Abstract
Acetyl-CoA carboxylases (ACC) 1 and 2 catalyze the carboxylation of acetyl-CoA to malonyl-CoA and depend on biotin as a coenzyme. ACC1 localizes in the cytoplasm and produces malonyl-CoA for fatty acid (FA) synthesis. ACC2 localizes in the outer mitochondrial membrane and produces malonyl-CoA that inhibits FA import into mitochondria for subsequent oxidation. We hypothesized that ACCs are checkpoints in adipocyte differentiation and tested this hypothesis using the ACC1 and ACC2 inhibitor soraphen A (SA) in murine 3T3-L1 preadipocytes. When 3T3-L1 cells were treated with 100 nM SA for 8 days after induction of differentiation, the expression of PPARγ mRNA and FABP4 mRNA decreased by 40% and 50%, respectively, compared with solvent controls; the decrease in gene expression was accompanied by a decrease in FABP4 protein expression and associated with a decrease in lipid droplet accumulation. The rate of FA oxidation was 300% greater in SA-treated cells compared with vehicle controls. Treatment with exogenous palmitate restored PPARγ and FABP4 mRNA expression and FABP4 protein expression in SA-treated cells. In contrast, SA did not alter lipid accumulation if treatment was initiated on day eight after induction of differentiation. We conclude that loss of ACC1-dependent FA synthesis and loss of ACC2-dependent inhibition of FA oxidation prevent lipid accumulation in adipocytes and inhibit early stages of adipocyte differentiation.
Keywords: acetyl-CoA carboxylase, adipogenesis, peroxisome-proliferator-activated receptor gamma, soraphen A, lipid metabolism
1. Introduction
Acetyl-CoA carboxylase 1 and 2 (ACC 1 and 2) catalyze the synthesis of malonyl-CoA through the carboxylation of acetyl-CoA (Zempleni et al., 2012). Malonyl-CoA synthesized from cytoplasmic ACC1 and mitochondrial ACC2 serves as the C2 donor in fatty acid synthesis and as a regulator of beta-oxidation of long chain fatty acids, respectively (Wakil, 1960; Wakil et al., 1983). Malonyl-CoA produced by ACC2 anchored in the outer mitochondrial membrane is an inhibitor of carnitine palmitoyltransferase I (CPT I); the binding of carnitine to fatty acids by CPT I is the rate-limiting step in mitochondrial fatty acid import for subsequent beta-oxidation (McGarry and Brown, 1997).
Due to their key roles in fatty acid metabolism and intracellular lipid accumulation, ACCs have attracted attention as potential targets for lipid lowering treatments. For example, ACC2−/− mice fed a high fat diet or high fat/high-carbohydrate diet for 4 months had lower body weights than wild-type controls (Abu-Elheiga et al., 2001; Choi et al., 2007; Oh et al., 2003). Additionally, ACC2−/− mice exhibited an increase in glucose uptake and insulin sensitivity and a decrease in triglyceride content in the liver and skeletal muscle compared with controls. However, these findings are controversial. Other investigators could not reproduce the differences in overall energy balance between skeletal muscle-specific and global ACC2 knockout (ACC2−/−) mice compared to controls (Hoehn et al., 2010; Olson et al., 2010). Body weights and food intake were similar in ACC2−/− and wild-type controls (Hoehn et al., 2010; Olson et al., 2010) despite a decrease in malonyl-CoA levels and increased fatty acid oxidation in skeletal muscle.
Mice lacking ACC1 are embryonically lethal (Abu-Elheiga et al., 2005) and two liver-specific ACC1 knockout mice (LACC1−/−) generated by Kusunoki’s and Wakil’s groups (Harada et al., 2007; Mao e t al., 2006) produced conflicting results. There were no differences in fatty acid synthesis and malonyl-CoA levels in Kusonoki’s LACC1−/− mice liver samples compared to wild-type mice, whereas the rate of fatty acid synthesis and levels of malonyl-CoA were lower in the liver of Wakil’s LACC1−/− mice compared with controls (Harada et al., 2007; Mao et al., 2006).
The above controversies notwithstanding, there is consensus that ACCs play key roles in fatty acid metabolism, and inhibitors of ACCs are useful tools to interrogate the enzymes’ roles in lipid metabolism. Soraphen A (SA), originally isolated from the myxobacterium Sorangium cellulosum, is a macrocylic polyketide that displays antifungal activity. Evidence suggests that SA targets eukaryotic ACCs (Gerth et al., 1994; Vahlensieck et al., 1994) and inhibits the enzymes at concentrations as low as 1 nM (Shen et al., 2004). Structural studies demonstrate that the compound acts by binding to the biotin carboxylase domain of the ACC enzymes preventing dimerization of eukaryotic ACCs (Shen et al., 2004). Male C57BL/6 mice fed a high fat diet supplemented with SA had reduced weight gain, adipose tissue and fasting plasma insulin levels compared to mice fed high fat diets without SA supplementation and mice on chow control diets (Schreurs et al., 2009). One plausible mechanism for the observed reduction in fat mass displayed by mice fed a high fat diet supplemented with SA is a reduction in ACC1-dependent fatty acid synthesis and ACC2-dependent fatty acid oxidation, potentially in combination with a decrease in adipocyte differentiation. Here, we assessed the temporal effects of SA on lipid metabolism and differentiation in 3T3-L1 murine adipocytes, a commonly used model in adipocyte biology (Student et al., 1980).
2. Materials and Methods
2.1 Cell cultures and differentiation
Mouse preadipocyte 3T3- L1 cells (passages 6–8) were purchased from American Type Culture Collection (catalog no. CL-173). Cells were seeded at a density of 1.5 × 105 in 12 well plates or 5 × 105 in T25 flasks and maintained at 37°C in a 5% CO2/95% air atmosphere. 3T3-L1 preadipocytes were cultured in DMEM containing 25 mM glucose and 10% bovine calf serum until 2 days after reaching confluence (designated as day 0) when the media was supplemented with one of the following differentiation cocktails. Cocktail 1 produced final concentrations of 1.75 µM insulin, 1 µM dexamethasone, and 500 µM 3-isobutyl-1-methylxanthine (IBMX), and cells were treated with the cocktail for 2 days; cultures were continued for another two days in media supplemented with 1.75 µM insulin, but no dexamethasone and IBMX. Cocktail 2 produced final concentrations of 1 µM rosiglitazone and 1.75 µM insulin, and was used for 4 days when media were continued in cocktail free media. Where indicated, SA was added to cells at a concentration of 100 nM or 1 µM. SA was a gift from Dr. Rolf Jansen of the Helmholtz-Centre for Infection Research in Braunschweig, Germany. Cell viability was routinely monitored using trypan blue exclusion and was greater than 90% (Zempleni and Mock, 1998).
2.2 RNA isolation and quantitative real-time PCR (RT-qPCR)
Total RNA was isolated using illustra RNA spin Mini RNA isolation kit (GE healthcare) and the ImProm-II™ reverse transcription system (Promega) was used to synthesize cDNAs. Relative mRNA expression was quantified by RT-qPCR and the ΔCt protocol as described previously (Kaur Mall et al., 2010) using the primers in Online Supplementary Table 1. Assays were performed in three independent biological replicates and results were normalized to 18S ribosomal RNA.
2.3 Fatty acid oxidation assay
The rate of fatty acid oxidation was assessed by measuring the release of tritiated water from [3H]palmitate (Manning et al., 1990). Briefly, [9,10–3H(N)]-palmitic acid (NEN Perkin Elmer) was mixed with unlabeled 20 mM sodium palmitate in 0.01 N NaOH (TCI America, Portland, OR) to achieve a specific radioactivity of abou 1.184 Gbq/mmol. The resulting [3H]palmitate was complexed to fatty acid free bovine serum albumin (BSA, Roche, Basel, Switzerland) at a 3:1 molar ratio using stock solutions of 2 mM BSA and 20 mM [3H]palmitate.
Six days days after induction of differentiation, media was changed to MEM containing 2% FBS for 12 h. Cells were treated with either 100 nM SA beginning at day 0, or 1 µM SA when cells were transferred into MEM media; negative controls were treated with solvent (dimethylsulfoxide, DMSO). Etomoxir (Tocris Biosciences,Minneapolis,MN) inhibits fatty acid oxidation (Horn et al., 2004) and was added to cell cultures one h prior to addition of palmitate (positive control). After 12 h, MEM media was replaced with serum free media and cells were incubated with [3H]palmitate at about18.5 kbq/well; background radioactivity was assessed by incubating cells in fatty acid-free BSA. Incubations were continued for two h. Media were collected and the release of tritiated water was assessed as described previously (Manning et al., 1990) with the following modifications. Trichloroacetic acid was added to the media to produce a final concentration of 1.6 M trichloracetic acid, and samples were allowed to precipitate at 4°C for 30 min. Samples were centrifuged at 16,000 g at 4°C for 10 min, and 250 µl of supernatant was transferred to new tubes containing 42 µl of 6 N NaOH. Samples were applied to a DOWEX™ 1×2,200–400 mesh, ion-exchange resin prepared using 1 ml of suspension; the resin retains the [3H]water whereas [3H]palmitate elutes near the solvent front. Columns were washed with 300 µl of ultrapure water and radioactive water was eluted with 2 ml ultrapure water for analysis in a liquid scintillation counter. Results were normalized by protein concentration in whole cell extra cts from cells cultured in parallel and using the bicinchoninic acid assay for protein analysis. Activity is expressed in units of percent of SA-free solvent control.
2.4 Protein expression
Cells were washed with PBS and lysed directly in the culture dish using 0.5 ml radioimmunoprecipitation assay buffer containing 5µl protease inhibitor cocktail (Sigma, catalog no. P8340). Cells were scraped off and the lysate was transferred to a 1.5-ml tube where they were let stand for 5 min at 4°C. The debris was removed by centrifugation at 14,000g and 4°C for 15 min, and the supernatant was transferred to another tube. The total protein concentration was measured by bicinchoninic acid assay and 4 µg of protein was mixed with NuPage loading dye (Invitrogen) and kept at 95°C for 10 min. Proteins were run on 3–8% Tris-acetate gels and transferred to polyvinylidene membranes. The expression of biotinylated ACC1 and 2 was assessed using streptavidin as a probe (Rios-Avila et al., 2011). To quantify expression of FABP4 and β-actin, proteins were run on 4–12% Bis-Tris gels, transferred to polyvinylidene membranes and probed with either A-FABP antibody (Santa Cruz Biotechnology, catalog no. sc-271529) or β-actin antibody (Thermo Scientific, catalog no. RB-9421-P1).
2.5 Oil Red O (ORO) staining and lipid quantitation
Cells were fixed with 4% paraformaldehyde and lipids were stained using ORO (Ramirez-Zacarias et al., 1992). Hematoxylin was used to stain the nuclear compartment (Ross et al., 2000). Microscopy images were obtained using a Nikon Ti-S Inverted Fluorescence Microscope. The relative accumulation of lipids was quantified by extracting ORO using 100% isopropanol and measuring the absorbance at 490 nm in a spectrophotometer.
2.6 Statistics
Data variances were homogenous as per Bartlett’s test; therefore data were not transformed prior to statistical analysis. One-way ANOVA was used to determine whether differences among treatment groups were significantly different. Dunnett’s multiple comparisons t-test was used to determine if differences between the control and treatment groups were statistically significant; the control groups are identified in Results. All data presented are means ± standard deviation from independent experiments (biological replicates). Statistical analysis was performed using Graph Pad Prism 6.0.
3. Results
3.1 Effects of SA on lipid accumulation and gene expression
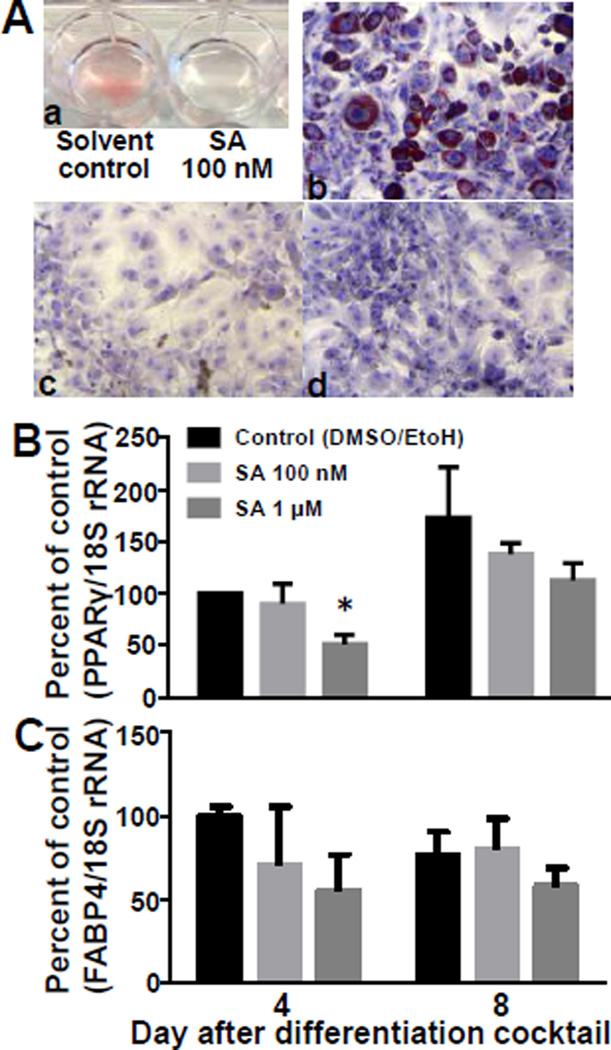
Inhibition of ACCs by SA caused a loss of lipid accumulation and adipogenic gene expression in 3T3-L1 cells. When cells were treated with 100 nM SA for 6 days, no ORO was visible in cells in 12-well plates compared to vehicle controls (Fig. 1Aa). There were moderate reductions in lipid accumulation with concentrations of 50 nM (data not shown). The same results were obtained when assessing the accumulation of lipid droplets in ORO-stained cells using a fluorescence microscope: droplets were easily detectable in control cells (Fig. 1Ab) whereas lipid droplets were not detectable in cells treated with 100 nM of 1 µM SA for 6 days (Fig. 1Ac,d). Note that SA inhibits two counteracting effects: ACC1-dependent Treatment with SA did not affect cell viability (units = % viability): 88.6 ± 9.6 % for solvent control, 88.6 ± 9.7 % for 100 nM SA, and 90.2 ± 4.8 % for 1 µM SA (P>0.05, n=3). Subsequent experiments were performed with 100 nM and 1 µM of SA.
Figure 1.
Soraphen A decreases lipid accumulation and adipogenic gene expression in 3T3-L1 cells. A) Cells were treated SA for 6 days and lipids were stained with Oil Red O: (a) cells stained in the well plate without magnification; (b – d) cells were fixed, stained with Oil Red O, and staining was captured by light microscopy (10× magnification): DMSO vehicle control (b), 100 nM SA (c), and 1 µM SA (d). Expression of PPARγ (B) and FABP4 (C) mRNA was measured 4 and 8 days after induction of differentiation (one-way ANOVA; *P<0.05 compared to vehicle control; n=3).
SA treatment caused a loss in PPARγ mRNA expression in 3T3-L1 cells. When cells were treated with 1 µM SA, the abundance of PPARγ mRNA was about 40% lower compared with solvent controls on both days 4 (P<0.05; n=3) and 8 (Fig. 1B). Treatment with a lower dose of SA (100 nM) did not elicit a statistically significant effect. The expression of PPARγ mRNA was significantly greater a day 8 compared with days 0 and 4 (P<0.05; n=3). Fatty acid binding protein 4 (FABP4) is a downstream target of PPARγ. SA treatment caused a pattern of FABP4 mRNA expression similar to that observed for PPARγ but none of these effects were significant (Fig. 1C).
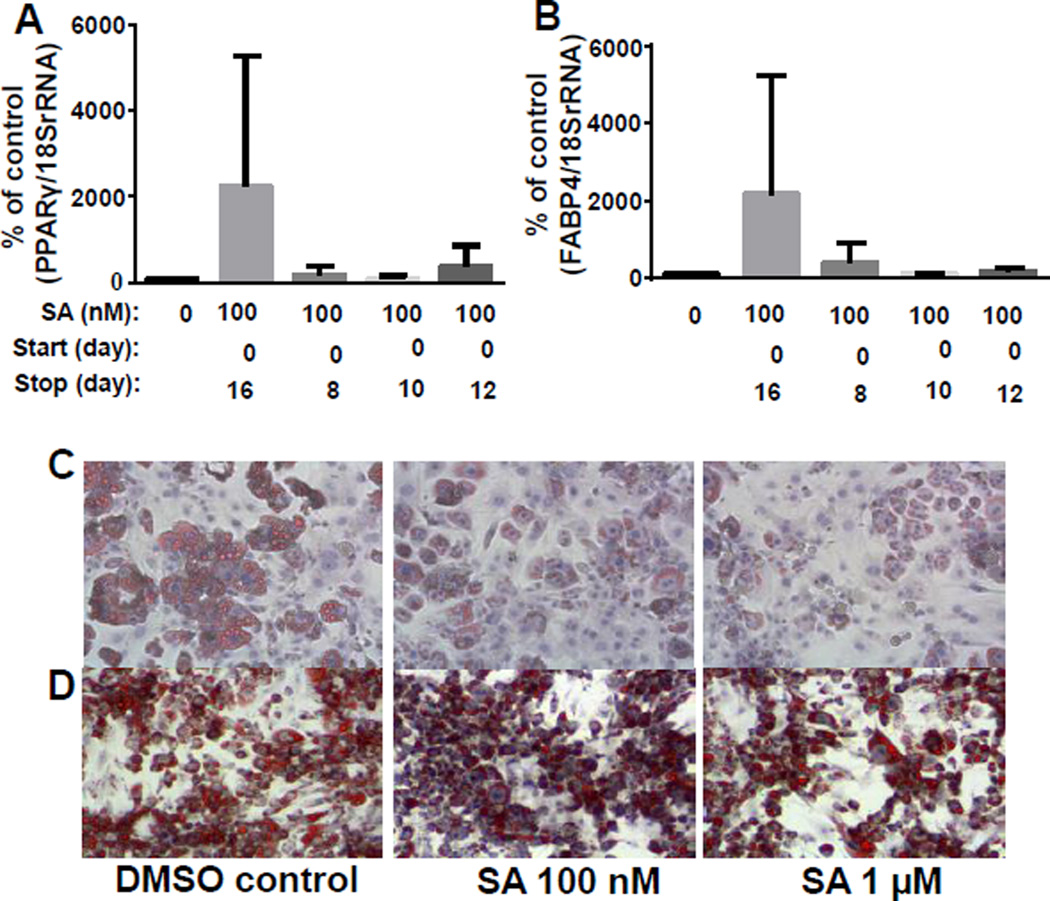
Continuous administration of SA was required to maintain an uncommitted phenotype. A series of time course experiments were performed to determine the temporal effects of SA. Cells were treated with SA for 8, 10, or 12 days after induction of differentiation and collected on day 16. PPARγ and FABP4 mRNA expression was not significantly different between treatment groups when measured at day 16 (P>0.05) (Fig. 2A,B). The unexpected increase in PPARγ and FABP4 mRNA expression in cells treated for the full 16 days was seen in all biological replicates but was not statistically significant. Cells began accumulating lipids two days after discontinuation of SA following a 14-day treatment course (Fig. 2C).
Figure 2.
Time courses of SA treatment in 3T3-L1 cells. Cells were treated with SA beginning on day 0, and treatment was discontinued on days 8, 10, or 12. Cells were harvested on day 16 for analysis of PPARγ mRNA (A) and FABP4 (B) (n=3). Cells were treated with 100 nM or 1 µM SA for 14 days. Media was replaced with SA-free media and cells were fixed on day 16 and ORO was visualized using a Nikon Ti-S Inverted Fluorescence Microscope at 4× (C). Cells were treated with either 100 nM or 1 µM SA beginning at 8 days post-differentiation and fixed on day 12. ORO staining was conducted as in panel C.
To determine if SA could revert the phenotype of terminally differentiated 3T3-L1 cells by downregulating the expression of PPARγ and lipid droplet accumulation, cells were treated with either 100 nM or 1 µM SA beginning at 8 days post-differentiation and collected on Day 12. There was no change in the size or the amount of lipid droplets after 4 days of SA administration at either concentration when administration started on day 8 (Fig. 2D).
3.2 Palmitate rescue experiments
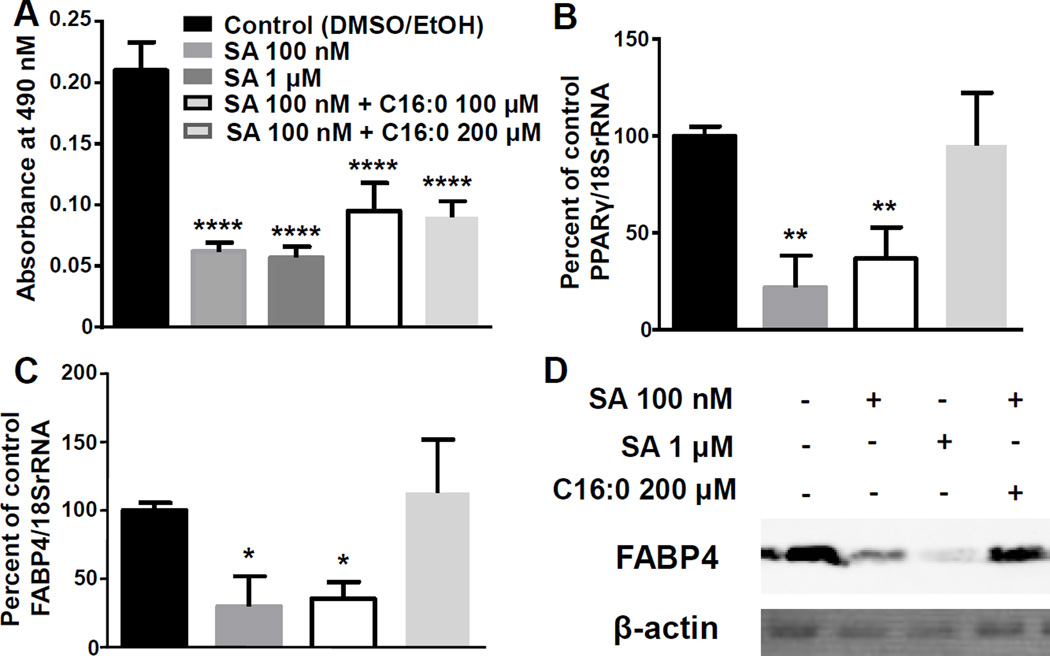
SA treatment caused a ~75% decrease in lipid accumulation compared with solvent controls, which was partially restored by exogenous palmitate in SA-treated 3T3-L1 cells (Fig. 3A). In contrast, exogenous palmitate fully restored PPARγ mRNA and FABP4 mRNA expression in SA-treated cells compared to solvent controls (Fig. 3B, C). Likewise, the expression of FABP4 protein was fully rescued by exogenous palmitate (Fig. 3D). Palmitate did not affect the viability of the cells (data not shown). Note that in these experiments SA was present in cell cultures for the entire observation period, whereas in the eight-day incubations in Fig. 1 SA was removed after six days.
Figure 3.
Palmitate rescue experiments. 3T3-L1 cells were treated with SA for 8 days with or without exogenous palmitate and stained with ORO (A). Expression of PPARγ mRNA (B), FABP4 mRNA (C), and FABP4 protein (representative blot, D). *P<0.05, **P<0.01, ****P<0.0001 compared to vehicle control; n=4 for lipid accumulation experiments; n=3 for mRNA and protein expression studies.
3.3 Rosiglitazone and insulin maintain adipogenic gene expression despite treatment with SA
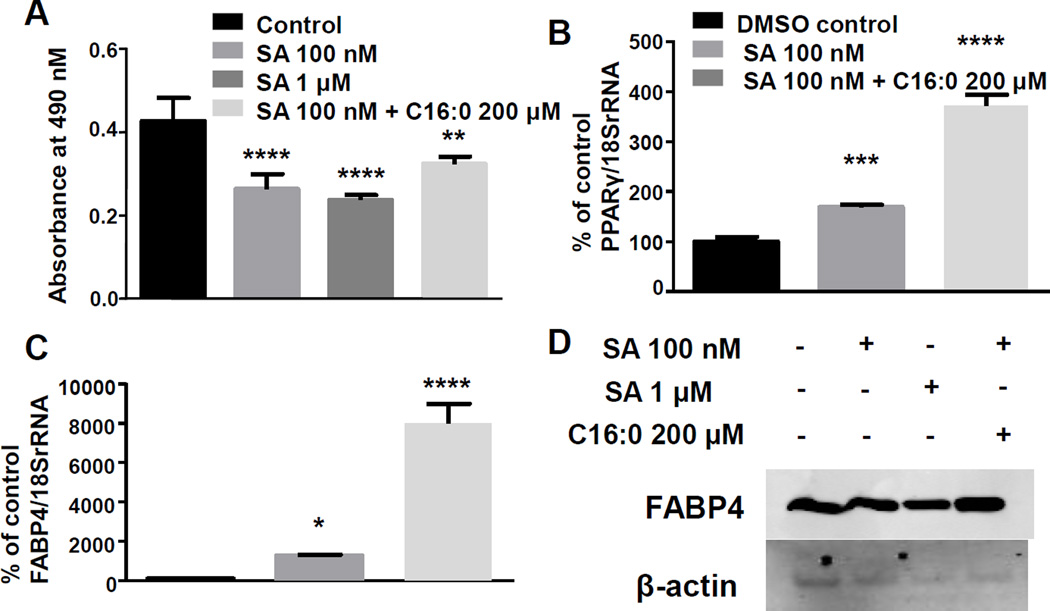
Differentiating 3T3-L1 cells using rosiglitazone, a PPARγ agonist, and insulin rescued adipogenic gene expression and lipid accumulation despite treatment with SA. Lipid accumulation was reduced by about 40% when cells were treated with 100 nM or 1 µM SA and palmitate restored lipid accumulation to 75% of that of the control (P<0.05; n=4) (Fig. 4A). Gene expression of PPARγ and FABP4 were increased compared with controls in cells treated with 100 nM SA and with or without 200 µM palmitate (Fig. 4 B,C). Treatment with 200 µM palmitate was particularly effective in elevating PPARγ and FABP4 mRNA expression (P<0.05; n=3) (Fig. 4 B,C). Protein expression of FABP4 was unchanged across treatment groups at day 8 (Fig. 4D). Note that SA caused a moderate increase in adipocyte differentiation when used in combination with rosiglitazone, whereas SA alone decreased adipocyte differentiation (see above).
Figure 4.
Rosiglitazone rescues adipogenic gene expression despite treatment with SA. Cells differentiated with a rosiglitazone/insulin cocktail were treated with either a DMSO vehicle control, or 100 nM SA with or without 200 µM palmitate. A) Cells were fixed at day 8 and stained with ORO and absorbance was measured at 490 nm. Gene expression of (B) PPARγ and (C) FABP4 was measured at 8 days after induction of differentiation. D) Representative western blot image of FABP4 protein expression. (One-way ANOVA; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared to vehicle control; n=4 for lipid accumulation experiments; n=3 for gene and protein expression studies).
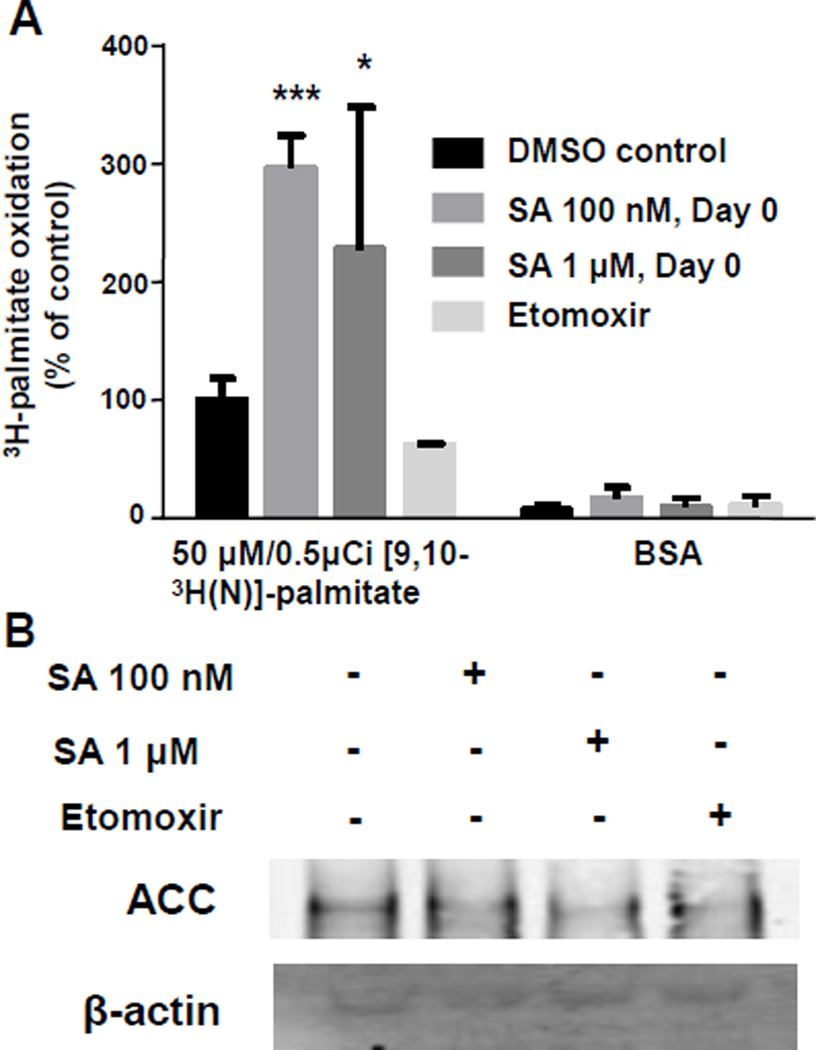
3.4 SA treatment increases fatty acid oxidation
Fatty acid oxidation is increased in 3T3-L1 cells upon treatment with SA. To measure fatty acid oxidation, cells were incubated with [9,10-3H]-palmitic acid after pretreatment with DMSO, 100 nM SA starting from day 0, or 1 µM SA for 12 h. When [9,10-3H]-palmitic acid undergoes dehydrogenation at the acyl-CoA dehydrogenase step, 3H-lableled H2O is released into the media and can be quantified. Cells treated with either 100 nM SA for 7 days or 1 µM SA for 12 h had a ~3-fold and ~2-fold increase in fatty acid oxidation, respectively (P<0.05; n=5). Administration of BSA resulted in no change in FAO between treatment groups. Etomoxir served as a negative control, inhibiting fatty acid oxidation by ~40% compared with controls (Fig. 5A). There was no change in ACC protein expression between the control and treatment groups (Fig. 5B).
Figure 5.
Fatty acid oxidation is increased in SA treated cells. A) 3T3-L1 cells were treated with or without 100 nM SA for 7 days beginning at time of induction of differentiation or SA 1 µM for 12 h prior to addition of either BSA or 18.5kbq/well palmitate for 2 h. Samples were processed as described and 3H-labeled H2O was quantified by liquid scintillation counting. B) Representative blot image of ACC expression. (One-way ANOVA; *P<0.05, **P<0.01, ***P<0.001, compared to vehicle control; n=5 for fatty acid oxidation assays, n=3 for protein expression studies).
4. Discussion
This is the first study to demonstrate that SA-dependent loss of lipid accumulation prevents adipocyte differentiation and this phenotype can be rescued by providing exogenous palmitate. Our finding that FABP4 gene and protein expression is decreased in 3T3-L1 cells upon treatment with SA is consistent with the observation that in FAS knockdown in primary mouse embryonic fibroblasts and 3T3-L1 cells, protein and gene expression of aP2 (FABP4) is decreased (Lodhi et al., 2012). However, we also saw a decrease in gene expression of PPARγ, the master regulator of adipogenesis, while Lodhi et al. reported no change in PPARγ protein expression (Lodhi et al., 2012). Those authors attributed changes in adipogenesis to a lack of PPARγ activation. Our finding that PPARγ gene expression is decreased in SA-treated cells agrees with a previous report suggesting that the ACC inhibitor, choloracetylated biotin derivative (CABI), inhibits adipogenesis, PPARγ protein expression, and lipid accumulation (Levert et al., 2002). However, CABI is less potent than SA in inhibiting lipid accumulation and adipogenic protein expression. For example, 8 µM of CABI were required to inhibit PPARγ protein expression and lipid accumulation (Levert et al., 2002), whereas 100 nM of SA was sufficient to inhibit adipogenic gene expression and lipid accumulation in this study.
In this study, exogenous palmitate restored adipogenic gene expression and FABP4 protein expression and resulted in a moderate increase in lipid accumulation. This is in agreement with previous studies suggesting that palmitate stimulates triacylglycerol accumulation and adipocyte differentiation in 3T3-L1 cells (Madsen et al., 1983) and activates PPARγ in-vitro (Kliewer et al., 1997). Interestingly, when we differentiated cells with the PPARγ agonist rosiglitazone and insulin in the presence of SA, the expression PPARγ and FABP4 was increased despite treatment with SA. Administering SA in combination with palmitate resulted in an even more dramatic increase in PPARγ and FABP4 expression. Additionally, the level of FABP4 protein expression remained constant across treatment groups. This contrasts with the results that we obtained when inducing 3T3-L1 cell differentiation with the classical differentiation cocktail of insulin, IBMX, and dexamethasone in the presence of SA. In that case, SA decreased adipogenic markers. We propose that treating 3T3-L1 cells with SA not only alters lipid metabolism but also PPARy activation and adipogenesis.
Inhibition of adipogenic genes and lipid accumulation by SA only occurred if SA was continuously added to the media at the beginning of differentiation. Surprisingly, at day 16 of SA administration, PPARγ and FABP4 gene expression were elevated compared to vehicle controls and cell cultures from which SA was removed at earlier time points. Likewise, when SA was administered for more than 16 days, cells would resume differentiation upon SA removal. The same phenomenon was reported previously with the ACC inhibitor, CABI (Levert et al., 2002). If SA was administered in later stages of differentiation, there was no change in the size or amount of lipid droplets and the adipogenic genes remain unchanged. Work by Camp et al. demonstrated that a novel potent antagonist, PD068235 was unable to cause de-differentiation of mature adipocytes indicating that PPARγ activation is minimal in differentiated adipocytes (Camp et al., 2001).
The role of ACC1 in de novo lipogenesis is well established (Wakil et al., 1983) and ACC1 is highly expressed in adipose tissue (Castle et al., 2009; Wakil et al., 1983). In contrast, ACC2 is abundantly expressed in skeletal muscle while mouse adipose tissue has low levels of ACC2 (Castle et al., 2009). Interestingly, we found a ~3- and ~2-fold increase in fatty acid oxidation with both chronic and acute treatment of SA, respectively; this increase is comparable to those achieved in other cell lines, i.e., a ~3-fold increase in HepG2 cells and a ~2-fold increase in LNCaP cells (Beckers et al., 2007; Jump et al., 2011). These results suggest that fatty acid oxidation is at least partially responsible for the loss of triacylglycerols in SA-treated cells and implicates ACC2 in lipid accumulation.
This study suggests that SA-dependent inhibition of FA oxidation elicits a decrease in lipid accumulation only before differentiation of adipocytes, but elicits no major decrease in lipid accumulation in differentiated adipocytes. This somewhat surprising observation is consistent with the following previous observations. Inhibition of PPARy blocks adipocyte differentiation but does not alter lipolysis in differentiated adipocytes (Camp et al., 2001). If rates of lipolysis are low, little substrate is available for SA-dependent increase in fatty acid oxidation in 3T3-L1 cells. We propose that the β-oxidation primarily depends on the exogenous supply of fatty acids, as opposed to lipolysis, in 3T3-L1 cells.
Obesity and related diseases, namely cardiovascular disease and diabetes, pose a substantial health risk for U.S. citizens and are an enormous economic burden for the health-care system (Centers for Disease Control and Prevention, 2011a, b, 2015). The prevalence of obesity (defined as a body mass index ≥30) is 20 to 30 percent among U.S. adults, depending on geographic region and results in $58.6 billion in lost productivity (Vijan et al., 2004). Note that SA may be cytotoxic at high concentrations and alternative ACC inhibitors have been developed (Borbeau et al., 2007). Deciphering t he physiological mechanisms underlying obesity is essential to the development of treatments and prevention strategies against obesity. A fat-specific fatty acid synthase (FAS) knockout mouse model was shown to lead to an increase in brown fat-like adipocytes in subcutaneous adipose tissue and increased energy expenditure (Lodhi et al., 2012). Lodhi et al. demonstrated that knocking out FAS blocked the production of endogenous ligands normally produced in the fatty acid synthesis pathway, which activate PPARγ and subsequently adipogenesis. The ACC enzymes lie upstream of FAS and produce acetyl-CoA, which serves as a substrate for FAS. Our results have demonstrated that SA not only alters lipid accumulation in 3T3-L1 cells but also plays a role in preventing adipogenesis. It is reasonable to propose that dual inhibition of the ACC enzymes is critical to eliminate lipid accumulation in 3T3-L1 cells and prevent the production of endogenous ligands for PPARγ activation. Previously, mice fed a high fat diet supplemented with SA, were found to have reduced adiposity and weight gain and improved insulin sensitivity, comparable to those fed a chow control diet (Schreurs et al., 2009). While it appears that SA protects mice from diet-induced obesity, the molecular and cellular mechanisms that could be responsible for this protection were not formally tested. Our studies provide one possible explanation for this observation, i.e., SA decreases fatty acid accumulation and PPARγ activation in adipose tissue.
The extent to which each ACC enzyme contributes to lipid accumulation and adipogenesis is unknown. Investigations into the role of both ACC1 and ACC2 are currently underway in our laboratory. Given that mice lacking ACC1 are embryonically lethal (Abu-Elheiga et al., 2005) it would be interesting to observe the effects of an adipose-specific knockout of ACC1 and whether or not this would yield similar results to treating animals with SA.
4.1 Conclusions
We report here that inhibition of ACCs by SA blocks adipogenic gene expression and lipid accumulation. Fatty acid oxidation is at least partially responsible for the lack of lipid droplet accumulation. This report is an important step in understanding the mechanisms that underlie obesity and identifies one potential therapeutic target.
Supplementary Material
Acknowledgments
Supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2015-67017-23181, Public Health Service grant NIH 1P20GM104320, the Gerber Foundation, the Egg Nutrition Center, the University of Nebraska Agricultural Research Division (Hatch Act), and USDA multistate group W3002 (all to J.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no conflicts of interest to disclose.
Author contributions
ELC and JZ designed experiments. ELC, SKJ, and FEH performed data collection and analysis. ELC and JZ interpreted the data and wrote the manuscript.
References
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, Wakil SJ. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc. Natl. Acad. Sci. USA. 2005;102:12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers A, Organe S, Timmermans L, Scheys K, Peeters A, Brusselmans K, Verhoeven G, Swinnen JV. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- Bourbeau MP, Bartberger MD. Recent advances in the development of acetyl-CoA carboxylase (ACC) inhibitors for the treatment of metabolic disease. J. Med. Chem. 2007;58:525–536. doi: 10.1021/jm500695e. [DOI] [PubMed] [Google Scholar]
- Camp HS, Chaudhry A, Leff T. A novel potent antagonist of peroxisome proliferator-activated receptor gamma blocks adipocyte differentiation but does not revert the phenotype of terminally differentiated adipocytes. Endocrinol. 2001;142:3207–3213. doi: 10.1210/endo.142.7.8254. [DOI] [PubMed] [Google Scholar]
- Castle JC, Hara Y, Raymond CK, Garrett-Engele P, Ohwaki K, Kan Z, Kusunoki J, Johnson JM. ACC2 is expressed at high levels in human white adipose and has an isoform with a novel N-terminus [corrected] PLoS ONE. 2009;4:e4369. doi: 10.1371/journal.pone.0004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Diabetes public health resource. [accessed 9/21/2011];2011a http://www.cdc.gov/diabetes/
- Centers for Disease Control and Prevention. Heart Disease. [accessed 9/25/2011];2011b http://www.cdc.gov/heartdisease/
- Centers for Disease Control and Prevention. Adult obesity facts. [accessed 10/2/2015];2015 http://www.cdc.gov/obesity/data/adult.html.
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc. Natl. Acad. Sci. USA. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth K, Bedorf N, Irschik H, Hofle G, Reichenbach H. The soraphens: a family of novel antifungal compounds from Sorangium cellulosum (Myxobacteria). I. Soraphen A1 alpha: fermentation, isolation, biological properties. J. Antibiot. (Tokyo) 1994;47:23–31. doi: 10.7164/antibiotics.47.23. [DOI] [PubMed] [Google Scholar]
- Harada N, Oda Z, Hara Y, Fujinami K, Okawa M, Ohbuchi K, Yonemoto M, Ikeda Y, Ohwaki K, Aragane K, Tamai Y, Kusunoki J. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol. Cell. Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn KL, Turner N, Swarbrick MM, Wilks D, Preston E, Phua Y, Joshi H, Furler SM, Larance M, Hegarty BD, Leslie SJ, Pickford R, Hoy AJ, Kraegen EW, James DE, Cooney GJ. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Ji H, Friedman MI. Etomoxir, a fatty acid oxidation inhibitor, increases food intake and reduces hepatic energy status in rats. Physiol. Behav. 2004;81:157–162. doi: 10.1016/j.physbeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Jump DB, Torres-Gonzalez M, Olson LK. Soraphen A, an inhibitor of acetyl CoA carboxylase activity, interferes with fatty acid elongation. Biochem. Pharmacol. 2011;81:649–660. doi: 10.1016/j.bcp.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur Mall G, Chew YC, Zempleni J. Biotin requirements are lower in human Jurkat lymphoid cells but homeostatic mechanisms are similar to those of HepG2 liver cells. J. Nutr. 2010;140:1086–1092. doi: 10.3945/jn.110.121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levert KL, Waldrop GL, Stephens JM. A biotin analog inhibits acetyl-CoA carboxylase activity and adipogenesis. J. Biol. Chem. 2002;277:16347–16350. doi: 10.1074/jbc.C200113200. [DOI] [PubMed] [Google Scholar]
- Lodhi IJ, Yin L, Jensen-Urstad AP, Funai K, Coleman T, Baird JH, El Ramahi MK, Razani B, Song H, Fu-Hsu F, Turk J, Semenkovich CF. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen NB, Shechosky S, Fletterick RJ. Site-site interactions in glycogen phosphorylase b probed by ligands specific for each site. Biochem. 1983;22:4460–4465. doi: 10.1021/bi00288a017. [DOI] [PubMed] [Google Scholar]
- Manning NJ, Olpin SE, Pollitt RJ, Webley J. A comparison of [9,10-3H]palmitic and [9,10-3H]myristic acids for the detection of defects of fatty acid oxidation in intact cultured fibroblasts. J. Inherit. Metab. Dis. 1990;13:58–68. doi: 10.1007/BF01799333. [DOI] [PubMed] [Google Scholar]
- Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc. Natl. Acad. Sci. USA. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- Oh SY, Park SK, Kim JW, Ahn YH, Park SW, Kim KS. Acetyl-CoA carboxylase beta gene is regulated by sterol regulatory element-binding protein-1 in liver. J. Biol. Chem. 2003;278:28410–28417. doi: 10.1074/jbc.M300553200. [DOI] [PubMed] [Google Scholar]
- Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB. Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc. Natl. Acad. Sci. USA. 2010;107:7598–7603. doi: 10.1073/pnas.0913492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- Rios-Avila L, Prince SA, Wijeratne SSK, Zempleni J. A 96-well plate assay for high-throughput analysis of holocarboxylase synthetase activity. Clin. Chim. Acta. 2011;412:735–739. doi: 10.1016/j.cca.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Schreurs M, van Dijk TH, Gerding A, Havinga R, Reijngoud DJ, Kuipers F. Soraphen, an inhibitor of the acetyl-CoA carboxylase system, improves peripheral insulin sensitivity in mice fed a high-fat diet. Diabetes Obes. Metab. 2009;11:987–991. doi: 10.1111/j.1463-1326.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- Shen Y, Volrath SL, Weatherly SC, Elich TD, Tong L. A mechanism for the potent inhibition of eukaryotic acetyl-coenzyme A carboxylase by soraphen A, a macrocyclic polyketide natural product. Mol. Cell. 2004;16:881–891. doi: 10.1016/j.molcel.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- Vahlensieck HF, Pridzun L, Reichenbach H, Hinnen A. Identification of the yeast ACC1 gene product (acetyl-CoA carboxylase) as the target of the polyketide fungicide soraphen A. Curr. Genet. 1994;25:95–100. doi: 10.1007/BF00309532. [DOI] [PubMed] [Google Scholar]
- Vijan S, Hayward RA, Langa KM. The impact of diabetes on workforce participation: results from a national household sample. Health Serv. Res. 2004;39:1653–1669. doi: 10.1111/j.1475-6773.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil S, Gibson DM. Studies on the mechanism of fatty acid synthesis. VIII. the participation of protein-bound biotin in the biosynthesis of fatty acids. Biochim. Biophys. Acta. 1960;41:122–129. [Google Scholar]
- Wakil SJ, Stoops JK, Joshi VC. Fatty acid synthesis and its regulation. Annu. Rev. Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Zempleni J, Mock DM. Uptake and metabolism of biotin by human peripheral blood mononuclear cells. Am. J. Physiol. Cell Physiol. 1998;275:C382–C388. doi: 10.1152/ajpcell.1998.275.2.C382. [DOI] [PubMed] [Google Scholar]
- Zempleni J, Wijeratne SSK, Kuroishi T. Biotin. In: Erdman JW Jr, Macdonald I, Zeisel SH, editors. Present Knowledge in Nutrition. Washington, D.C.: International Life Sciences Institute; 2012. pp. 587–609. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.