Abstract
The peptide hormone oxytocin (OT) plays an important role in social behaviors, including social bond formation. In different contexts, however, OT is also associated with aggression, social selectivity, and reduced affiliation. Female meadow voles form social preferences for familiar same-sex peers under short, winter-like day lengths in the laboratory, and provide a means of studying affiliation outside the context of reproductive pair bonds. Multiple lines of evidence suggest that the actions of OT in the lateral septum (LS) may decrease affiliative behavior, including greater density of OT receptors in the LS of meadow voles that huddle less. We infused OT into the LS of female meadow voles immediately prior to cohabitation with a social partner to determine its effects on partner preference formation. OT prevented the formation of preferences for the partner female. Co-administration of OT with a specific OT receptor antagonist did not reverse the effect, but co-administration of OT with a specific vasopressin 1a receptor (V1aR) antagonist did, indicating that OT in the LS likely acted through V1aRs to decrease partner preference. Receptor autoradiography revealed dense V1aR binding in the LS of female meadow voles. These results suggest that the LS is a brain region that may be responsible for inhibitory effects of OT administration on affiliation, which will be important to consider in therapeutic administrations of OT.
Keywords: Oxytocin, Vasopressin, Vasopressin 1a receptor, Meadow vole, Social behavior, Lateral septum
1. Introduction
The neuropeptide hormone oxytocin (OT) is well known for its effects on social behaviors ranging from maternal behavior to monogamous pair bonding and peer affiliation (reviewed in Anacker and Beery, 2013; Carter, 2014; Johnson and Young, 2015). A wealth of studies show that while OT often facilitates social behavior, specific effects vary with species, sex, social context, timing, and brain region, among other factors. In some circumstances, OT is even associated with enhanced aggression, fear, and/or avoidance (reviewed in Beery, 2015; Shen, 2015; van Anders et al., 2013; Yong, 2012; Zik and Roberts, 2015).
Meadow voles (Microtus pennsylvanicus) are seasonally social rodents that provide an opportunity to study mechanisms supporting non-reproductive affiliative behavior. Meadow voles are promiscuous breeders (Boonstra et al., 1993; Getz, 1972), and females are highly territorial during the summer breeding season. This behavioral pattern changes in winter as territories collapse, and voles form mixed-sex groups, including non-kin (Madison et al., 1984). These peer groups become selective, closing to immigration by Spring (Madison et al., 1984; McShea, 1990); similarly, meadow voles housed in short day lengths do not form preferences for a new same-sex cagemate following separation from an original cagemate (Parker and Lee, 2003). In the laboratory, female meadow voles form partner preferences for cohabiting females under winter-like, short day length conditions (Beery et al., 2008b, 2009; Parker and Lee, 2003). Day length is a sufficient cue to prompt shifts in both olfactory preferences (Ferkin and Zucker, 1991), and huddling behavior (Beery et al., 2008b), with temperature further affecting social preferences (Ondrasek et al., 2015). Several studies have begun to investigate the hormonal and neural pathways potentially involved in these behavioral shifts (Anacker et al., 2016; Beery et al., 2008b, 2014; Beery and Zucker, 2010; Ferkin and Zucker, 1991; Leonard et al., 2005; Leonard and Ferkin, 1999; Parker et al., 2001).
OT and the OT receptor (OTR) have been implicated in the modulation of social behavior between same-sex peers in meadow voles. Intracerebroventricular infusion of OT during pairing with another female produces a significant enhancement in the fraction of time spent huddling with the partner over an unfamiliar female, and this enhancement is blocked by addition of OTR antagonist (OTRA) (Beery and Zucker, 2010). This effect is not accounted for by known mechanisms of opposite-sex bond formation: in female prairie voles, partner preference for an opposite-sex mate relies on OT binding to OTRs in the nucleus accumbens (Liu and Wang, 2003; Young et al., 2001); in meadow voles, however, OTR density in the nucleus accumbens is not related to same-sex (Beery and Zucker, 2010) or opposite-sex partner preferences, even when expressed at supraphysiological levels via viral vector-driven expression (Ross et al., 2009). Thus it is of particular interest where and how OT influences partner preferences in meadow voles.
OTRs in brain regions other than the nucleus accumbens likely modulate partner preferences in response to OT. We previously demonstrated that female meadow voles housed under short-day conditions conducive to affiliative behavior exhibited higher levels of OTR density in the central nucleus of the amygdala (Beery and Zucker, 2010), indicating that this is one region with the potential to influence the effects of OT on partner preference. In contrast, we also showed that individual variation in OTR density in the lateral septum (LS) of meadow voles is negatively correlated with time spent huddling in partner preference tests (PPT), suggesting that OT acting in this region might have effects opposing those demonstrated by intracerebroventricular administration (Beery and Zucker, 2010). The LS has been highlighted as an important part of the vertebrate social behavior network (Goodson, 2005; Newman, 1999), and neuropeptide signaling in the LS has been implicated in social recognition, fear, territoriality, and aggression (e.g. Lukas et al., 2013; Veenema et al., 2010; Zoicas et al., 2014). OT action in the LS, in particular, has been linked to withdrawal behaviors including social defeat-induced enhancement of fear (Guzman et al., 2013). Increased OTR density in the LS is also associated with reduced juvenile alloparenting behavior in prairie voles (Olazabal and Young, 2006) and correlates with maternal behaviors in rats (Curley et al., 2012). Actions of the closely related neuropeptide arginine vasopressin (AVP) in the LS have been studied at greater length, and are associated with aggression in male rats (Veenema et al., 2010), and also with pair-bonding in male prairie voles (Liu et al., 2001), while activation of the vasopressin 1a receptor (V1aR) is necessary for social recognition (Landgraf et al., 1995; Lukas et al., 2013; Veenema et al., 2012). The similar structures of OT and AVP allow for substantial affinity at each other’sreceptors (Manning et al., 2012), and recent studies have noted instances in which these peptides act principally through each others’receptors (Qiu et al., 2014; Sala et al., 2011; Schorscher-Petcu et al., 2010; Song et al., 2014), thus testing effects of OT administration at both receptors aids in the identification of potential mechanisms of action.
These findings suggest that nonapeptide hormones may influence behaviors relevant to pair bonding through actions in the LS. We tested the effects of site-specific infusion of OT and receptor antagonists into the LS in order to assess the hypothesis that OT reduces huddling behavior—either in general, or specifically toward the partner.
2. Materials and methods
2.1. Subjects
Meadow voles were bred locally at Smith College. Voles were transferred at weaning on postnatal day 18–20 to a short day light cycle (10 h:14 h, light:dark). Average room temperature was 20 °C, and water and food (Labdiet, Mouse Diet 5015) were available ad libitum. Nesting materials (Lab Supply Enviro-dri bedding, a nestlet, and PVC tubing) were used to supplement bedding (Harlan TekLab aspen shavings) for these burrowing animals.
Subjects were adult females, 79 ± 7 days old, weighing 35.9 ± 1.17 g (mean ± standard error) at the time of behavioral testing. All procedures were approved by the Smith College Institutional Animal Care and Use Committee and were conducted in accordance with national guidelines.
2.2. Experimental treatment
Subjects were pre-assigned one of four infusion treatments: oxytocin (OT), OT with OT receptor antagonist (OT + OTRA), OT with vasopressin 1a receptor antagonist (OT + V1aRA), or artificial cerebrospinal fluid (aCSF) control. The aCSF consisted of 125.2 mM sodium chloride, 3.8 mM potassium chloride, 1.2 mM potassium dihydrogen phosphate, 24.8 mM sodium bicarbonate, 10 mM dextrose, 1 mM magnesium sulfate, and 1.8 mM calcium chloride at pH 7.4. All compounds were dissolved in aCSF, with a total injected volume of 200 nL. Doses were as follows: OT (Bachem): 1 ng, n = 10. OT + OTRA: 1 ng OT with 30 ng specific oxytocin receptor antagonist desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT, n = 9. OT + V1aRA: 1 ng OT with 30 ng specific vasopressin receptor antagonist d(CH2)5[D-Tyr(Me)2,Dab5]AVP, n = 8. Vehicle (aCSF): n = 10. The OTRA and V1aRA compounds were generously provided by Dr. Maurice Manning, University of Toledo (Chan et al., 1996; Manning et al., 2012, 1995).
The peptide receptor antagonists were chosen due to their specificity for the OTR and V1aR, respectively. The OTRA used in the present study and in an earlier study (Beery and Zucker, 2010) is 95 times more potent as an OTR antagonist than as a V1aR antagonist, unlike the OTR antagonists that have been more often used in other laboratories (Manning et al., 2012). The V1aRA used in the present study is selective for the V1a receptor, and exhibits no antagonist effects at either the vasopressin 2 receptors or the OTR (Manning et al., 2012). While the use of peptide antagonists is inadvisable with peripheral administration without a known mechanism by which the peptides can cross the blood brain barrier, non-peptide antagonists hold no advantage with infusion directly into the brain, as in the present study.
2.3. Surgical procedure
General anesthesia was maintained through approximately 3% isoflurane (IsoFlo, Abbott Lab) while a stereotaxic apparatus (Kopf) was used for guide cannula placement. To target the LS, a single guide cannula was inserted at a 10° angle (as in Bredewold et al., 2014), placed in the right hemisphere at +0.8 mm anterior −0.5 mm lateral, and lowered −2.5 mm ventral from bregma. One screw (0–80 × 1/16, PlasticsOne) was inserted into the skull for secure attachment with dental cement (GC Fuji Plus, Smartpractice). The guide cannula was 1.8 cm in length, cut from 26 gauge stainless steel hypodermic tubing (Small Parts, Inc.). A dummy cannula (31 gauge, Small Parts, Inc.) was placed in the guide cannula following surgery and drug infusion.
Buprenorphine (0.05 mg/kg of body weight) and ketoprofen (5 mg/kg) were administered subcutaneously for analgesic and anti-inflammatory effects before and after surgery. Postoperative animals were monitored daily. Subjects with a compromised cannula were euthanized without inclusion in the study.
2.4. Infusion and cohabitation
Following three days of recovery from cannula placement, treatments were administered under brief isoflurane anesthesia approximately 2 h after onset of the light cycle. A syringe (Neurosyringe microliter 7001, Hamilton) was inserted into the guide cannula, extending 2 mm beyond the end. 200 nL of solution was administered continuously over a period of 1 min, and the syringe was held in place for an additional minute to allow the solution to diffuse into the tissue. Immediately after infusion and recovery from anesthesia, the subject was moved to a clean cage and a 24 h cohabitation began with a novel partner (an age-matched, non-sibling female).
2.5. Partner preference testing
The PPT apparatus was a linear 3-chambered cage that has been described previously (Ahern et al., 2009; Anacker et al., 2016). The PPT has been described previously (Ahern et al., 2009; Williams et al., 1992) and is described briefly here. Immediately after the cohabitation, the partner was tethered at one end of the apparatus while an age- and sex-matched stranger was tethered at the other end. The focal animal was then placed in the center of the chamber and allowed to roam throughout the apparatus for the duration of the 3 h test. Tests were video recorded with a ceiling-mounted camera for later scoring. After testing, focal animals were euthanized under carbon dioxide and the brain was dissected for verification of the cannula placement.
2.6. Cannula placement verification
For visualization and verification of the cannula placement, 250 nL India ink were infused using a Neurosyringe extending 2 mm past the end of the guide cannula, immediately after euthanasia. Brains were frozen on crushed dry ice and then stored at −80° C until they were sliced at 40 µm on a cryostat (Leica CM 1900). Targeting was evaluated without knowledge of treatment group, and improper targeting (n = 11) or lack of visible dye to allow confirmation of placement (n = 5) resulted in removal of that subject from analysis.
2.7. Behavior analysis
A trained observer, blind to subject treatment, scored videos using custom software (Intervole Timer v. 1.6, AKB, available upon request). The amount of time the subject spent huddling with the stimulus animals was the primary outcome measure; time spent in each chamber and number of crossings between chambers were also recorded.
2.8. Receptor autoradiography
Receptor autoradiography was performed to assess V1aR binding in the brains of 27 female meadow voles. Subjects were long-day and short-day housed females with previously collected brain tissue (Beery et al., 2014; Beery and Zucker, 2010). Subjects had prior experience as focal voles or partners in PPTs, and huddling data were available for the focal voles (n = 11). One series of 20 µm coronal sections was assayed with 50pM linear V1aRA radioligand [125I]-phenylacetyl-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-NH2(NEX310, PerkinElmer, Waltham, MA). Adjacent sections were processed for non-specific binding using radioligand together with 500 nM vasopressin receptor antagonist d(CH2)51[Tyr(Me)2,Arg8]vasopressin (H-5350, Bachem, Torrance, CA). The assay was performed exactly as described elsewhere (Beery et al., 2008a). Slides were apposed to film (Kodak BioMax MR film, Carestream Health) for 48 h before developing. 125I-labeled autoradiographic standards (ARI 0133, American Radiolabeled Chemicals Inc.) were used to convert optical density to nCi/mg tissue equivalent.
Binding for each subregion of the LS was quantified in three adjacent sections and averaged for each brain. For each region, a fixed area was sampled in each section. Anterior LS binding was sampled in the dorsolateral region of highest binding within anterior sections. Posterior LS binding was sampled in sections near the anterior commissure fusion, throughout the vertical extent of binding. Non-specific binding was subtracted from total binding to yield specific binding values. Anterior and posterior LS binding densities were comparable, and these regions were averaged for the LS analysis below.
2.9. Statistical analysis
The time spent huddling in the PPT was analyzed using a two-way ANOVA with drug treatment as the between-subjects variable and stimulus animal as a within-subjects variable. A Mann–Whitney U-test was used for analysis of non-normally distributed samples to compare the time spent huddling with partners compared to strangers in each group, and to perform a post hoc comparison between partner huddling time in the aCSF and OT groups.
V1aR density in short day- and long day-housed voles was analyzed by t-test. Correlation between V1aR density and huddling behavior in the PPT was assessed using correlation coefficient (r) in the subset of subjects used as focal voles in the test. Correlations between V1aR density and OTR, or corticotropin releasing factor receptor (type 1 or 2) measured previously (Beery et al., 2014; Beery and Zucker, 2010) were also assessed using Pearson’s r.
All statistical tests were performed using JMP 8.0 (SAS Institute Inc.) and the α level was set at 0.05 for statistical significance.
3. Results
3.1. Effects of oxytocin on partner preference
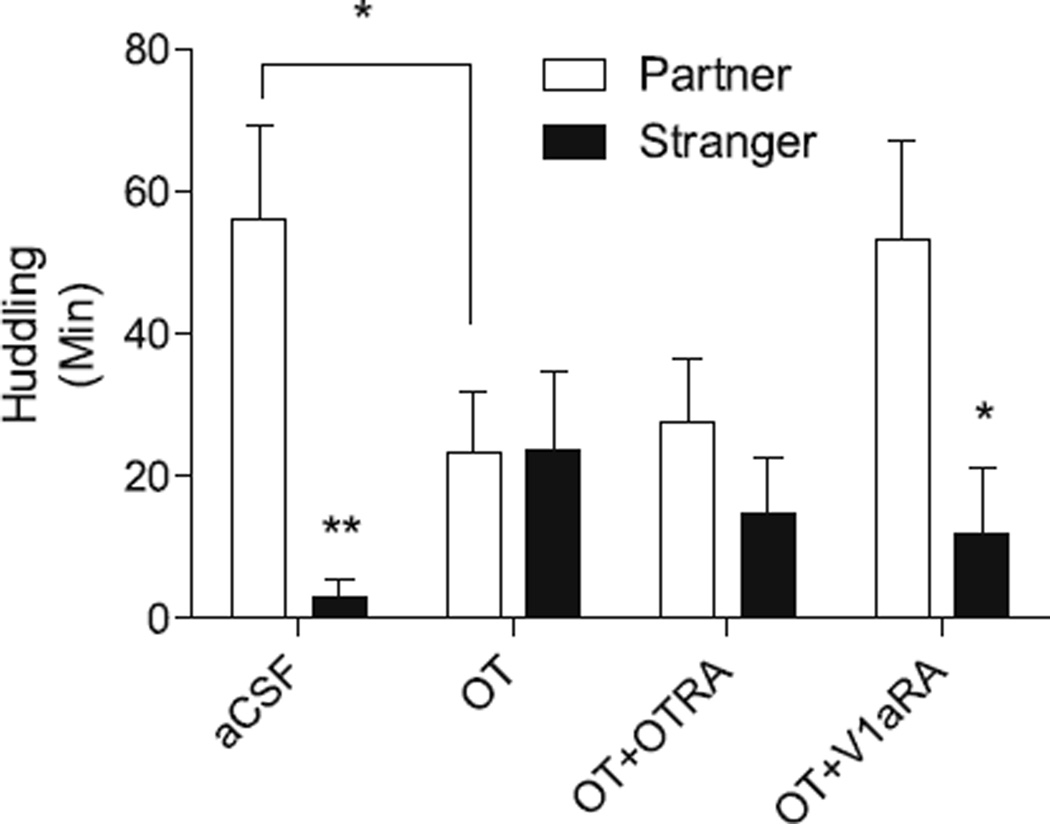
Huddling behavior in the PPT following infusion into the LS is shown in Fig. 1. There was no main effect of infusion treatment alone on total huddling time (F(366) = 0.527; p = 0.665). There was a significant main effect of stimulus animal on huddling time (F(166) = 14.6; p = 0.0003), with more partner huddling than stranger huddling. There was a significant interaction between infusion treatment and stimulus animal (F(366) = 3.32; p = 0.025), indicating that infusion treatment group altered huddling with the partner versus the stranger. The aCSF-treated subjects spent significantly more time huddling with the partner than the stranger (U = 4, p = 0.0006), while subjects with OT infused into the LS exhibited no difference in huddling with the partner compared to the stranger (U = 45.5, p = 0.762). Co-administration of the OTRA with OT did not block this effect of OT; huddling was not significantly different with the partner versus stranger (U = 24, p = 0.162). Co-administration of the V1aRA with OT reversed the effect, as there was a significant difference between the time spent huddling with the partner and stranger in this group (U = 12, p = 0.0404). In addition, OT subjects spent significantly less time huddling with the partner compared to vehicle-treated controls (U = 23, p = 0.04), and a lower fraction of time huddling with the partner relative to total huddling (U = 21, p = 0.03; 0.952 ± 0.031 for controls versus 0.493 ± 0.143 for the OT group, mean ± SEM, voles with <5 min huddling excluded). The fraction of time huddling with the partner relative to total huddling was 0.5410 ± 0.152 for the OT + OTRA group, and 0.797 ± 0.129 for the OT + V1aRA group (mean ± SEM).
Fig. 1.
Effects of oxytocin infusion on partner preferences for a same-sex cage-mate. A significant preference for huddling with the partner over the stranger was observed in the aCSF group, while OT inhibited the partner preference, and significantly less time was spent huddling with the partner in the OT group compared to the aCSF group. OTRA co-administration did not block the OT-induced inhibition of the partner preference, whereas V1aRA co-administration rescued the partner preference. *p < 0.05, **p < 0.001. aCSF: artificial cerebrospinal fluid (vehicle); OT: oxytocin (1 ng); OT + OTRA: oxytocin (1 ng) + oxytocin receptor antagonist (30 ng); OT + V1aRA: oxytocin (1 ng) + vasopressin receptor antagonist (30 ng).
3.2. Vasopressin 1a receptor binding in the lateral septum
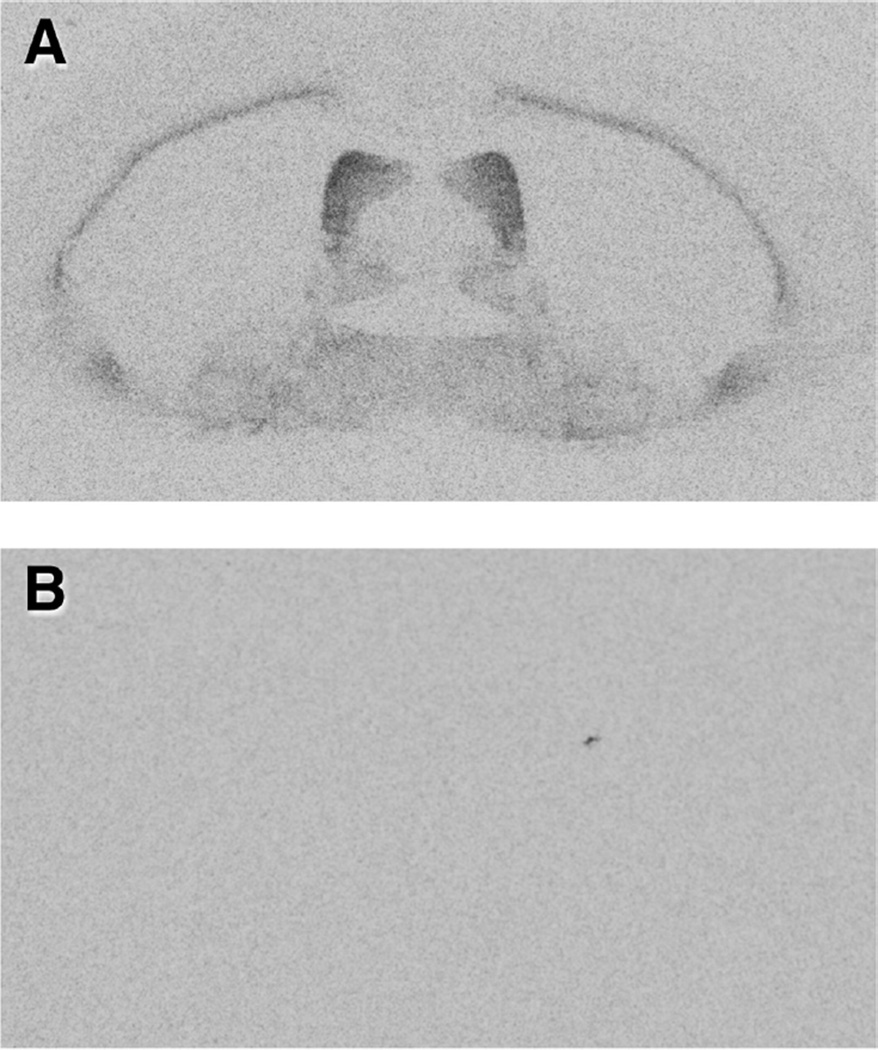
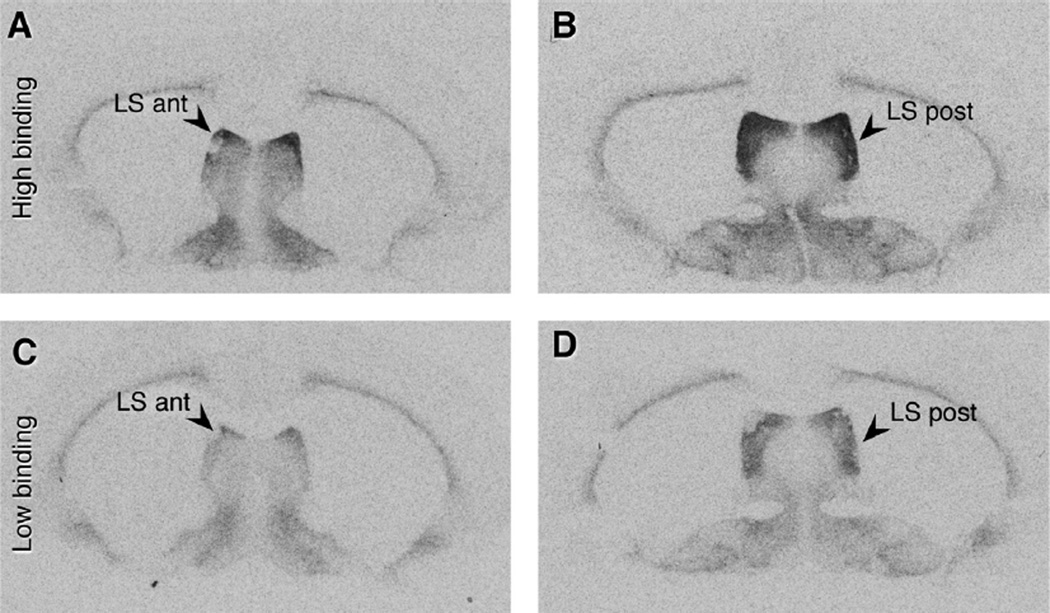
Binding of radioactive ligand to V1a receptors was detected in several brain regions, and background binding was eliminated by co-incubation with non-radioactive selective V1aR ligand (Fig. 2). V1aR binding was very dense in the LS of female meadow voles compared to other brain regions, consistent with previous reports in male meadow voles (Insel et al., 1994; Parker et al., 2001) and diverse rodent species (surveyed in Beery, 2015; Beery et al., 2008a). Substantial binding was also observed in the ventral pal-lidum, bed nucleus of the stria terminalis, medial preoptic area, and thalamus. A high level of binding was evident throughout the LS, from anterior to posterior sections, with individual variation in density (Fig. 3). In many subjects, the dorsal LS appeared to have more dense binding than the rest of the LS.
Fig. 2.
Vasopressin 1a receptor binding in the presence and absence of a selective receptor antagonist. (A) Incubation with the radioligand [125I]-phenylacetyl-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-NH2. (B) Co-incubation with radioligand and an excess of unlabeled selective vasopressin 1a receptor ligand d(CH2)51[Tyr(Me)2,Arg8]vasopressin in an adjacent section from the same brain. Exposure time was optimized to keep lateral septum binding within the linear range of iodinated standards; in this range non-specific binding was almost undetectable.
Fig. 3.
Vasopressin 1a receptor binding in the lateral septum. One representative individual (top row) displays dense V1aR binding in anterior (A) and posterior (B) sections of the LS. A second representative individual (bottom row) demonstrates the low end of observed V1aR binding in anterior (C) and posterior (D) sections of the LS, while binding in other brain regions remains consistent. Binding in the anterior LS was highest in the dorsal region, while binding was more uniform in the posterior LS.
There was no significant difference in V1aR levels between subjects housed under long day and short day conditions (14L versus 10L; t(19) = 0.447, p = 0.660, Table 1). In the subsample of individuals used as focal voles in PPTs, individual differences in V1aR binding density within the LS were unrelated to total huddling time (r = 0.032, n = 11, p = 0.93), huddling time with the partner (r = 0.028, n = 11, p = 0.93), or huddling time with the stranger (r = 0.164, n = 11, p = 0.63). V1aR binding levels in the LS were not significantly correlated with levels of OTR or corticotropin releasing factor receptor (type 1 or 2) binding in the lateral septum measured in the same individuals in previous assays (Beery et al., 2014; Beery and Zucker, 2010).
Table 1.
Vasopressin 1a receptor radioligand binding (nCi/mg tissue equivalent) with non-specific binding subtraction. Receptor binding did not differ across the two sub-regions with high binding (anterior/dorsal versus posterior) or by day length (short days versus long days).
| Day Length |
V1aR LS ant |
V1aR LS post |
n |
|---|---|---|---|
| SD | 3.70 ± 0.31 | 3.20 ± 0.23 | 13 |
| LD | 3.57 ± 0.17 | 3.29 ± 0.15 | 14 |
4. Discussion
This study is the first to show that OT acting in a specific brain region can acutely reduce affiliative behavior in adult peer relationships. Control subjects exhibited a strong preference for huddling with their cagemate over an unfamiliar individual; infusion of OT into the LS abolished this preference for the partner. These findings contrast with the effects of OT infusion into the lateral ventricles, which result in enhancement of partner preferences (Beery and Zucker, 2010). This supports the growing evidence that OT does not always promote affiliation, and that the brain region and/or timing in which it acts is essential for predicting the behavioral outcome (Beery, 2015; van Anders et al., 2013).
The effects of OT were not blocked by administration of a saturating amount of highly specific OTR antagonist. Instead, a saturating amount of specific V1aR antagonist blocked the effect of OT when co-administered in the LS. This suggests that the effects of OT were V1aR-dependent, not OTR-dependent. The present results do not conclusively demonstrate this as the mechanism of action, and it remains plausible that the V1aR antagonist in the LS affected partner preference through a separate mechanism of action that was not due to blocking OT binding. However, work from other laboratories contributes strong evidence for the actions of OT via V1aRs. A similar effect was recently reported in which OT administered intracerebroventricularly induced flank marking in adult male hamsters, and the V1aR antagonist, but not OTR antagonist, blocked this effect. Importantly, the differing effects of the receptor antagonists held true even when OT was produced endogenously, induced by administration of α-melanocyte-stimulating hormone (Song et al., 2014). OT administration induced analgesia in mice, but not in V1aR knockouts or when a V1aR antagonist was administered (Qiu et al., 2014; Schorscher-Petcu et al., 2010). OT rescued social behavioral deficits in OTR knockout mice, but not when pretreated with a V1aR antagonist (Sala et al., 2011). Finally, blocking either OTR or V1aR in the LS of male prairie voles leads to a deficit in partner preferences for a familiar female (Liu et al., 2001), in contrast to restoration of preference only by V1aR blockade in same-sex partner preferences in female meadow voles demonstrated here.
One initially plausible explanation for these effects of OT in the LS is that OT is altering social recognition. OT knockout mice are socially amnestic (Ferguson et al., 2000), and while OT signaling in the medial amygdala has been shown to be most important for social recognition (Ferguson et al., 2001; Gur et al., 2014), V1aR signaling in the LS of adult rats is necessary for recognition (Landgraf et al., 1995; Lukas et al., 2013; Veenema et al., 2012). However, if OT binding to V1aRs in the LS were enhancing recognition, one would expect to see increased preference for the partner in the OT treatment group, and loss of distinction between partner and stranger in the V1aRA group—opposite the present findings.
The LS, and particularly the V1aRs in this region, have been associated with aggressive behavior in many studies. AVP is released in the LS during inter-male aggression in rats, and blockade of V1aR prevents this aggression (Veenema et al., 2010). Similar findings were demonstrated in male zebra finches, where vasotocin increased aggression and an antagonist reduced it (Goodson and Adkins-Regan, 1999). In female rats, both OTR and V1aR levels in the LS change over time in a manner that positively correlates with the development of maternal aggression (Caughey et al., 2011). It is possible that activation of V1aR in the LS of female meadow voles by administration of OT in the present study induced aggressive tendencies or negative affect just prior to pairing, preventing formation of a partner preference. Future studies could look at the effect of OT in the LS on aggressive behaviors immediately after infusion in female meadow voles. Additionally, future tests of OT administration after pairing but before partner preference testing could assess whether the effect on the expression of the preference for the partner is similar to the observed effects on formation, which could also give more information regarding the behavioral mechanism of action of OT, i.e. increasing aggression or affecting social recognition.
It is unknown whether OT action at V1aRs is an endogenous signaling mechanism impacting the development of the partner preferences. OT release has been measured by microdialysis in the dorsal lateral septum in mice (Zoicas et al., 2014), and OT fibers are present in the ventral LS in meadow voles (Beery, 2015), indicating that OT is delivered to this region under some circumstances. This OT may bind to OTRs, as well as to dense V1aRs. Affinity (Ki) of OT for the V1aR is approximately 46–71 nM in mice and rats, compared to 0.6–1 nM at the OTR, while the affinity of vasopressin for the V1aR is 1.3–2.6 nM, and 1.7–1.8 nM at the OTR (Manning et al., 2012). OT binding to V1aRs is more likely to occur when present at high concentrations, and the dose given in the present study—while similar to those used in previous reports (e.g. Liu and Wang, 2003)—is likely still supraphysiological, and much higher than other doses recently found effective at modulating social behaviors (Dumais et al., 2016). The use of high doses of administered OT is useful for understanding potential responses of brain circuitry to OT, as well as possible effects of exogenous OT (such as are currently used in human clinical trials), however it may not reflect the normal endogenous signaling in the brain.
Vasopressin immunoreactivity in the LS is testosterone-dependent, and typically lower in females than in males (Bamshad et al., 1993; de Vries et al., 1981; De Vries et al., 1992; Wang and Devries, 1993), but may also play a role in activating V1aRs in female meadow voles. V1aR density in untreated voles did not relate to time spent huddling in PPTs, although behavioral data were only available for 11 of the voles assayed, which would not provide sufficient power to detect a moderate relationship. Furthermore, correlations between individual receptor binding and behavior are not always present, even when manipulations of receptor density affect behavior in question (Keebaugh et al., 2015). Therefore, the role of endogenous vasopressin should also be considered in the formation of the partner preference among female meadow voles.
While the endogenous peptide signaling effects on social behavior are important to understand, the role of exogenously administered OT is also critical to inform current studies and therapeutic applications of OT. Intranasal OT is given acutely in clinical trials and other circumstances, and although there is uncertainty about how intranasal OT leads to elevated brain OT, it remains essential to understand the range of possible effects of this drug, regardless of which downstream receptor pathways mediate the effects. Importantly, there is a growing body of evidence showing that OT, contrary to its long-standing reputation as the “love hormone”, has effects that are not always pro-social, and vary depending on a variety of factors including social target (group member versus perceived outsider), prior history, sex, and target region within the brain (see Beery, 2015 for review; De Dreu et al., 2010, 2011; Declerck et al., 2014; Goodson and Thompson, 2010; Scheele et al., 2014; Sheng et al., 2013). These context, sex, and region-specific effects are extremely important to understand, especially as intranasal OT becomes more widely administered in human populations. In an animal model of chronic intranasal OT administration from a young age, male prairie voles showed decreased social bonding behavior in adulthood (Bales et al., 2013). In a study of juvenile social behavior, female rats showed inhibited play in the home cage when OT was administered in the LS (Bredewold et al., 2014). Now we show that OT in the LS inhibits the development of peer social preferences in female meadow voles in adulthood. These studies suggest that the LS may be an important region for the regulation of effects of OT on social behavior.
5. Conclusions
This study adds to the growing body of evidence that oxytocin does not always enhance social behavior. This is the first experiment to demonstrate that oxytocin, acting through vasopressin 1a receptors in the lateral septum, can interfere with normally expressed affiliation for a social partner. Further study of the nuances in OT’seffects on social behaviors should help to elucidate the roles of specific brain regions and contexts in mediating these effects.
Acknowledgments
Role of the funding source
This work was supported by grants from the National Science Foundation, the National Institutes of Health, and the Eveillard Postdoctoral Fellowship at Smith College. Funding sources played no further role in study design, data collection, data analysis, or decision to publish.
We are grateful to Dr. Maurice Manning for the generous donation of the oxytocin receptor antagonist and vasopressin 1a receptor antagonist used in the present studies. Nastacia Goodwin assisted with autoradiography and figure preparation. Nastacia Goodwin, Lucy Bicks, and Mursal Nader acted as surgery assistants, and Dr. Alexa Veenema and Remco Bredewold consulted on surgical techniques. Chase Freschlin scored the social behavior tests and assisted with cannula placement verification. Emily Starr–Phillips contributed to the early design of the studies. This work was supported by National Science Foundation grant #1257162 to A. Beery, the Eveillard Postdoctoral Fellowship to A. Anacker, and National Institutes of Health T32 training grant #MH016434.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to report.
Contributions
AMJA designed the experiments, performed surgeries, behavioral testing and receptor autoradiography, analyzed data, and drafted, revised and approved the manuscript.
JDC performed surgeries and behavioral testing, contributed to data analysis and manuscript preparation, and critically revised and approved the manuscript.
EML performed surgeries and behavioral testing, and critically revised and approved the manuscript.
DMG analyzed receptor autoradiography, and critically revised and approved the manuscript.
AKB designed the experiments, analyzed data, and drafted, revised and approved the manuscript.
Contributor Information
Allison M.J. Anacker, Email: allison.anacker@gmail.com.
Jennifer D. Christensen, Email: j3nnchrstnsn@gmail.com.
Elyssa M. LaFlamme, Email: eml97@georgetown.edu.
Diana M. Grunberg, Email: diana.ms.grunberg@gmail.com.
Annaliese K. Beery, Email: abeery@smith.edu.
References
- Ahern TH, Modi ME, Burkett JP, Young LJ. Evaluation of two automated metrics for analyzing partner preference tests. J. Neurosci. Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Beery AK. Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci. 2013;7:185. doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Reitz KM, Goodwin NL, Beery AK. Stress impairs new but not established relationships in seasonally social voles. Horm. Behav. 2016;79:52–57. doi: 10.1016/j.yhbeh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol. Psychiatry. 2013;74:180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J. Neuroendocrinol. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Beery AK. Antisocial oxytocin: complex effects on social behaviors. Curr. Opin. Behav. Sci. 2015;6 [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J. Comp. Neurol. 2008a;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Beery AK, Loo TJ, Zucker I. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Horm. Behav. 2008b;54:153–159. doi: 10.1016/j.yhbeh.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Routman DM, Zucker I. Same-sex social behavior in meadow voles: multiple and rapid formation of attachments. Physiol. Behav. 2009;97:52–57. doi: 10.1016/j.physbeh.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Vahaba DM, Grunberg DM. Corticotropin-releasing factor receptor densities vary with photoperiod and sociality. Horm. Behav. 2014;66:779–786. doi: 10.1016/j.yhbeh.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience. 2010;169:665–673. doi: 10.1016/j.neuroscience.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Xia X, Pavone L. Mating system of the meadow vole, Microtus pennsylvanicus. Behav. Ecol. 1993;4:83–89. [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front. Behav. Neurosci. 2014;8:216. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, Meddle SL. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J. Neuroendocrinol. 2011;23:1113–1124. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- Chan WY, Wo NC, Cheng LL, Manning M. Isosteric substitution of Asn5 in antagonists of oxytocin and vasopressin leads to highly selective and potent oxytocin and V1a receptor antagonists: new approaches for the design of potential tocolytics for preterm labor. J. Pharmacol. Exp. Ther. 1996;277:999–1003. [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Franks B, Champagne FA. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav. 2012;61:454–461. doi: 10.1016/j.yhbeh.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proc. Natl. Acad. Sci. U.S.A. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain–presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Crenshaw BJ, al-Shamma HA. Gonadal steroid modulation of vasopressin pathways. Ann. N.Y. Acad. Sci. 1992;652:387–396. doi: 10.1111/j.1749-6632.1992.tb34369.x. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. The effect of oxytocin on cooperation in a prisoner’s dilemma depends on the social context and a person’s social value orientation. Soc. Cogn. Affect Neurosci. 2014;9:802–809. doi: 10.1093/scan/nst040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH. Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology. 2016;64:79–88. doi: 10.1016/j.psyneuen.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Zucker I. Seasonal control of odour preferences of meadow voles (Microtus pennsylvanicus) by photoperiod and ovarian hormones. J. Reprod. Fertil. 1991;92:433–441. doi: 10.1530/jrf.0.0920433. [DOI] [PubMed] [Google Scholar]
- Getz LL. Social structure and aggressive behavior in a population of Microtus pennsylvanicus. J. Mamm. 1972:53. [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J. Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Gur R, Tendler A, Wagner S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol. Psychiatry. 2014;76:377–386. doi: 10.1016/j.biopsych.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat. Neurosci. 2013;16:1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J. Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci. 2015;10:561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding social discrimination abilities, and anxiety-related behavior in rats. J. Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard ST, Alizadeh-Naderi R, Stokes K, Ferkin MH. The role of prolactin and testosterone in mediating seasonal differences in the self-grooming behavior of male meadow voles, Microtus pennsylvanicus. Physiol. Behav. 2005;85:461–468. doi: 10.1016/j.physbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Ferkin MH. Prolactin and testosterone affect seasonal differences in male meadow vole, Microtus pennsylvanicus, odor preferences for female conspecifics. Physiol. Behav. 1999;68:139–143. doi: 10.1016/s0031-9384(99)00161-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav. Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38:916–926. doi: 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Madison DM, FitzGerald RW, McShea WJ. Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): possible consequences for population cycling. Behav. Ecol. Sociobiol. 1984;15:9–17. [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY. Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with Atosiban. Int. J. Pept. Protein Res. 1995;46:244–252. doi: 10.1111/j.1399-3011.1995.tb00596.x. [DOI] [PubMed] [Google Scholar]
- McShea WJ. Social tolerance and proximate mechanisms of dispersal among winter groups of meadow voles, Microtus pennsylvanicus. Anim. Behav. 1990;39:346–351. [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann. N.Y Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm. Behav. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ondrasek NR, Wade A, Burkhard T, Hsu K, Nguyen T, Post J, Zucker I. Environmental modulation of same-sex affiliative behavior in female meadow voles (Microtus pennsylvanicus) Physiol. Behav. 2015;140:118–126. doi: 10.1016/j.physbeh.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Kinney LF, Phillips KM, Lee TM. Paternal behavior is associated with central neurohormone receptor binding patterns in meadow voles (Microtus pennsylvanicus) Behav. Neurosci. 2001;115:1341–1348. doi: 10.1037//0735-7044.115.6.1341. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Lee TM. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J. Comp. Psychol. 2003;117:283–289. doi: 10.1037/0735-7036.117.3.283. [DOI] [PubMed] [Google Scholar]
- Qiu F, Qiu CY, Cai H, Liu TT, Qu ZW, Yang Z, Li JD, Zhou QY, Hu WP. Oxytocin inhibits the activity of acid-sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br. J. Pharmacol. 2014;171:3065–3076. doi: 10.1111/bph.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol. Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Kendrick KM, Schwering C, Noelle J, Wille A, Schlapfer TE, Maier W, Hurlemann R. Opposing effects of oxytocin on moral judgment in males and females. Hum. Brain Mapp. 2014;35:6067–6076. doi: 10.1002/hbm.22605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J. Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. Neuroscience: the hard science of oxytocin. Nature. 2015;522:410–412. doi: 10.1038/522410a. [DOI] [PubMed] [Google Scholar]
- Sheng F, Liu Y, Zhou B, Zhou W, Han S. Oxytocin modulates the racial bias in neural responses to others’suffering. Biol. Psychol. 2013;92:380–386. doi: 10.1016/j.biopsycho.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JKt, Larkin TE, 2nd, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine–vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anders SM, Goodson JL, Kingsbury MA. Beyond oxytocin = good: neural complexities and the flipside of social bonds. Arch. Sex Behav. 2013;42:1115–1118. doi: 10.1007/s10508-013-0134-9. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav. 2012;61:50–56. doi: 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Devries GJ. Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster) Brain Res. 1993;631:156–160. doi: 10.1016/0006-8993(93)91203-5. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Yong E. Dark side of the love hormone. New Sci. 2012;213:39–41. [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm. Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Zik JB, Roberts DL. The many faces of oxytocin: implications for psychiatry. Psychiatry Res. 2015;226:31–37. doi: 10.1016/j.psychres.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacol. 2014;39:3027–3035. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]