF7 cells in soft agar. In mouse xenograft experiments, SPCA2 knockdown drastically reduced tumor formation relative to the control. Furthermore, SPCA2 was able to confer increased proliferation as well as ability for colony formation in soft agar upon overexpression in MCF10A cells, a nonmalignant mammary epithelial cell line. SPCA2 knockdown and low calcium conditions conferred decreased activity in the ERK1/2 pathway, which may explain the decrease in proliferation. Rao and coworkers also showed that SPCA2 has the unique ability to elicit store-independent calcium entry (SICE) through its physical interaction and activation of Orai1. With its ability to pump calcium and its ability to activate SICE, SPCA2 may have an important role in the proliferative potential of cancer by enabling calcium entry for various calcium-dependent processes such as cell cycle progression. Mutations in the SPCA have not been linked to cancer but their constitutive levels of overexpression may have an important consequence in tumorigenesis.
5. Clinical Relevance
Despite clear evidence linking Ca2+-ATPases and calcium signaling to tumorigenesis and malignancy, pharmacological targeting of calcium transporters is still at its infancy [94]. The most promising development in this regard is the exploitation of the potent and selective inhibition of SERCA pumps by thapsigargin. As the active ingredient in the ancient medicinal Mediterranean plant Thapsia garganica, thapsigargin is responsible for intense skin irritation upon contact, resulting from Ca2+-induced histamine release from mast cells [95]. When bound to thapsigargin, SERCA is locked in an inactive E2-like conformation and calcium entry into the ER is abrogated, leaving the IP3R calcium channel to leak calcium from the ER into the cytoplasm. Emptying of ER Ca2+ leads to organelle stress and induces programmed cell death pathways [96]. To avoid widespread cell death, thapsigargin may be targeted to cancer cells in the form of a prodrug: for example, a peptide-conjugated form of thapsigargin cannot enter cells to inhibit SERCA until the peptide is cleaved by a specific protease [95]. When targeted for prostate cancer, the carboxypeptidase protease is the prostate specific membrane antigen (PMSA) found in the vicinity of prostate cells. Currently, the prodrug compound (G202) is in Phase II clinical trials for glioblastoma [97], having successfully concluded Phase I [98].
The overexpression of Ca2+-ATPase genes in breast cancer patients could have important clinical significance in defining specific cancer subtype. Subtype specific preference in expression has also been observed in cell culture lines derived from various cancers. The majority of breast cancer patients are classified as either luminal A/B or ERBB2 (HER2), which are estrogen receptor (ER) positive and HER2 positive, respectively. These subtypes have treatment options that target the receptors to halt downstream activation of tumorigenic pathways. Analysis of patient tumor databases by Feng et al. [39] showed that SPCA2 was up regulated in ERBB2 subtype and down regulated in basal-like tumors. On the other hand, SPCA1 is overexpressed in basal-like tumors and down regulated in luminal subtypes [93]. Tumors derived from basal cells that lack receptor targets have a poorer prognosis than other subtypes due to fewer treatment options. Thus, expression levels of SPCA genes could be differential markers for basal-like tumors and ERBB2 tumors, respectively. This leads to the question of whether this inverse correlation of isoform expression and cancer subtype-specificity has functional relevance. A recent study found that high SPCA2 expression was correlated with epithelial genes in cancer cell lines, whereas SPCA2 expression was low in cell lines that exhibited mesenchymal phenotypes [99]. Epithelial gene expression is a hallmark of more differentiated cancers, whereas cancer cells that have acquired mesenchymal phenotypes are less differentiated and associated with metastasis. Patients with metastatic tumors have poor prognosis and may have developed drug-resistance. Epithelial-mesenchymal transition (EMT) is critical to initiate cancer metastasis, allowing cells of a primary tumor to detach from its neighbors and from the basement membrane, and migrate to form secondary tumors in distant organs, such as the brain, bone, or liver [100]. EMT should not be confused with mesenchymal-derived tumors, which are sarcomas that can develop in nonepithelial tissues such as the bone and muscle [101]. It remains to be determined whether SPCA2 down regulation facilitates EMT, allowing tumor cells to lose their epithelial characteristics and acquire markers of less differentiated or mesenchymal cells.
In summary, the role of SPCA and other calcium pump isoforms in breast and other cancers is an area of active investigation not only as biomarkers for characterizing tumors and their subtypes, but also as potential candidates for novel drug targeting.
Highlights.
Three distinct subtypes of Ca2+-ATPases maintain calcium homeostasis
Gene mutations in ATP2A-C underlie disorders of skin, muscle and development
Ca2+-ATPases show isoform specific dysregulation in cancer subtypes
SPCA2 (ATP2C2) is highly upregulated in epithelial cancers
Ca2+-ATPases may be markers of differentiation and drug targets in cancer therapy
Acknowledgments
This work was funded by a grant from the National Institutes of General Medicine (NIGMS) of the National Institutes of Health, R01GM62142 to R.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews Molecular cell biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Brini M, Carafoli E. Calcium signalling: a historical account, recent developments and future perspectives. Cellular and molecular life sciences : CMLS. 2000;57:354–370. doi: 10.1007/PL00000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mooren FC, Kinne RK. Cellular calcium in health and disease. Biochimica et biophysica acta. 1998;1406:127–151. doi: 10.1016/s0925-4439(98)00006-4. [DOI] [PubMed] [Google Scholar]
- 5.Orrenius S, Gogvadze V, Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochemical and biophysical research communications. 2015;460:72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 6.Palmgren MG, Nissen P. P-type ATPases. Annual review of biophysics. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 7.Palmgren MG, Axelsen KB. Evolution of P-type ATPases. Biochimica et biophysica acta. 1998;1365:37–45. doi: 10.1016/s0005-2728(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 8.Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nature reviews Molecular cell biology. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 9.Vangheluwe P, Sepulveda MR, Missiaen L, Raeymaekers L, Wuytack F, Vanoevelen J. Intracellular Ca2+- and Mn2+-transport ATPases. Chem Rev. 2009;109:4733–4759. doi: 10.1021/cr900013m. [DOI] [PubMed] [Google Scholar]
- 10.Brini M, Cali T, Ottolini D, Carafoli E. Calcium pumps: why so many? Compr Physiol. 2012;2:1045–1060. doi: 10.1002/cphy.c110034. [DOI] [PubMed] [Google Scholar]
- 11.McFarland BH, Inesi G. Solubilization of sarcoplasmic reticulum with Triton X-100. Archives of biochemistry and biophysics. 1971;145:456–464. doi: 10.1016/s0003-9861(71)80005-x. [DOI] [PubMed] [Google Scholar]
- 12.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiological reviews. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 13.Bergner A, Huber RM. Regulation of the endoplasmic reticulum Ca(2+)-store in cancer. Anticancer Agents Med Chem. 2008;8:705–709. doi: 10.2174/187152008785914734. [DOI] [PubMed] [Google Scholar]
- 14.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Ogawa H, Yonekura S, Mitsuhashi H, Mitsuhashi S, Nishino I, Toyoshima C, Ishiura S. Functional analysis of SERCA1b, a highly expressed SERCA1 variant in myotonic dystrophy type 1 muscle. Biochimica et biophysica acta. 2015;1852:2042–2047. doi: 10.1016/j.bbadis.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell calcium. 2005;38:291–302. doi: 10.1016/j.ceca.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Baba-Aissa F, Raeymaekers L, Wuytack F, Dode L, Casteels R. Distribution and isoform diversity of the organellar Ca2+ pumps in the brain. Mol Chem Neuropathol. 1998;33:199–208. doi: 10.1007/BF02815182. [DOI] [PubMed] [Google Scholar]
- 18.Martin V, Bredoux R, Corvazier E, Van Gorp R, Kovacs T, Gelebart P, Enouf J. Three novel sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) 3 isoforms. Expression, regulation, and function of the membranes of the SERCA3 family. The Journal of biological chemistry. 2002;277:24442–24452. doi: 10.1074/jbc.M202011200. [DOI] [PubMed] [Google Scholar]
- 19.Dode L, De Greef C, Mountian I, Attard M, Town MM, Casteels R, Wuytack F. Structure of the human sarco/endoplasmic reticulum Ca2+-ATPase 3 gene. Promoter analysis and alternative splicing of the SERCA3 pre-mRNA. The Journal of biological chemistry. 1998;273:13982–13994. doi: 10.1074/jbc.273.22.13982. [DOI] [PubMed] [Google Scholar]
- 20.Toyoshima C, Cornelius F. New crystal structures of PII-type ATPases: excitement continues. Curr Opin Struct Biol. 2013;23:507–514. doi: 10.1016/j.sbi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Toyoshima C, Mizutani T. Crystal structure of the calcium pump with a bound ATP analogue. Nature. 2004;430:529–535. doi: 10.1038/nature02680. [DOI] [PubMed] [Google Scholar]
- 22.Olesen C, Picard M, Winther AM, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–1042. doi: 10.1038/nature06418. [DOI] [PubMed] [Google Scholar]
- 23.Krebs J. The plethora of PMCA isoforms: Alternative splicing and differential expression. Biochimica et biophysica acta. 2015;1853:2018–2024. doi: 10.1016/j.bbamcr.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Chicka MC, Strehler EE. Alternative splicing of the first intracellular loop of plasma membrane Ca2+-ATPase isoform 2 alters its membrane targeting. The Journal of biological chemistry. 2003;278:18464–18470. doi: 10.1074/jbc.M301482200. [DOI] [PubMed] [Google Scholar]
- 25.Strehler EE. Plasma membrane calcium ATPases: From generic Ca(2+) sump pumps to versatile systems for fine-tuning cellular Ca(2.) Biochemical and biophysical research communications. 2015;460:26–33. doi: 10.1016/j.bbrc.2015.01.121. [DOI] [PubMed] [Google Scholar]
- 26.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. The Journal of biological chemistry. 2004;279:42369–42373. doi: 10.1074/jbc.M407788200. [DOI] [PubMed] [Google Scholar]
- 27.Burette A, Weinberg RJ. Perisynaptic organization of plasma membrane calcium pumps in cerebellar cortex. J Comp Neurol. 2007;500:1127–1135. doi: 10.1002/cne.21237. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao JI, Moir DT. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 29.Sorin A, Rosas G, Rao R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. The Journal of biological chemistry. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 30.Durr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav J, Muend S, Zhang Y, Rao R. A phenomics approach in yeast links proton and calcium pump function in the Golgi. Mol Biol Cell. 2007;18:1480–1489. doi: 10.1091/mbc.E06-11-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ton VK, Rao R. Functional expression of heterologous proteins in yeast: insights into Ca2+ signaling and Ca2+-transporting ATPases. Am J Physiol Cell Physiol. 2004;287:C580–589. doi: 10.1152/ajpcell.00135.2004. [DOI] [PubMed] [Google Scholar]
- 33.Pestov NB, Dmitriev RI, Kostina MB, Korneenko TV, Shakhparonov MI, Modyanov NN. Structural evolution and tissue-specific expression of tetrapod-specific second isoform of secretory pathway Ca2+-ATPase. Biochemical and biophysical research communications. 2012;417:1298–1303. doi: 10.1016/j.bbrc.2011.12.135. [DOI] [PubMed] [Google Scholar]
- 34.Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, Raeymaekers L, Wuytack F. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. The Journal of biological chemistry. 2005;280:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- 35.Fairclough RJ, Dode L, Vanoevelen J, Andersen JP, Missiaen L, Raeymaekers L, Wuytack F, Hovnanian A. Effect of Hailey-Hailey Disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1) The Journal of biological chemistry. 2003;278:24721–24730. doi: 10.1074/jbc.M300509200. [DOI] [PubMed] [Google Scholar]
- 36.Dode L, Andersen JP, Vanoevelen J, Raeymaekers L, Missiaen L, Vilsen B, Wuytack F. Dissection of the functional differences between human secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and 2 isoenzymes by steady-state and transient kinetic analyses. The Journal of biological chemistry. 2006;281:3182–3189. doi: 10.1074/jbc.M511547200. [DOI] [PubMed] [Google Scholar]
- 37.Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+,Mn(2+)-ATPase, hSPCA2, has unusual properties and is expressed in the brain. The Journal of biological chemistry. 2005;280:11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- 38.Garside VC, Kowalik AS, Johnson CL, DiRenzo D, Konieczny SF, Pin CL. MIST1 regulates the pancreatic acinar cell expression of Atp2c2, the gene encoding secretory pathway calcium ATPase 2. Experimental cell research. 2010;316:2859–2870. doi: 10.1016/j.yexcr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunzelmann K, Mehta A. CFTR: a hub for kinases and crosstalk of cAMP and Ca2+ Febs J. 2013;280:4417–4429. doi: 10.1111/febs.12457. [DOI] [PubMed] [Google Scholar]
- 41.Proks P, de Wet H, Ashcroft FM. Sulfonylureas suppress the stimulatory action of Mg-nucleotides on Kir6.2/SUR1 but not Kir6.2/SUR2A KATP channels: a mechanistic study. J Gen Physiol. 2014;144:469–486. doi: 10.1085/jgp.201411222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neville MC. Calcium secretion into milk. Journal of mammary gland biology and neoplasia. 2005;10:119–128. doi: 10.1007/s10911-005-5395-z. [DOI] [PubMed] [Google Scholar]
- 43.Cross BM, Breitwieser GE, Reinhardt TA, Rao R. Cellular calcium dynamics in lactation and breast cancer: from physiology to pathology. Am J Physiol Cell Physiol. 2014;306:C515–526. doi: 10.1152/ajpcell.00330.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross BM, Hack A, Reinhardt TA, Rao R. SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS One. 2013;8:e67348. doi: 10.1371/journal.pone.0067348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinhardt TA, Horst RL. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am J Physiol. 1999;276:C796–802. doi: 10.1152/ajpcell.1999.276.4.C796. [DOI] [PubMed] [Google Scholar]
- 46.Reinhardt TA, Lippolis JD. Mammary gland involution is associated with rapid down regulation of major mammary Ca2+-ATPases. Biochemical and biophysical research communications. 2009;378:99–102. doi: 10.1016/j.bbrc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Davis FM, Janoshazi A, Janardhan KS, Steinckwich N, D’Agostin DM, Petranka JG, Desai PN, Roberts-Thomson SJ, Bird GS, Tucker DK, Fenton SE, Feske S, Monteith GR, Putney JW., Jr Essential role of Orai1 store-operated calcium channels in lactation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:5827–5832. doi: 10.1073/pnas.1502264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. The Journal of biological chemistry. 2004;279:33742–33750. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi Y, Hirawa N, Tabara Y, Muraoka H, Fujita M, Miyazaki N, Fujiwara A, Ichikawa Y, Yamamoto Y, Ichihara N, Saka S, Wakui H, Yoshida S, Yatsu K, Toya Y, Yasuda G, Kohara K, Kita Y, Takei K, Goshima Y, Ishikawa Y, Ueshima H, Miki T, Umemura S. Mice lacking hypertension candidate gene ATP2B1 in vascular smooth muscle cells show significant blood pressure elevation. Hypertension. 2012;59:854–860. doi: 10.1161/HYPERTENSIONAHA.110.165068. [DOI] [PubMed] [Google Scholar]
- 50.Lu X, Wang L, Chen S, He L, Yang X, Shi Y, Cheng J, Zhang L, Gu CC, Huang J, Wu T, Ma Y, Li J, Cao J, Chen J, Ge D, Fan Z, Li Y, Zhao L, Li H, Zhou X, Chen L, Liu D, Chen J, Duan X, Hao Y, Wang L, Lu F, Liu Z, Yao C, Shen C, Pu X, Yu L, Fang X, Xu L, Mu J, Wu X, Zheng R, Wu N, Zhao Q, Li Y, Liu X, Wang M, Yu D, Hu D, Ji X, Guo D, Sun D, Wang Q, Yang Y, Liu F, Mao Q, Liang X, Ji J, Chen P, Mo X, Li D, Chai G, Tang Y, Li X, Du Z, Liu X, Dou C, Yang Z, Meng Q, Wang D, Wang R, Yang J, Schunkert H, Samani NJ, Kathiresan S, Reilly MP, Erdmann J, Peng X, Wu X, Liu D, Yang Y, Chen R, Qiang B, Gu D ADG-WR Coronary, C Meta-Analysis. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet. 2012;44:890–894. doi: 10.1038/ng.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bortolozzi M, Brini M, Parkinson N, Crispino G, Scimemi P, De Siati RD, Di Leva F, Parker A, Ortolano S, Arslan E, Brown SD, Carafoli E, Mammano F. The novel PMCA2 pump mutation Tommy impairs cytosolic calcium clearance in hair cells and links to deafness in mice. The Journal of biological chemistry. 2010;285:37693–37703. doi: 10.1074/jbc.M110.170092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. The Journal of biological chemistry. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- 53.Spiden SL, Bortolozzi M, Di Leva F, de Angelis MH, Fuchs H, Lim D, Ortolano S, Ingham NJ, Brini M, Carafoli E, Mammano F, Steel KP. The novel mouse mutation Oblivion inactivates the PMCA2 pump and causes progressive hearing loss. PLoS Genet. 2008;4:e1000238. doi: 10.1371/journal.pgen.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoue Y, Matsumura Y, Inoue K, Ichikawa R, Takayama C. Abnormal synaptic architecture in the cerebellar cortex of a new dystonic mutant mouse, Wriggle Mouse Sagami. Neurosci Res. 1993;16:39–48. doi: 10.1016/0168-0102(93)90007-d. [DOI] [PubMed] [Google Scholar]
- 55.Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochemical and biophysical research communications. 2004;322:1192–1203. doi: 10.1016/j.bbrc.2004.07.156. [DOI] [PubMed] [Google Scholar]
- 56.Brody IA. Muscle contracture induced by exercise. A syndrome attributable to decreased relaxing factor. N Engl J Med. 1969;281:187–192. doi: 10.1056/NEJM196907242810403. [DOI] [PubMed] [Google Scholar]
- 57.Benders AA, Veerkamp JH, Oosterhof A, Jongen PJ, Bindels RJ, Smit LM, Busch HF, Wevers RA. Ca2+ homeostasis in Brody’s disease. A study in skeletal muscle and cultured muscle cells and the effects of dantrolene an verapamil. The Journal of clinical investigation. 1994;94:741–748. doi: 10.1172/JCI117393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Odermatt A, Barton K, Khanna VK, Mathieu J, Escolar D, Kuntzer T, Karpati G, MacLennan DH. The mutation of Pro789 to Leu reduces the activity of the fast-twitch skeletal muscle sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA1) and is associated with Brody disease. Hum Genet. 2000;106:482–491. doi: 10.1007/s004390000297. [DOI] [PubMed] [Google Scholar]
- 59.Odermatt A, Taschner PE, Khanna VK, Busch HF, Karpati G, Jablecki CK, Breuning MH, MacLennan DH. Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat Genet. 1996;14:191–194. doi: 10.1038/ng1096-191. [DOI] [PubMed] [Google Scholar]
- 60.Pan Y, Zvaritch E, Tupling AR, Rice WJ, de Leon S, Rudnicki M, McKerlie C, Banwell BL, MacLennan DH. Targeted disruption of the ATP2A1 gene encoding the sarco(endo)plasmic reticulum Ca2+ ATPase isoform 1 (SERCA1) impairs diaphragm function and is lethal in neonatal mice. The Journal of biological chemistry. 2003;278:13367–13375. doi: 10.1074/jbc.M213228200. [DOI] [PubMed] [Google Scholar]
- 61.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 62.Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40–50. doi: 10.1016/0190-9622(92)70154-8. [DOI] [PubMed] [Google Scholar]
- 63.Foggia L, Aronchik I, Aberg K, Brown B, Hovnanian A, Mauro TM. Activity of the hSPCA1 Golgi Ca2+ pump is essential for Ca2+-mediated Ca2+ response and cell viability in Darier disease. Journal of cell science. 2006;119:671–679. doi: 10.1242/jcs.02781. [DOI] [PubMed] [Google Scholar]
- 64.Dhitavat J, Cobbold C, Leslie N, Burge S, Hovnanian A. Impaired trafficking of the desmoplakins in cultured Darier’s disease keratinocytes. J Invest Dermatol. 2003;121:1349–1355. doi: 10.1046/j.1523-1747.2003.12557.x. [DOI] [PubMed] [Google Scholar]
- 65.Prasad V, Lorenz JN, Lasko VM, Nieman ML, Huang W, Wang Y, Wieczorek DW, Shull GE. SERCA2 Haploinsufficiency in a Mouse Model of Darier Disease Causes a Selective Predisposition to Heart Failure. Biomed Res Int. 2015;2015:251598. doi: 10.1155/2015/251598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varadi A, Lebel L, Hashim Y, Mehta Z, Ashcroft SJ, Turner R. Sequence variants of the sarco(endo)plasmic reticulum Ca(2+)-transport ATPase 3 gene (SERCA3) in Caucasian type II diabetic patients (UK Prospective Diabetes Study 48) Diabetologia. 1999;42:1240–1243. doi: 10.1007/s001250051298. [DOI] [PubMed] [Google Scholar]
- 67.Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, Ikeda S, Mauro T, Epstein EH., Jr Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 68.Sudbrak R, Brown J, Dobson-Stone C, Carter S, Ramser J, White J, Healy E, Dissanayake M, Larregue M, Perrussel M, Lehrach H, Munro CS, Strachan T, Burge S, Hovnanian A, Monaco AP. Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca(2+) pump. Hum Mol Genet. 2000;9:1131–1140. doi: 10.1093/hmg/9.7.1131. [DOI] [PubMed] [Google Scholar]
- 69.Ton VK, Rao R. Expression of Hailey-Hailey disease mutations in yeast. The Journal of investigative dermatology. 2004;123:1192–1194. doi: 10.1111/j.0022-202X.2004.23437.x. [DOI] [PubMed] [Google Scholar]
- 70.Dhitavat J, Fairclough RJ, Hovnanian A, Burge SM. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 2004;150:821–828. doi: 10.1111/j.1365-2133.2004.05904.x. [DOI] [PubMed] [Google Scholar]
- 71.Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, Prasad V, Shull GE. Loss of the Atp2c1 secretory pathway Ca(2+)-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. The Journal of biological chemistry. 2007;282:26517–26527. doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
- 72.Smith AW, Holden KR, Dwivedi A, Dupont BR, Lyons MJ. Deletion of 16q24.1 supports a role for the ATP2C2 gene in specific language impairment. J Child Neurol. 2015;30:517–521. doi: 10.1177/0883073814545113. [DOI] [PubMed] [Google Scholar]
- 73.Newbury DF, Winchester L, Addis L, Paracchini S, Buckingham LL, Clark A, Cohen W, Cowie H, Dworzynski K, Everitt A, Goodyer IM, Hennessy E, Kindley AD, Miller LL, Nasir J, O’Hare A, Shaw D, Simkin Z, Simonoff E, Slonims V, Watson J, Ragoussis J, Fisher SE, Seckl JR, Helms PJ, Bolton PF, Pickles A, Conti-Ramsden G, Baird G, Bishop DV, Monaco AP. CMIP and ATP2C2 modulate phonological short-term memory in language impairment. Am J Hum Genet. 2009;85:264–272. doi: 10.1016/j.ajhg.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stewart TA, Yapa KT, Monteith GR. Altered calcium signaling in cancer cells. Biochimica et biophysica acta. 2015;1848:2502–2511. doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 75.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 76.Burnside ES, Ochsner JE, Fowler KJ, Fine JP, Salkowski LR, Rubin DL, Sisney GA. Use of microcalcification descriptors in BI-RADS 4th edition to stratify risk of malignancy. Radiology. 2007;242:388–395. doi: 10.1148/radiol.2422052130. [DOI] [PubMed] [Google Scholar]
- 77.Cox RF, Morgan MP. Microcalcifications in breast cancer: Lessons from physiological mineralization. Bone. 2013;53:437–450. doi: 10.1016/j.bone.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 78.Morgan MP, Cooke MM, McCarthy GM. Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors? Journal of mammary gland biology and neoplasia. 2005;10:181–187. doi: 10.1007/s10911-005-5400-6. [DOI] [PubMed] [Google Scholar]
- 79.James AD, Patel W, Butt Z, Adiamah M, Dakhel R, Latif A, Uggenti C, Swanton E, Imamura H, Siriwardena AK, Bruce JI. The Plasma Membrane Calcium Pump in Pancreatic Cancer Cells Exhibiting the Warburg Effect Relies on Glycolytic ATP. The Journal of biological chemistry. 2015;290:24760–24771. doi: 10.1074/jbc.M115.668707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reinhardt TA, Filoteo AG, Penniston JT, Horst RL. Ca(2+)-ATPase protein expression in mammary tissue. Am J Physiol Cell Physiol. 2000;279:C1595–1602. doi: 10.1152/ajpcell.2000.279.5.C1595. [DOI] [PubMed] [Google Scholar]
- 81.Lee WJ, Roberts-Thomson SJ, Monteith GR. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochemical and biophysical research communications. 2005;337:779–783. doi: 10.1016/j.bbrc.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 82.Ribiczey P, Tordai A, Andrikovics H, Filoteo AG, Penniston JT, Enouf J, Enyedi A, Papp B, Kovacs T. Isoform-specific up-regulation of plasma membrane Ca2+ATPase expression during colon and gastric cancer cell differentiation. Cell calcium. 2007;42:590–605. doi: 10.1016/j.ceca.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aung CS, Kruger WA, Poronnik P, Roberts-Thomson SJ, Monteith GR. Plasma membrane Ca2+-ATPase expression during colon cancer cell line differentiation. Biochemical and biophysical research communications. 2007;355:932–936. doi: 10.1016/j.bbrc.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 84.Saito K, Uzawa K, Endo Y, Kato Y, Nakashima D, Ogawara K, Shiba M, Bukawa H, Yokoe H, Tanzawa H. Plasma membrane Ca2+ ATPase isoform 1 down-regulated in human oral cancer. Oncology reports. 2006;15:49–55. [PubMed] [Google Scholar]
- 85.Roti G, Carlton A, Ross KN, Markstein M, Pajcini K, Su AH, Perrimon N, Pear WS, Kung AL, Blacklow SC, Aster JC, Stegmaier K. Complementary genomic screens identify SERCA as a therapeutic target in NOTCH1 mutated cancer. Cancer Cell. 2013;23:390–405. doi: 10.1016/j.ccr.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brouland JP, Gelebart P, Kovacs T, Enouf J, Grossmann J, Papp B. The loss of sarco/endoplasmic reticulum calcium transport ATPase 3 expression is an early event during the multistep process of colon carcinogenesis. The American journal of pathology. 2005;167:233–242. doi: 10.1016/S0002-9440(10)62968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gelebart P, Kovacs T, Brouland JP, van Gorp R, Grossmann J, Rivard N, Panis Y, Martin V, Bredoux R, Enouf J, Papp B. Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. The Journal of biological chemistry. 2002;277:26310–26320. doi: 10.1074/jbc.M201747200. [DOI] [PubMed] [Google Scholar]
- 88.Launay S, Gianni M, Kovacs T, Bredoux R, Bruel A, Gelebart P, Zassadowski F, Chomienne C, Enouf J, Papp B. Lineage-specific modulation of calcium pump expression during myeloid differentiation. Blood. 1999;93:4395–4405. [PubMed] [Google Scholar]
- 89.Ait-Ghezali L, Arbabian A, Jeibmann A, Hasselblatt M, Hallaert GG, Van den Broecke C, Gray F, Brouland JP, Varin-Blank N, Papp B. Loss of endoplasmic reticulum calcium pump expression in choroid plexus tumours. Neuropathol Appl Neurobiol. 2014;40:726–735. doi: 10.1111/nan.12098. [DOI] [PubMed] [Google Scholar]
- 90.Dellis O, Arbabian A, Brouland JP, Kovacs T, Rowe M, Chomienne C, Joab I, Papp B. Modulation of B-cell endoplasmic reticulum calcium homeostasis by Epstein-Barr virus latent membrane protein-1. Mol Cancer. 2009;8:59. doi: 10.1186/1476-4598-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan L, Li A, Li W, Cai P, Yang B, Zhang M, Gu Y, Shu Y, Sun Y, Shen Y, Wu X, Hu G, Xu Q. Novel role of Sarco/endoplasmic reticulum calcium ATPase 2 in development of colorectal cancer and its regulation by F36, a curcumin analog. Biomed Pharmacother. 2014;68:1141–1148. doi: 10.1016/j.biopha.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 92.Liu LH, Boivin GP, Prasad V, Periasamy M, Shull GE. Squamous cell tumors in mice heterozygous for a null allele of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump. The Journal of biological chemistry. 2001;276:26737–26740. doi: 10.1074/jbc.C100275200. [DOI] [PubMed] [Google Scholar]
- 93.Grice DM, Vetter I, Faddy HM, Kenny PA, Roberts-Thomson SJ, Monteith GR. Golgi calcium pump secretory pathway calcium ATPase 1 (SPCA1) is a key regulator of insulin-like growth factor receptor (IGF1R) processing in the basal-like breast cancer cell line MDA-MB-231. The Journal of biological chemistry. 2010;285:37458–37466. doi: 10.1074/jbc.M110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prevarskaya N, Skryma R, Shuba Y. Targeting Ca(2)(+) transport in cancer: close reality or long perspective? Expert Opin Ther Targets. 2013;17:225–241. doi: 10.1517/14728222.2013.741594. [DOI] [PubMed] [Google Scholar]
- 95.Doan NT, Paulsen ES, Sehgal P, Moller JV, Nissen P, Denmeade SR, Isaacs JT, Dionne CA, Christensen SB. Targeting thapsigargin towards tumors. Steroids. 2015;97:2–7. doi: 10.1016/j.steroids.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schonthal AH. Targeting endoplasmic reticulum stress for cancer therapy. Front Biosci (Schol Ed) 2012;4:412–431. doi: 10.2741/s276. [DOI] [PubMed] [Google Scholar]
- 97.GenSpera Efficacy, Safety and CNS Exposure of G-202 in Patients With Recurrent or Progressive Glioblastoma, Clinical Trials.gov, (2015).
- 98.Denmeade SR, Mhaka AM, Rosen DM, Brennen WN, Dalrymple S, Dach I, Olesen C, Gurel B, Demarzo AM, Wilding G, Carducci MA, Dionne CA, Moller JV, Nissen P, Christensen SB, Isaacs JT. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci Transl Med. 2012;4:140ra186. doi: 10.1126/scitranslmed.3003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kohn KW, Zeeberg BM, Reinhold WC, Pommier Y. Gene expression correlations in human cancer cell lines define molecular interaction networks for epithelial phenotype. PLoS One. 2014;9:e99269. doi: 10.1371/journal.pone.0099269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]