Abstract
Successful navigation of the environment requires attending and responding efficiently to objects and conspecifics with the potential to benefit or harm (i.e., that have value). In humans, this function is subserved by a distributed large-scale neural network called the “salience network”. We have recently demonstrated that there are two anatomically and functionally dissociable salience networks anchored in the dorsal and ventral portions of the human anterior insula (Touroutoglou et al., 2012). In this paper, we test the hypothesis that these two subnetworks exist in rhesus macaques (Macaca mulatta). We provide evidence that a homologous ventral salience network exists in macaques, but that the connectivity of the dorsal anterior insula in macaques is not sufficiently developed as a dorsal salience network. The evolutionary implications of these finding are considered.
Keywords: intrinsic functional connectivity, resting state fMRI, salience network, rhesus macaques
1.1 Introduction
Salience processing is crucial for survival. It enables animals to successfully navigate the detection of, attention to, and action planning for stimuli that are potentially rewarding or harmful (i.e., that are relevant for allostasis). Allostasis, as the continually adjustment of the body’s internal milieu to promote survival and reproduction, is a fundamental feature of the mammalian nervous system (Sterling, 2012; Sterling and Laughlin, 2015). Affect, characterized as valence (hedonicity) and arousal (physiological activation), is a cue to the value of stimuli for allostasis (Barrett and Bliss-Moreau, 2009) and is also thought to be a general feature of the mammalian nervous system (Anderson and Adolphs, 2014). The broadly distributed neural networks that subserve salience should, then, be present, in some form, in all mammals, but the existence of such networks across mammalian species remains an open question.
A “salience network” (SN) has been identified within the intrinsic architecture of the human brain (Seeley et al., 2007) and its function linked to affect and attention (Barrett and Satpute, 2013; Touroutoglou et al., 2012). Major hubs of the salience network, including anterior insula (AI), anterior cingulate cortex (ACC), and amygdala, show spontaneous, low frequency blood oxygen level-dependent (BOLD) activity that fluctuates in a correlated manner in task independent periods (i.e., in the absence of external stimuli or tasks). We recently demonstrated (Touroutoglou et al., 2012) that the SN can be decomposed into two subnetworks that together represent salience in humans (see Fig. 1). Other neuroimaging studies have shown similar distinctive patterns of connectivity within the dorsal and ventral anterior insula (Chang et al., 2013; Deen et al., 2011; Kelly et al., 2012; Kurth et al., 2010; Taylor et al., 2009; Uddin et al., 2014). The ventral salience subnetwork, anchored in with the agranular ventral AI (vAI), is connected to visceromotor regions that regulate allostasis, as well as regions that represent interoceptive and other sensory inputs linked to affective experience. Connectivity strength variation in this subnetwork uniquely predicted affective experience intensity when viewing unpleasant images (Touroutoglou et al., 2012). In contrast, the dysgranular dorsal anterior AI (dAI) anchors the dorsal salience network; this network is similar to the so-called ventral attention network (Corbetta et al., 2008; Corbetta and Shulman, 2002). Connectivity strength variation in this subnetwork predicted attentional processing— people with greater connectivity were better at switching their attention between sets (Touroutoglou et al., 2012). Thus, the SN can be thought of as an integrated system in which affect and attention interact to encode sensory stimuli that, in the past, have had allostatic consequences.
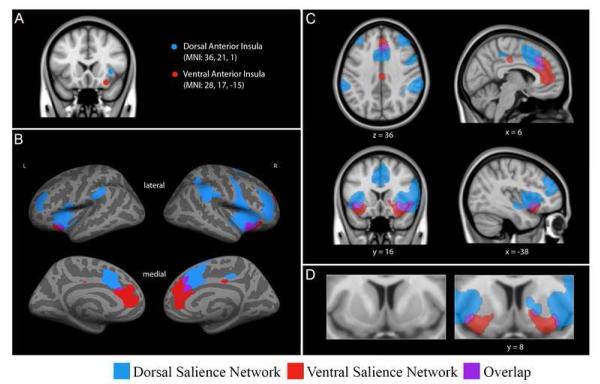
Figure 1.
Dissociable dorsal and ventral salience networks (right dorsal anterior insula seed, blue; right ventral anterior insula seed, red) in humans previously published by our laboratory (Touroutoglou et al., 2012). In B, C and D regions that preferentially correlate with the right dAI seed are shown in blue, regions that preferentially correlate with the right vAI seed are shown in red, and regions that correlate with both seeds are shown in purple. For display purposes, the binarized correlation maps, z(r)>0.2, were overlaid on (B) the inflated cortical surfaces of the left and right hemisphere (the fsaverage template in FreeSurfer) and (C and D) the 1mm MNI152 T1-standard template image in FSL (Adapted figure from Touroutoglou et al., 2012).
There has been extensive study of the neurobiological systems that support attentional processing in macaques (for reviews, see Desimone and Duncan, 2995; Squire et al., 2013). Also, a good deal is known about the macaque neural systems that code for value (i.e., whether a stimulus has been disruptive to allostasis in the past) (for reviews see Wallis , 2007; Morrison and Salzman, 2010). There is still much to learn about how value signals entrain attention to incoming sensory inputs that have been important to allostasis in the past (this has been called “salience”). Some regions that regulate allostasis (i.e., active during reward processing) are also active during spatial attention, such as ventromedial prefrontal cortex, ACC (Kaping et al., 2011) and the amygdala (Peck et al., 2013). Bidirectional anatomical connections (Mesulam and Mufson, 1982a; Mufson and Mesulam, 1982) and intrinsic connections (Hutchison et al., 2011; Hutchison et al., 2012) between two major nodes of the salience network, AI and ACC, have been identified in macaques, but studies have thus far failed to identify a fully developed salience network in monkeys (Mantini et al., 2013). Failure to find any evidence of comparable SNs would call into question the use of macaques as a good model for human brain function, as well as limit their translational value for studying human diseases in which salience or the anatomy or connectivity of the SN is perturbed [e.g., multiple neuropsychiatric disorders(Menon and Uddin, 2010; Uddin, 2014)].
In this paper, we tested the hypothesis that a homologous ventral salience subnetwork exists in macaques, but that a dorsal salience subnetwork would be less in evidence. We used a “seed-based” analysis, specifying two regions in anterior insula as anchor regions (Biswal et al., 1995; Vincent et al., 2007) would reveal Given the evolutional patterns of cortical expansion in humans relative to macaques [e.g., (Hill et al., 2010; Preuss, 2012; Sherwood et al., 2012)] and, in particular, the cortical layers in which the expansion is thought to be focused (Finlay and Uchiyama, 2014), we reasoned that structures that constitute the ventral salience network are largely homologous across macaques and humans, while the dorsal salience network in humans included areas of frontal and parietal cortex that are substantially less developed in macaques (Orban et al., 2004; Passingham, 2009; Vanduffel et al., 2002).
2. Materials and Methods
2.1 Subjects
Subjects were four rhesus macaques (Macaca mulatta, one female, 4-6 kg, 4-7 years old) who had been extensively trained and tested for other magnetic resonance imaging (MRI) studies (Mantini et al., 2012a; Mantini et al., 2013; Mantini et al., 2011; Mantini et al., 2012b). Animal care standards were maintained according to all Belgian and European guidelines (European Union Directive on the Protection of Animals Used for Scientific Purposes 2010/63/EU). Experimental procedures were approved by the KU Leuven Medical School. Animals were socially housed (in pairs or small groups) and provided access to a large group socialization enclosure equipped with toys and enrichment devices. The monkeys received food ad libitum and were allowed to drink water until satiated during the experimental tests.
2.2 Resting state procedure
The current analyses used the same data as in (Mantini et al., 2013). Briefly, monkeys were first trained to continuously fixate on a point (red dot centered on screen 0.3° visual angle in size) on a blank screen in a mock scanner until they reached criterion of at least 95% fixation performance (Vanduffel et al., 2001). Once they reached criterion, they advanced to scanning sessions during which they performed the fixation task while MRI data was collected. During the fixation tasks, monkeys were rewarded with juice when they were successfully fixating. Immediately prior to fMRI data acquisition, animals were injected with microcrystalline iron-oxide nanoparticles (Sinerem; Guerbet; 6-10mg/kg) to increase signal-to-noise ratio beyond that of the normal BOLD signal. During each scan, monkeys sat in a sphinx position in a custom nonhuman primate chair that allowed their heads to be affixed to the chair via implanted MRI compatible head-posts [see (Vanduffel et al., 2001)]. Each subject completed twenty 10-min resting state scans on 5 or 6 different occasions over a period of 6 months.
2.3 fMRI data acquisition
Data were collected on a 3T Siemens Trio scanner in Leuven, Belgium [additional details can be found in (Mantini et al., 2013)]. Monkeys were imaged using an in-house built 8-channel head coil with SENSE reconstruction and a saddle-shaped, radial transmit-only surface coil made specifically for use with nonhuman primates (Kolster et al., 2009). The functional images were collected using a gradient-echo T2*-weighted echo-planar sequence (40 slices, 84 × 84 in-plane matrix, TR/TE = 2000/19ms, flip angle = 75°, voxel size = 1.25 × 1.25 × 1.25 mm3). Additionally, T1-weighted anatomical scans were collected during different scanning sessions while animals were sedated with ketamine/xylazine (induction ketamine 10mg/kg I.M. + Xylazine 0.5 mg/kg I.M., maintenance dose of 0.01 to 0.05 mg ketamine per minute I.V.). Nine whole brain volumes were collected with a MP-RAGE sequence (TR/TE = 2200/4.06, voxel size = 0.5 × 0.5 × 0.5 mm3) and averaged to create each animal’s anatomical composite.
2.4 fMRI data preprocessing
Preprocessing of the resting state fMRI data involved a series of standard procedures previously described in Mantini et al., 2011. Preprocessing was performed with the SPM5.0 software package (http://www.fil.ion.ucl.ac.uk/spm). These steps were: 1) slice-dependent time shifts correction, 2) motion correction, 3) linear detrending, 4) co-registration to the anatomical image, and 5) spatial normalization to macaque F99 (http://sumsdb.wustl.edu/sums/macaquemore.do) atlas space using the 112RM template (http://www.brainmap.wisc.edu/monkey.html), and 6) spatial smoothing with a Gaussian kernel at 2mm full-width-half-maximum. The method is the same as that used in Mantini et al. (2011)—in addition to signal detrending, cerebral spinal fluid and white matter signals and their first derivatives were regressed out of the signal. We did not elect to perform global signal regression. While there is a general consensus about the use of white matter and cerebrospinal fluid regression (Giove et al., 2009; Van Dijk et al., 2010), the importance of global signal regression (i.e. the average signal across the brain) is still debated. It has been demonstrated that the use of global signal regression potentially alters the whole-brain distribution of connectivity values, since the average correlation across the brain is forced to zero. This means that negative correlations may artifactually emerge as a result of global signal regression (Gotts et al., 2013; Murphy et al., 2009; Saad et al., 2012). Other studies disagree, and suggest that the global signal may be at least in part of artifactual origin (He & Liu, 2012) so that the negative correlations that emerge after global signal regression are physiologically meaningful (Fox et al., 2009). Given the controversy, we decided to adopt a conservative approach and not perform global signal regression.
2.5 Seed selection
Salience network analyses were conducted by placing 2 seeds in the right anterior insula. Seed locations were selected based on evaluation of the macaque neuroanatomical literature (Mesulam and Mufson, 1982a, b; Mufson and Mesulam, 1982) and two macaque brain atlases (Paxinos et al., 2000; Saleem and Logothetis, 2012) relative to a human brain atlas (Mai et al., 2008). Seed locations in the macaque brain were selected based on the established sulcal and gyral boundaries of the insula and other landmarks at the appropriate anterior-posterior levels such that they were at anatomically corresponding cortical locations as the human seed locations previously used to identify dorsal and ventral salience networks in two independent samples of healthy young adults (N = 89 and N = 31) (Touroutoglou et al., 2012). The dorsal and ventral seeds were spaced maximally so that they did not overlap, resulting in their placement slightly more dorsal and ventral than might have been expected based on (Touroutoglou et al., 2012). Two spherical seeds [2-mm radius, as in (Mantini et al., 2011)] were used as regions of interest (ROIs) and right vAI (17.5, 5.5, −3.5) (Fig. 2) and right dAI (20, 2.5, 1.5) (Fig. 3) (see Fig. 3S and Fig. 4S Supplementary Materials for left vAI and left dAI seeds).
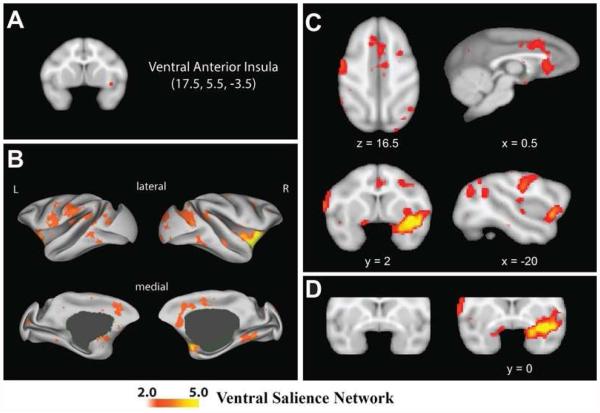
Figure 2.
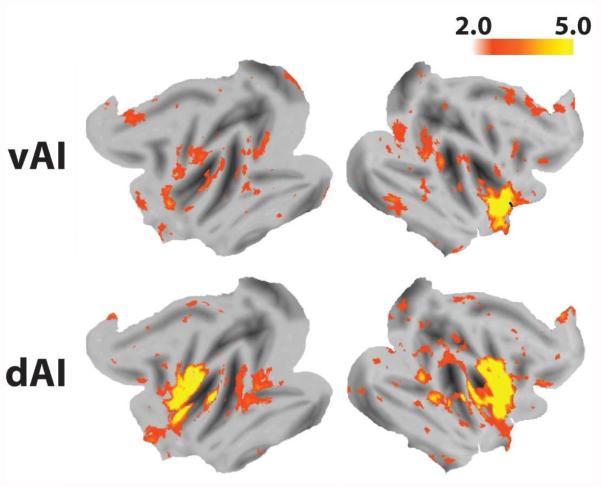
A ventral salience network in macaques. Regions that correlate with the right vAI seed (A) are shown in B, C, and D. Color bars displayed at the bottom of the figure indicate the z-values of correlated voxels. For display purposes, the correlation maps, z > 2, were overlaid on (B) the inflated cortical surfaces of the left and right hemisphere (the surface template in Caret 5.61) and (C and D) the F99 T1 template in neurological convention.
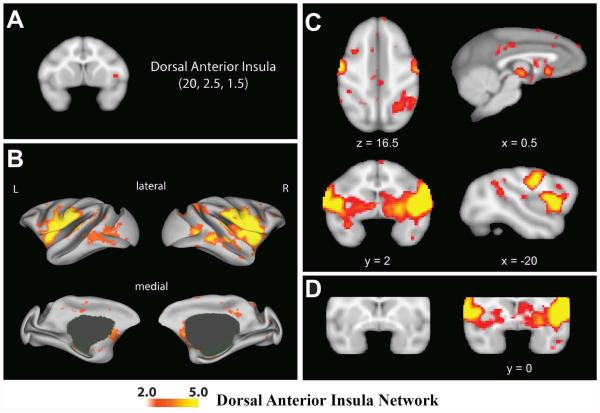
Figure 3.
A dorsal anterior insula network in macaques. Regions that correlate with the right dAI seed (A) are shown in B, C, and D. Color bars displayed at the bottom of the figure indicate the z-values of correlated voxels. For display purposes, the correlation maps, z > 2, were overlaid on (B) the inflated cortical surfaces of the left and right hemisphere (the surface template in Caret 5.61) and (C and D) the F99 T1 template in neurological convention.
2.6. Seed-based intrinsic connectivity network analyses
2.6.1. Spatial topography analysis
We used whole brain seed-based analysis as used in Mantini et al. (2011) to explore the spatial topography of the intrinsic connectivity of vAI and dAI subregions. In a manner similar to seed-based intrinsic connectivity analyses previously published in nonhuman primates (Mantini et al., 2011; Vincent et al., 2007), we extracted the average signal across voxels within each seed region of interest (ROI) and calculated the correlations between the signals in the seed ROI and each voxel of the rest of the brain. Such methods stand in contrast to independent component analysis (Hutchison et al., 2012; Mantini et al., 2013; Moeller et al., 2009) which derives networks that are independent from each other by comparing correlated BOLD signal within and between networks using voxel-wise signals from the whole brain without regard to an anatomically defined point of origin (Joel et al., 2011). Individual whole brain connectivity maps were converted to Z-scores by the Fisher’s r-to-z transformation. It is worth noting that the calculation of the z-scores in the present study differed from that in Touroutoglou et al. (2012) insofar as the calculations used here (from Mantini et al., 2011) account for the number of volumes per scan. As a result, the z-scores from the present study are different than the z-scores used to threshold the previous human study (for equation of z-score calculation see also Supplementary Materials). Group correlation maps were created using a fixed-effect analysis (Genest, 1992). Similarly to the whole brain seed-based analysis used in Touroutoglou et al. (2012), we focused only on positive connectivity as both the dorsal and the ventral salience networks have previously shown positive correlations (Seeley et al., 2007; Touroutoglou et al., 2012).
The ventral and dorsal anterior insula maps were represented in the volume space and in the surface space. The conversion from volumes to surfaces was performed with Caret 5.61 (http://brainvis.wustl.edu/wiki/index.php/Caret:Download) (Van Essen, 2012).
2.6.2. Functional connectivity strength analysis
To quantify the strength of intrinsic connectivity, we calculated the pair-wise correlations between each seed and its correlated target anatomical regions, as in Mantini and colleagues (2011). The target anatomical regions—those that showed connectivity with the vAI and dAI seeds—were determined through visual inspection of the dorsal salience and ventral salience maps independently, as in Touroutoglou et al. (2012), using the Freesurfer viewing tool Freeview (http://surfer.nmr.mgh.harvard.edu/). A minimum connectivity threshold was set to z = 2, p < 0.0062, corrected, with false discovery rate. After localizing regions based on a combination of anatomically defined ROIs (Makris et al., 2010) and functional connectivity clusters, we quantified the connectivity strength for each ROI using a series of statistical steps previously published in Mantini et al. (2011).
To quantify the specificity of the dorsal and ventral salience networks, we compared within- and between-network connectivity. To do this, we computed two summary connectivity measures for each individual target region: 1) a dorsal anterior insula network connectivity measure, by averaging z(r) values between the dAIseed and each target region bilaterally across all monkeys; 2) a ventral anterior insula network connectivity measure, by averaging z(r) values between the vAIseed and each target region bilaterally across all monkeys. Paired-sample t-tests compared the within- and between-network connectivity for both the dAI targets and vAI targets.
3. Results
Connectivity for the ventral AI
The topological correspondence of the ventral salience network between the monkey and the humans is presented in Fig. 1S in the Supplementary Materials. As in the human network (Touroutoglou et al., 2012), intrinsic BOLD signal within the macaque right vAI seed was correlated with intrinsic signal in the anterior and mid cingulate cortices [dorsal ACC (dACC), subgenual ACC (sgACC), mid cingulate cortex (MCC)], orbitofrontal cortex, amygdala, and putamen, as well as in the frontoinsula. As such, the network appeared to be largely homologous with the ventral salience network in humans. Unlike the human network, the macaque ventral salience network also included structures in temporal and dorsolateral striate cortex (e.g., superior temporal sulcus, lunate sulcus, etc.) as well as areas in frontal cortex (e.g., precentral and postcentral gyrus) and mid and posterior insula (see Fig. 2). The mean and standard deviation of correlations between the vAI seed and the anatomical targets of the right ventral salience network across subjects are shown in Table 1S in Supplementary Materials. Similar functional connectivity patterns were observed for the left vAI seed (see Fig. 3S in Supplementary Materials).
Connectivity for the dorsal AI
The topological correspondence of the dorsal anterior insula network between the monkey and the humans is presented in Figure 2S in the Supplementary Materials. As in the human network, intrinsic BOLD signal within the macaque right dAI was correlated with intrinsic BOLD signal in other regions of the insula (bilateral vAI, mid and posterior insula), as well as areas in the frontal cortex located in the precentral and postcentral gyrus, and areas of the cingulate. While the major anatomical nodes of the human network in the cingulate were the dACC/MCC, the macaque network dAI seed evidenced extensive connectivity with the bilateral sgACC and a cluster in posterior cingulate cortex (for functional connectivity of different subregions within the dAI see Fig. 7S in Supplementary Materials; for functional connectivity of the mid-cingulate cortex see Fig. 8S in Supplementary Materials). While both the human and macaque dAI seeds were connected to putamen, connectivity between the macaque dAI seed and putamen was much more extensive insofar as it was bilateral, the clusters in the putamen were large, and the connectivity strength between them and the dAI seed was high.
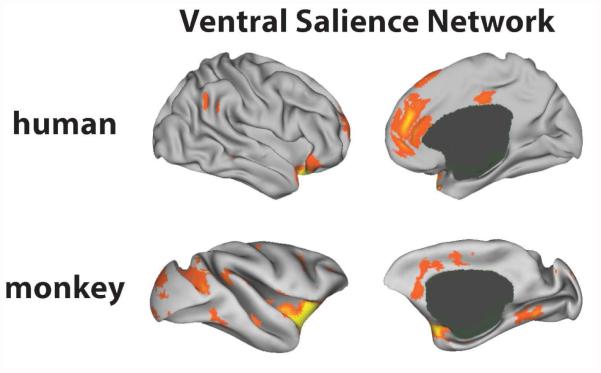
Figure 7.
A homologous ventral salience network in the human and macaque. Folded cortical maps of the ventral salience network in humans (z > .2) and monkeys (z > 2) plotted over a representative cortical surface in lateral and medial views.
In contrast to the human network, which showed connectivity to the inferior parietal lobule, the supramarginal gyrus, and the middle frontal gyrus, the macaque dAI seed had connectivity only with the right intraparietal sulcus of the parietal cortex. In addition, the macaque dAI seed evidenced significant connections with areas of temporal and occipital cortex that was not present in the human network (Fig. 3). Another significant difference was the involvement of additional subcortical structures including the pallidum, basal forebrain, caudate, thalamus, hippocampus, and cerebellum (see Table 2S in Supplementary Materials). Similar functional connectivity patterns were observed for the left dAI seed (see Fig. 4S in Supplementary Materials).
Figure 4.
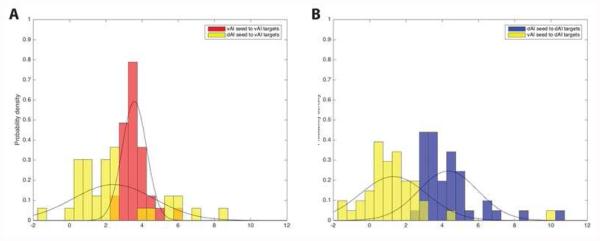
The distributions of within- and between-network connectivity for each pair. In (A) the central tendencies of the connectivity distributions between the right vAI seed and the vAI targets (red) and between the right dAI seed and the nodes of the ventral salience network (vAI targets) (yellow) are non-overlapping. Similarly, in (B) the central tendencies of the connectivity distributions between the right dAI seed and the nodes of the dorsal anterior insula network (dAI targets) (blue) and between the right vAI seed and the dAI targets (yellow) are not overlapping. ROI pairs that are present in both the right dorsal and right ventral salience networks are indicated in dark yellow.
Dissociability of the ventral and dorsal AI connectivity
It was important to determine whether the networks that emerged in the macaque were dissociable from each other. Figure 4 lists connectivity strength between the anterior insula seeds and their respective targets across four subjects (see also Fig. 2 in Touroutoglou et al. (2012) for comparison with the human data and Supplementary Materials for a quality control analysis).
The average within-network correlations were significantly stronger than the between network correlations providing evidence that networks are dissociable [t(40)=9.952, p < 0.001 for the dAIseed-dAItargets as compared to vAIseed-dAItargets connectivity and t(32)=3.26, p < 0.005 for the vAIseed-vAItargets as compared to dAIseed-vAItargets connectivity].
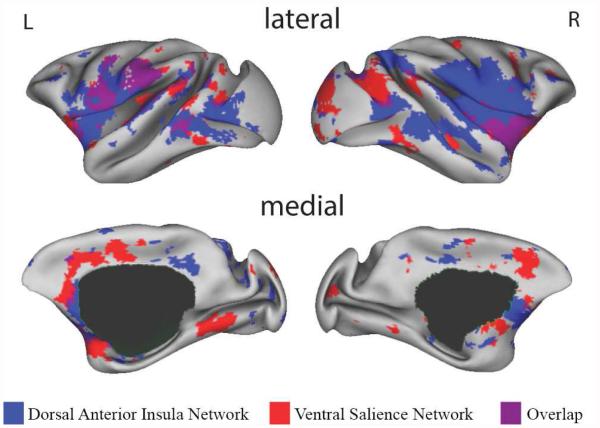
While the human dorsal and ventral salience networks share some regions in common (as do all intrinsic networks; van den Heuvel & Sporsn, 2013), the regions belonging to both subnetworks differed between species (see Fig. 5). In macaques, the ventral salience network and dAI connectivity both contained bilateral vAI, sgACC, and postcentral and precentral gyrus. In contrast, regions of overlap between the two salience subnetworks in the human included bilaterally the lateral OFC, vAI and adjacent frontal operculum, dACC/MCC and putamen (see Fig. 6; also see Fig. 5S for overlapping regions between the two salience networks at each individual monkey). A voxel wise statistical comparison of species differences is presented in Fig. 6S in Supplementary Materials.
Figure 5.
The monkey ventral salience network and dorsal anterior insula network are represented over flattened cortical surfaces (threshold at z > 2) (Caret 5.61).
Figure 6.
The monkey dorsal anterior insula network and ventral salience network are represented on the inflated cortical surfaces of the left and right hemisphere at z = 2 (the surface template in Caret 5.61). Regions that preferentially correlate with the right dAI seed are shown in blue, regions that preferentially correlate with the right vAI seed are shown in red, and regions that correlate with both seeds are shown in purple.
4. Discussion
This study demonstrates that some, but not all, aspects of salience-related intrinsic connectivity are homologous across macaques and humans. Using seed-based intrinsic connectivity analyses, we identified a homologous ventral salience subsystem generated by vAI that was anatomically dissociable from the connections to the dAI. The topography of the human and macaque ventral salience subnetworks was very similar (see Figure 7).
The presence of a homologous ventral salience network is consistent with observations that representations of affect macaques and humans share a number of striking similarities. For example, the parasympathetic and sympathetic branches of the macaque autonomic nervous system respond to evocative stimuli in a manner consistent with the responsivity of the human autonomic nervous system (Bliss-Moreau et al., 2013). Cardiac parasympathetic activity increased and sympathetic activity decreased as macaque subjects viewed videos stimuli that became less unpleasant and more pleasant. Similarly, individual differences in resting state (rather than task-evoked) cardiac activity in macaques relates to individual differences in affective processing (i.e., affective reactivity; Bliss-Moreau, in prep) as is the case in humans [e.g., (Licht et al., 2009; Licht et al., 2008; Oveis et al., 2009; Watkins et al., 1998)]. In humans, functional neuroimaging studies also indicate that activity within the vAI and other regions of salience network, such as amygdala and MCC, contribute to cardiac responsivity to evocative stimuli (Beissner et al., 2013; Craig, 2009; Critchley et al., 2004; Koelsch et al., 2007). We hypothesize that connectivity and activation in the ventral salience network is responsible for representing high-dimensional interoceptive changes (related to allostasis) as lower dimensional affective experience (Chanes and Barrett, 2016). Evidence for this hypothesis comes from studies documenting that individuals with stronger connectivity between vAI and pregenual ACC (Touroutoglou et al., 2012) as well as between the dorsal amygdala and the rest of the salience network (Touroutoglou et al., 2014) predicts more intense self-reported arousal experience while viewing negative pictures. As such, it is possible that connectivity strength in the ventral salience network could be used as a correlate of affective experience in animals who cannot report on their experiences.
While there were some similarities in the connectivity associated with the macaque dAI seed and the human dorsal salience network, the lack of extensive connectivity with parietal and frontal regions suggests that the network that is associated with the dAI in the macaque is only a nascent homolog of the network associated with human dAI. These differences are likely the result of variation between macaque and human neuroanatomy. Macaque brains are thought to be comparable to human brains in terms of their general neuroanatomical regions, the structure of cortex, and global connections between brain areas [and arguably the best neuroscience model for human brains; (Capitanio and Emborg, 2008; Phillips et al., 2014)], but a number of substantial differences between macaque and human brains exist. It is clear, for example, that frontal and parietal areas associated with the dorsal salience network have grown in size and increased in connectivity in humans relative to macaques; the nature of those changes (e.g., whether or not the microstructure and connectivity of frontal and parietal regions have expanded in a way that is greater than what would be expected by scaling up of the brain alone [e.g., (Finlay and Uchiyama, 2015; Herculano-Houzel, 2012; Hill et al., 2010; Orban et al., 2004; Passingham, 2009; Passingham and Smaers, 2014; Preuss, 2012; Rilling, 2006; Sherwood et al., 2012)] is the source of considerable debate [see also (Barton and Venditti, 2013; Vanduffel et al., 2014)]. It is possible that the expansion of insula connectivity with these parietal and other frontal regions in the human allow for the integration of salient information into some cognitive functions that appear to be unique to humans.
Our findings are consistent with growing evidence of intrinsic connectivity between the AI and the ACC in nonhuman primates [i.e., macaques:(Hutchison et al., 2011; Hutchison et al., 2012); marmosets:(Belcher et al., 2013)] and therefore provide evidence that the choice of analysis method may be important in identifying cross-species similarities and differences. We found evidence of some salience network related connectivity in this macaque sample using a seed-based analysis method, whereas the original report utilizing ICA-based analytical methods on the same data (Mantini et al., 2013) did not. There are a number of methodological reasons why this might be the case including sample size and requirements of filtering and univariate cross-correlations in seed-based approaches that are not requirements of ICA. The more important factor, however, was that our analyses were guided by prior findings about the meaningful dorsal-ventral dissociation in the salience network (Touroutoglou et al., 2014; Touroutoglou et al., 2012) combined with knowledge of anatomical differences between the human and macaque brains. Taken together, this combination led us to make specific a-priori predictions about where we would find potential homologies across species. This seems particularly important when working with such small samples of subjects in nonhuman primate studies. Other methodological differences between prior studies in nonhuman primates, such whether the animals are anesthetized, as in (Hutchison et al., 2011; Hutchison et al., 2012) awake but resting without performing an attention task [as in (Belcher et al., 2013)], or awake and performing a fixation task (as in the present study and (Mantini et al., 2013) are important to note, of course. These issues, however, now seem of secondary importance to the issue of whether or not a salience network can be found in the macaque brain.
Taken together, previous findings, in concert with our findings, point to clear homologies between the human and nonhuman primate salience subsystems that arise regardless of methodological variation. The findings are also consistent with new evidence that demonstrates that the microstructure of two major nodes in the SN, the vAI and dACC, may not differ between species as much as previously thought. It was long believed that macaques lacked “von Economo” neurons or “spindle cells” in the AI and ACC that are present in great apes (Allman et al., 2002). However, the presence of “von Economo” neurons in the AI has recently been documented in macaques (Evrard et al., 2012).
There are two logical next steps in this research program—one addressing a limitation of the present study and one expanding the scope of the investigation. First, the number of subjects in the present study is consistent with other nonhuman primate studies, but an important future direction is demonstrating the presence of the network in larger samples of macaques. In concert with demonstrating that the network is present in larger samples, a logical next step of this study is to investigate whether variation in intrinsic connectivity in the ventral salience network is associated with variation in affective processing in monkeys as it is in humans (Touroutoglou et al., 2012). To establish the link between ventral salience network connectivity and affect, it will be important to evaluate the same animals on tests of affective reactivity (e.g., induced changes to sympathetic and parasympathetic activity as in Bliss-Moreau et al., 2013) and network connectivity. After establishing that a relationship exists between affect and network connectivity, it would then be critical to evaluate the magnitude of the relationship between the two variables in order to understand how connectivity in the ventral salience network calibrates with affective experience. This provides an empirical approach for investigating affective processing in a wide-variety of animal species. The development of effective treatments and early interventions for affect-related psychopathology hinges on such animal models.
5. Conclusions
The salience network is important for encoding stimuli that have had allostatic relevance in the past (e.g., what is good, and what is bad). Using seed-based resting state functional connectivity analysis, we provide the first evidence that an affective salience network anchored in the ventral anterior insula exists in the macaque brain. The connectivity of the dorsal anterior insula in macaques did not constitute a network that is homologous to that observed in humans. Our results have important implications for translational systems neuroscience as they speak to issues of whether monkeys can experience affect and to the utility of non-human animal neuroimaging models of psychiatric illness.
Supplementary Material
Highlights.
A salience large-scale brain network is identified in the macaque brain.
The ventral salience subnetwork is homologous in humans and macaques.
The dorsal salience subnetwork is less developed in macaques.
Acknowledgements
We thank Ioannis Sanidas, Ph.D for his assistance in preparing the figures of the manuscript and Olamide Abiose for her help in preparing the Supplementary Materials. Preparation of this manuscript was supported by funding from the National Institutes of Health: R01AG030311 to LFB and BCD and K99MH10138 to EBM; from Research Foundation Flanders (G0A5613, G083111), GOA/10/19; IUAP VII/11; PFV/10/008; and the Hercules foundation to WV, from the Wellcome Trust and the Royal Society: Sir Henry Dale Fellowship grant 101253/Z/13/Z to DM.
Footnotes
Conflicts of Interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Allman J, Hakeem A, Watson K. Two Phylogenetic Specializations in the Human Brain. The Neuroscientist. 2002;8:335–346. doi: 10.1177/107385840200800409. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. Affect as a Psychological Primitive. Advances in experimental social psychology. 2009;41:167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Current Opinion in Neurobiology. 2013:1–12. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton RA, Venditti C. Human frontal lobes are not relatively large. Proc Natl Acad Sci U S A. 2013;110:9001–9006. doi: 10.1073/pnas.1215723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, Yen CC, Stepp H, Gu H, Lu H, Yang Y, Silva AC, Stein EA. Large-scale brain networks in the awake, truly resting marmoset monkey. J Neurosci. 2013;33:16796–16804. doi: 10.1523/JNEUROSCI.3146-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Machado CJ, Amaral DG. Macaque Cardiac Physiology Is Sensitive to the Valence of Passively Viewed Sensory Stimuli. PLoS ONE. 2013;8:e71170. doi: 10.1371/journal.pone.0071170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Emborg ME. Contributions of non-human primates to neuroscience research. Lancet. 2008;371:1126–1135. doi: 10.1016/S0140-6736(08)60489-4. [DOI] [PubMed] [Google Scholar]
- Chanes L, Barrett LF. Redefining the Role of Limbic Areas in Cortical Processing. Trends Cogn Sci. 2016;20:96–106. doi: 10.1016/j.tics.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theo… - PubMed - NCBI. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Forro T, Logothetis NK. Von Economo Neurons in the Anterior Insula of the Macaque Monkey. Neuron. 2012;74:482–489. doi: 10.1016/j.neuron.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Uchiyama R. Developmental mechanisms channeling cortical evolution. Trends Neurosci. 2014 doi: 10.1016/j.tins.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Uchiyama R. Developmental mechanisms channeling cortical evolution. Trends Neurosci. 2015;38:69–76. doi: 10.1016/j.tins.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Genest C. Vincentization Revisited. The Annals of Statistics. 1992;20:1137–1142. [Google Scholar]
- Giove F, Gili T, Iacovella V, VMacaluso E, Maraviglia B. Images-based suppression of unwanted global signals in resting-state functional connectivity studies. Magnetic resonance imaging. 2009;27:1058–1064. doi: 10.1016/j.mri.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Leung LS, Mirsattari SM, Gati JS, Menon RS, Everling S. Resting-state networks in the macaque at 7T. Neuroimage. 2011;56:1546–1555. doi: 10.1016/j.neuroimage.2011.02.063. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Leung LS, Menon RS, Everling S. Resting-State Connectivity Identifies Distinct Functional Networks in Macaque Cingulate Cortex. Cerebral Cortex. 2012;22:1294–1308. doi: 10.1093/cercor/bhr181. [DOI] [PubMed] [Google Scholar]
- Joel SE, Caffo BS, van Zijl PCM, Pekar JJ. On the relationship between seedDbased and ICADbased measures of functional connectivity. Magnetic Resonance in Medicine. 2011;66:644–657. doi: 10.1002/mrm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaping D, Vinck M, Hutchison RM, Everling S, Womelsdorf T. Specific contributions of ventromedial, anterior cingulate, and lateral prefrontal cortex for attentional selection and stimulus valuation. PLoS Biol. 2011;9:e1001224. doi: 10.1371/journal.pbio.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 2012;61:1129–1142. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Remppis A, Sammler D, Jentschke S, Mietchen D, Fritz T, Bonnemeier H, Siebel WA. A cardiac signature of emotionality. European Journal of Neuroscience. 2007;26:3328–3338. doi: 10.1111/j.1460-9568.2007.05889.x. [DOI] [PubMed] [Google Scholar]
- Kolster H, Mandeville JB, Arsenault JT, Ekstrom LB, Wald LL, Vanduffel W. Visual field map clusters in macaque extrastriate visual cortex. J Neurosci. 2009;29:7031–7039. doi: 10.1523/JNEUROSCI.0518-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht CMM, De Geus EJC, van Dyck R, Penninx BWJH. Association between Anxiety Disorders and Heart Rate Variability in The Netherlands Study of Depression and Anxiety (NESDA) Psychosomatic medicine. 2009;71:508–518. doi: 10.1097/PSY.0b013e3181a292a6. [DOI] [PubMed] [Google Scholar]
- Licht CMM, de Geus EJC, Zitman FG, Hoogendijk WJG, van Dyck R, Penninx BWJH. Association Between Major Depressive Disorder and Heart Rate Variability in the Netherlands Study of Depression and Anxiety (NESDA) Archives of general psychiatry. 2008;65:1358. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- Mai J, Paxinos G, Voss T. Atlas of the Human Brain. 3rd Academic Press; San Diego, California: 2008. [Google Scholar]
- Makris N, Kennedy DN, Boriel DL, Rosene DL. Methods of MRI-based structural imaging in the aging monkey. Methods. 2010;50:166–177. doi: 10.1016/j.ymeth.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. Data-driven analysis of analogous brain networks in monkeys and humans during natural vision. Neuroimage. 2012a;63:1107–1118. doi: 10.1016/j.neuroimage.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. Evolutionarily Novel Functional Networks in the Human Brain? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:3259–3275. doi: 10.1523/JNEUROSCI.4392-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand J-B, Joly O, Simone L, Sawamura H, Wardak C, Orban GA, Buckner RL, Vanduffel W. Default mode of brain function in monkeys. Journal of Neuroscience. 2011;31:12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Hasson U, Betti V, Perrucci MG, Romani GL, Corbetta M, Orban GA, Vanduffel W. Interspecies activity correlations reveal functional correspondence between monkey and human brain areas. Nature Methods. 2012b;9:277–282. doi: 10.1038/nmeth.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. The Journal of Comparative Neurology. 1982a;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. The Journal of Comparative Neurology. 1982b;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Moeller S, Nallasamy N, Tsao DY, Freiwald WA. Functional connectivity of the macaque brain across stimulus and arousal states. Journal of Neuroscience. 2009;29:5897–5909. doi: 10.1523/JNEUROSCI.0220-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. Re-valuing the amygdala. Current Opinion Neurobiology. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. The Journal of Comparative Neurology. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9:265–270. doi: 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Passingham R. How good is the macaque monkey model of the human brain? - PubMed - NCBI. Current Opinion in Neurobiology. 2009;19:6–11. doi: 10.1016/j.conb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Smaers JB. Is the prefrontal cortex especially enlarged in the human brain allometric relations and remapping factors. Brain Behav Evol. 2014;84:156–166. doi: 10.1159/000365183. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; 2000. [Google Scholar]
- Peck CJ, Lau B, Salzman CD. The primate amygdala combines information about space and value. Nat Neurosci. 2013;16:340–348. doi: 10.1038/nn.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. Why primate models matter. Am J Primatol. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Human brain evolution: from gene discovery to phenotype discovery. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10709–10716. doi: 10.1073/pnas.1201894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK. Human and nonhuman primate brains: are they allometrically scaled versions of the same design? Evolutionary Anthropology: Issues, Reviews, and Reviews. 2006;15:65–77. [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. Elsevier; 2012. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Bauernfeind AL, Bianchi S, Raghanti MA, Hof PR. Human brain evolution writ large and small. Prog Brain Res. 2012;195:237–254. doi: 10.1016/B978-0-444-53860-4.00011-8. [DOI] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ, Moore T. Prefrontal contributions to visual selective attention. Annual Review of Neuroscience. 2013;36:451–466. doi: 10.1146/annurev-neuro-062111-150439. [DOI] [PubMed] [Google Scholar]
- Sterling P. Allostasis: a model of predictive regulation. Physiol Behav. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Sterling P, Laughlin S. Principles of Neural Design. The MIT Press; Cambridge, Massachusetts: 2015. [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Bickart KC, Barrett LF, Dickerson BC. Amygdala taskDevoked activity and taskDfree connectivity independently contribute to feelings of arousal. Human Brain Mapping. 2014;35:5316–5327. doi: 10.1002/hbm.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett LF. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience. 2014 doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kinnison J, Pessoa L, Anderson ML. Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J Cogn Neurosci. 2014;26:16–27. doi: 10.1162/jocn_a_00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. Cortical cartography and Caret software. Neuroimage. 2012;62:757–764. doi: 10.1016/j.neuroimage.2011.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RBH, Orban GA. Visual Motion Processing Investigated Using Contrast Agent-Enhanced fMRI in Awake Behaving Monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Peuskens H, Denys K, Sunaert S, Todd JT, Orban GA. Extracting 3D from motion: differences in human and monkey intraparietal cortex. Science. 2002;298:413–415. doi: 10.1126/science.1073574. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Zhu Q, Orban GA. Monkey cortex through fMRI glasses. Neuron. 2014;83:533–550. doi: 10.1016/j.neuron.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annual Reviews Neuroscience. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosomatic medicine. 1998;60:498–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.