Abstract
Background
Blood stream infection (BSI) among Neonatal Intensive Care Unit (NICU) infants is a frequent problem associated with poor outcomes. Monitoring for abnormal heart rate characteristics (HRC) may decrease infant mortality by alerting clinicians to sepsis before it becomes clinically apparent.
Methods
HRC scores were acquired using the HRC (HeRO) monitor system from Medical Predictive Science Corporation and entered into the electronic medical record by bedside staff. We retrospectively analyzed HRC scores recorded twice daily in the medical record during a 30-month period (January 1, 2010 through June 30, 2012) for infants in the NICU at the Monroe Carell Jr. Children’s Hospital at Vanderbilt. We identified infants that met CDC criteria for late-onset BSI (>3 days of life) during the study period.
Results
During the study period, we recorded 127,673 HRC scores from 2384 infants. We identified 46 infants with BSI. Although 8% (9701/127,673) of the HRC scores were ≥2 and 1% (1387/127,673) were ≥5, BSI (at any time) was observed in just 5% of patients with HRC scores ≥2, and 9% of patients with HRC scores ≥5. Of infants with BSI, 5/46 (11%) had at least one HRC score ≥5 and 17/46 (37%) had at least one score ≥2 recorded in the 48 hour period prior to the evaluation that resulted in the first positive blood culture of the episode.
Conclusion
In our single center retrospective study, elevated HRC scores had limited ability to detect BSI. BSI was infrequent at any time during hospitalization in infants with significantly elevated HRC scores.
Keywords: HRC, neonate, sepsis
Introduction
In Neonatal Intensive Care Unit (NICU) patients, abnormal heart rate characteristics (HRCs) that reflect decreased variability and transient decelerations have been associated with the presence of potential infection[1–5]. A large (3003 patient) randomized controlled trial (RCT) of HRC monitoring demonstrated a reduced rate of mortality primarily in extremely low birth weight infants (ELBWs) as a secondary outcome[6]. In a secondary analysis of the RCT that specifically examined the impact of HRC monitoring on sepsis-associated mortality between 2004 and 2010, 30-day mortality was reduced specifically for coagulase negative Staphylococcus (CoNS)[7]. In a site-specific analysis of patients enrolled in the RCT, 22% of patients with “spikes”, defined as sharp rises (>3) in the HRC index, developed necrotizing enterocolitis or culture positive infection (urine or blood)[8]. Infants with spikes were more likely to have antimicrobial treatment initiated. In an editorial that accompanied the publication of the RCT, the authors asked “will the benefits transfer beyond the clinical trial environment in general neonatal practice”[9]? The purpose of this study was to examine the relationship between BSI and HRC elevations in clinical practice.
Methods
This study was approved by the Vanderbilt Institutional Review Board (IRB#111600). HRC scores were acquired using the HRC (HeRO) monitor system from Medical Predictive Science Corporation and entered into the electronic medical record by staff. We performed a retrospective nested cohort analysis using all HRC scores recorded in the electronic medical record (EMR) at Monroe Carell Jr. Children’s Hospital at Vanderbilt (MCJCHV) from January 1, 2010 through June 30, 2012. HRC scores are recorded in the EMR at least once every 12 hours. After retrieval of all available scores, non-numeric scores (e.g. “HRC not picking up”) and scores that were out of the range of possible scores (<0 or >7) were removed (404 occurrences). The remaining HRC scores were used for all subsequent analyses.
For our analyses, we chose cutoff HRC scores of 2 and 5. These scores represent a 2- and 5-fold increase in the risk of illness, would be expected to capture a high percentage of infants with sepsis, and typically generate clinician concern and action including sepsis evaluation[4]. In our cohort, we previously identified patients who met the criteria for the Centers for Disease Control (CDC) definition of blood stream infection (BSI)[10 11]. Briefly, BSI was defined as a single blood culture positive for non-contaminant, non-CoNS bacteria or for Candida species. CoNS infections were considered BSI if at least 1 repeat positive blood culture positive for CoNS within 72 hours of the initial positive blood culture. We excluded cases of early onset sepsis [<3 days after birth[12]]. In our cohort, we collected HRC scores for the period 48 hours prior to the first positive blood culture of the BSI episode [7]. We also did a subgroup analysis of HRC scores among the patients with BSI (with exclusion of all CoNS) that had serum CRP (≥45mg/L) during the episode[13].
Results
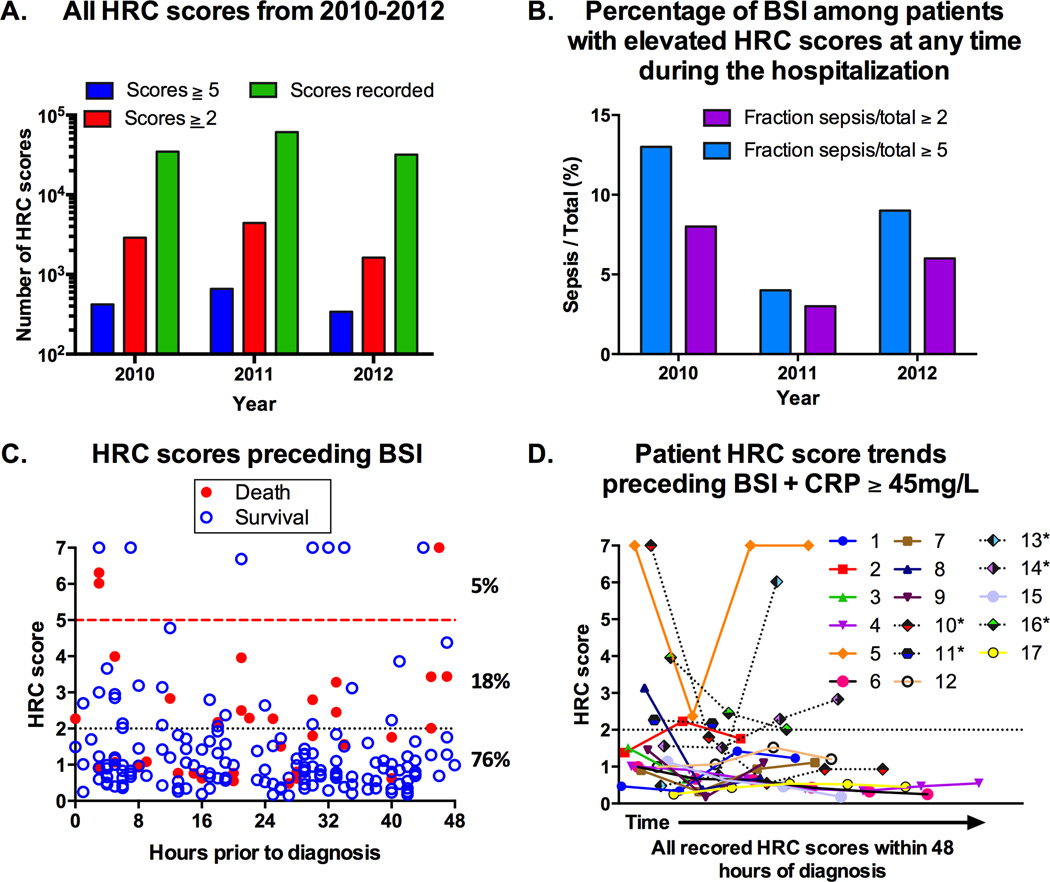
We extracted 127,673 HRC scores from 2384 patients during the study period (Figure 1A). The distribution of scores was consistent across the study period and the distribution of total scores above and below our cutoffs of interest was similar by year. HRC scores ≥5 were rare [1387/127,673 (~1%)] and occurred principally (91%) in patients that did not have BSI at any time during their hospitalization (Figure 1B). Furthermore, we found 9701/127,673 (8%) HRC scores ≥2 in 791 patients of which 95% did not have BSI at any time.
Figure 1.
A. Distribution of HRC scores by year studied. B. Among the patients with HRC scores ≥5 at any time during their hospitalization, the percentage of patients that had BSI were 13% (2010), 4% (2011), and 9% (2012). Among the patients with HRC scores ≥2 at any time during their hospitalization, the percentage of patients that had BSI were 8% (2010), 3% (2011), 6% (2012). C. HRC scores 48 hours prior to the evaluation that resulted in the first positive culture among patients with BSI. Open circles: survivors, Filled circles: non-survivors. Among the 187 scores, 10 (5%) were ≥5, 34 (18%) were 2–4.99, and 143 (76%) were <2. 5/46 (12%) patients had HRC scores ≥5 and 17/46 (37%) patients had scores ≥2. D. HRC score trends 48 hours prior to the first positive culture among patients with BSI (excluding CoNS) and a CRP ≥45mg/L during the episode. * non-survivors.
Because each episode of HRC score elevation ≥2 was not accompanied by an evaluation for BSI, we were unable to calculate diagnostic parameters of interest (e.g. sensitivity, specificity, positive predictive value, negative predictive value). However, we identified 46 patients with BSI among those with HRC scores available for analysis (Figure 1C)[11]. Among those with BSI, the median gestational age was 27 weeks (25th percentile: 25, 75th percentile: 35), median birth weight 940g (740–25480g), median day of life for BSI was 31 (14–52) and 61% were male. Mortality among patients with BSI was 22% (10/46). Necrotizing enterocolitis occurred in 4/46 (8.7%). Pathogens identified are shown in Table 1. Five of these 46 infants (11%), had at least one score ≥5 and 17 (37%) had at least one score ≥2 within 48 hours prior to diagnosis. In our subgroup analysis of infants with BSI (with exclusion of all CoNS) and HRC scores, 17 had CRP ≥ 45mg/L supporting significant systemic inflammation (Figure 1D, Table 1). Three of these 17 infants (18%) had HRC scores ≥5 and 8 (47%) had a HRC score ≥2 in the 48 hours prior to the diagnostic evaluation.
Table 1.
Pathogen representation among infants with BSI.
| Pathogens among patients with BSI* |
Pathogens among patients with non-CoNS BSI + CRP ≥ 45mg/L** |
||
|---|---|---|---|
| Gram positive | 28 | ||
| CoNS | 14 | ||
| Enterococcus faecalis | 6 | 2 | |
| Staphylococcus aureus | 5 | 3 | |
| Group B Streptococcus | 3 | 2 | |
| Gram negative | 22 | ||
| Enterobacter cloacae | 5 | 2 | |
| Escherichia coli | 5 | 2 | |
| Klebsiella spps | 3 | 1 | |
| Serratia marcescens | 3 | 1 | |
| Acinetobacterspps | 2 | 1 | |
| Pseudomonas aeruginosa | 2 | 2 | |
| Delftiaacidovorans | 1 | 1 | |
| Neiserria polysaccharea | 1 | ||
| Fungus | 1 | ||
| Candida albicans | 1 | 1 | |
-5 cases of polymicrobial infection (2 valid pathogens) were included;
-single patient had both E. fecalis and S. aureus CoNS-Coagulase negative Staphylococcus, BSI-Blood Stream Infection, CRP-C-reactive protein, spps-species
Discussion
In our study of 2384 NICU infants, significant elevations of HRC index (≥5) represented 1% of the total HRC scores. Of note, BSI at any time during the hospitalization occurred in a mean of 9% of patients with scores ≥5. The majority of the 46 infants with BSI, including those with a robust systemic inflammatory response, had no elevations in the recorded HRC scores in the 48-hour period prior to the evaluation that confirmed suspected sepsis. Similar to other reports[8 14–18], we found high HRC scores were most often not attributable to documented infection. Thus, these data suggest that HRC score is neither very sensitive nor very specific for BSI.
The predictive value of HRC monitoring in clinical practice is uncertain. Although an abnormal HRC index has been compared to laboratory or clinical parameters for predicting the odds of sepsis[1 3], we were unable to identify a published report that included commonly reported indices for diagnostic testing. In our cohort, the majority of HRC elevations occurred in patients without BSI, and patients with BSI infrequently experienced elevations.
In the HRC study that led to predictive modeling for sepsis[4] two cohorts of patients were studied to establish the HRC index: a training set and validation set. The training set consisted of 110 patients that had 155 episodes of sepsis or sepsis-like illness. Most episodes [(123/155 (79%)] in that study were not associated with a positive blood culture. Sixteen of the 22 positive blood cultures (73%) were CoNS and it was not stated whether multiple cultures were required for confirmation. The validation cohort was similar (93 infants with 118 episodes of sepsis and sepsis-like illness, 91/118 (77%) had negative blood cultures, 16/27 (59%) positive cultures with CoNS). The area under the curve for regression modeling ranged from 0.65–0.77, including the regression equation that yielded the HRC index (0.70). In contrast, confirmed BSI was the gold standard in our study and all CoNS infections had at least two positive cultures.
Our retrospective study has some limitations but reflects the daily practice environment in the NICU where evaluation of an infant for sepsis involves clinical judgment as well as laboratory and monitoring data. Unlike other studies, we included all NICU infants regardless of gestational and postnatal age. HRC scores used for our analyses represented values recorded in the electronic medical record, not the hourly values in real-time presented on the HRC-specific monitors, and so are subject to recording bias. This limitation is outweighed in part by the large number of scores recorded over a 2.5-year period, and the detailed examination of scores recorded on patients with documented BSI. Our cohort with BSI experienced 22% mortality; none of which was related to CoNS. The report of reduced 30-day mortality in ELBW infants associated with HRC monitoring was a secondary outcome in a trial with no difference in the primary outcome (number of days alive and ventilator-free in the 120 days after randomization). This reduction in mortality was primarily related to CoNS infection [18.1% (HRC non-display) and 6.3% (HRC visible)[7]. The mortality rate in the control group was 3-fold higher than the rates found in a recent study of 4364 infants with CoNS BSI[19]. Thus, it is possible that the benefit of a reduction in CoNS-mediated sepsis mortality associated with HRC monitoring may not translate to NICUs with typical CoNS mortality rates. Our data did not allow calculation of accurate predictive indices by episode of HRC elevation in our cohort. Reporting sensitivity, specificity, positive and negative predictive values for HRC and infection in hospitalized infants using the large cohort studied in detail in the RCT would be informative, increase our understanding of the utility of this technology, and perhaps justify the cost.
Conclusion
In our single center, large, retrospective study, elevated HRC scores had limited ability to detect BSI and BSI was infrequent in infants with significantly elevated HRC indices.
What is known
In Neonatal Intensive Care Unit (NICU) patients, abnormal heart rate characteristics (HRCs) that reflect decreased variability and transient decelerations have been associated with potential blood stream infection (BSI).
High HRC scores among NICU patients have many potential causes.
What this study adds
HRC scores are often elevated in NICU patients who do not have BSI.
Among patients with definitive BSI, HRC elevations ≥2 or ≥5 are infrequent in the 48 hours preceding the time of sepsis evaluation.
Acknowledgments
Grant support:
The project was supported by National Institutes of Health (NIH) GM106143 (to JLW), UL1 TR000445 from NCATS/NIH (REDCap) and the Department of Pediatrics at Vanderbilt University.
Footnotes
Disclosures: The authors declare no competing financial interests.
License for Publication statement
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ and co-owners or contracting owning societies (where published by the BMJ on their behalf), and its Licensees to permit this article (if accepted) to be published in Archives of Disease in Childhood and any other BMJ products and to exploit all subsidiary rights, as set out in our license.
Contributorship statement
Drs. Coggins and Wynn wrote the first draft of the manuscript. Ms. Grunwald extracted the relevant data from the database. All other authors edited the manuscript.
References
- 1.Griffin MP, Lake DE, O'Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res. 2007;61(2):222–227. doi: 10.1203/01.pdr.0000252438.65759.af. 00006450-200702000-00018 [pii][published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.Griffin MP, Lake DE, Bissonette EA, Harrell FE, Jr, O'Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116(5):1070–1074. doi: 10.1542/peds.2004-2461. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Griffin MP, Lake DE, Moorman JR. Heart rate characteristics and laboratory tests in neonatal sepsis. Pediatrics. 2005;115(4):937–941. doi: 10.1542/peds.2004-1393. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 4.Griffin MP, O'Shea TM, Bissonette EA, Harrell FE, Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatric research. 2003;53(6):920–966. doi: 10.1203/01.PDR.0000064904.05313.D2. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Griffin MP, Moorman JR. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics. 2001;107(1):97–104. doi: 10.1542/peds.107.1.97. [DOI] [PubMed] [Google Scholar]
- 6.Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. The Journal of pediatrics. 2011;159(6):900–906. e1. doi: 10.1016/j.jpeds.2011.06.044. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairchild KD, Schelonka RL, Kaufman DA, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatric research. 2013;74(5):570–575. doi: 10.1038/pr.2013.136. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan BA, Grice SM, Lake DE, Moorman JR, Fairchild KD. Infection and other clinical correlates of abnormal heart rate characteristics in preterm infants. The Journal of pediatrics. 2014;164(4):775–780. doi: 10.1016/j.jpeds.2013.11.038. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groves AM, Edwards AD. Heart rate characteristic monitoring-HeRO or villain? The Journal of pediatrics. 2011;159(6):885–886. doi: 10.1016/j.jpeds.2011.08.049. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. doi: S0196-6553(08)00167-3 [pii] 10.1016/j.ajic.2008.03.002[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 11.Coggins SA, Wynn JL, Hill ML, et al. Use of a computerized C-reactive protein (CRP) based sepsis evaluation in very low birth weight (VLBW) infants: a five-year experience. PLoS One. 2013;8(11):e78602. doi: 10.1371/journal.pone.0078602. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early Onset Neonatal Sepsis: The Burden of Group B Streptococcal and E. coli Disease Continues. Pediatrics. 2011;127(5):817–826. doi: 10.1542/peds.2010-2217. doi: peds.2010-2217 [pii] 10.1542/peds.2010-2217[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn JL, Guthrie SO, Wong HR, et al. Post-natal age is a critical determinant of the neonatal host response to sepsis. Mol Med. 2015 doi: 10.2119/molmed.2015.00064. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairchild KD. Predictive monitoring for early detection of sepsis in neonatal ICU patients. Curr Opin Pediatr. 2013;25(2):172–179. doi: 10.1097/MOP.0b013e32835e8fe6. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vergales BD, Zanelli SA, Matsumoto JA, et al. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. American journal of perinatology. 2014;31(10):855–862. doi: 10.1055/s-0033-1361937. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Addison K, Griffin MP, Moorman JR, Lake DE, O'Shea TM. Heart rate characteristics and neurodevelopmental outcome in very low birth weight infants. Journal of perinatology : official journal of the California Perinatal Association. 2009;29(11):750–756. doi: 10.1038/jp.2009.81. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairchild KD, Sinkin RA, Davalian F, et al. Abnormal heart rate characteristics are associated with abnormal neuroimaging and outcomes in extremely low birth weight infants. Journal of perinatology : official journal of the California Perinatal Association. 2014;34(5):375–379. doi: 10.1038/jp.2014.18. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone ML, Tatum PM, Weitkamp JH, et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. Journal of perinatology : official journal of the California Perinatal Association. 2013;33(11):847–850. doi: 10.1038/jp.2013.63. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ericson JE, Thaden J, Cross HR, et al. No survival benefit with empirical vancomycin therapy for coagulase-negative staphylococcal bloodstream infections in infants. The Pediatric infectious disease journal. 2015;34(4):371–375. doi: 10.1097/INF.0000000000000573. [published Online First: pub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]