Abstract
Purpose
Older women with early-stage disease comprise the most rapidly growing breast cancer demographic, yet it is not known which local therapy strategies are most favored by this population in the current era. Understanding utilization trends and cost of local therapy is important for informing design of bundled payment models as payers migrate away from fee-for-service. We therefore utilized the SEER-Medicare database to determine patterns of care and costs for local therapy among older women with breast cancer.
Materials and Methods
Treatment strategy and covariables were determined in 55,327 women age≥66 with Tis-T2 N0-1 M0 breast cancer who underwent local therapy between 2000 and 2008. Trends in local therapy were characterized using Joinpoint. Polychotomous logistic regression determined predictors of local therapy. Median aggregate cost over the first 24 months after diagnosis was determined from Medicare claims through 2010 and reported in 2014 dollars.
Results
Median age was 75. Local therapy distribution was as follows: 27,896 (50.3%) lumpectomy with external beam radiation; 18,356 (33.1%) mastectomy alone; 6,159 (11.1%) lumpectomy alone; 1,488 (2.7%) mastectomy with reconstruction; and 1,455 (2.6%) lumpectomy with brachytherapy. Mastectomy alone declined from 39.0% in 2000 to 28.2% in 2008 while use of breast conserving local therapies rose from 58.7% to 68.2%. Mastectomy with reconstruction was more common among the youngest, healthiest patients, whereas mastectomy alone was more common among patients living in rural, low income regions. By 2008, cost was $36,749 for lumpectomy with brachytherapy, $35,030 for mastectomy with reconstruction, $31,388 for lumpectomy with external beam radiation, $21,993 for mastectomy alone, and $19,287 for lumpectomy alone.
Conclusions
The use of mastectomy alone in older women declined in favor of breast conserving strategies between 2000 and 2008. Using these cost estimates, price points for local therapy bundles can be constructed to incentivize treatment strategies which confer the highest value.
Introduction
Older women with early breast cancer comprise the most rapidly growing breast cancer demographic, with an estimated 114,000 cases annually and 57% growth forecast from 2010 to 2030 (1). Historically, the most common local-regional treatments for such women were lumpectomy followed by approximately 6 weeks of external beam radiation or mastectomy without reconstruction. However, recent literature suggests that many older women may be appropriate candidates for either brachytherapy, which conveniently decreases the radiation treatment course to 1 week, or complete omission of any radiation, which confers even more convenience (2–4). Despite increasing support for these convenient options for breast conservation, recent patterns of care studies have demonstrated increasing use of mastectomy in the overall breast cancer population, potentially driven by greater availability and utilization of breast reconstruction (5–9).
For older women with early breast cancer, it is not known whether the increasing availability of more convenient breast conservation strategies has led overall to increased use of breast conservation, or whether increasing availability of breast reconstruction has led to increased use of mastectomy. Understanding utilization and cost trends in this large and growing population of older women with early breast cancer is critically important for promoting value, defined as the quality of outcomes achieved per dollar spent, as payers migrate away from fee-for-service reimbursement toward bundled care payment models (10–12). We therefore used the SEER-Medicare cohort to characterize population trends in local therapy utilization and to characterize predictors and cost of local therapy for older women with early breast cancer who are Medicare beneficiaries.
Methods
Data Source
The SEER-Medicare database captures clinical, pathological, and insurance claims data for incident cancers diagnosed in Medicare beneficiaries who reside within one of 16 geographic areas that account for 26% of the US population. The case ascertainment rate is approximately 98% (13). In this study, demographic and tumor characteristics for incident malignancies diagnosed from January 1, 2000 to December 31, 2008 were linked to Medicare treatment claims from January 1, 1999 to December 31, 2010.
Study Sample
From 2000–2008, 195,217 women age ≥ 66 years were diagnosed with invasive or in situ breast cancer and reported in the SEER-Medicare cohort. We applied standard exclusions as outlined in eTable 1 to create an analytic cohort of 55,327 patients with early stage disease (Tis-T2 N0–1). We required that all patients maintain fee-for-service Medicare coverage from 12 months prior through 24 months after diagnosis to permit ascertainment of comorbid illness before diagnosis and delayed breast reconstruction after diagnosis.
Outcomes
The primary outcome for this study was type of local treatment, defined as one of the following: (1) lumpectomy followed by external beam radiation, (2) mastectomy without reconstruction within 2 years of diagnosis, (3) mastectomy with reconstruction within 2 years of diagnosis, (4) lumpectomy followed by brachytherapy, or (5) lumpectomy with no adjuvant radiation therapy. For patients treated with mastectomy, we also required that they did not receive radiation within 12 months of surgery, as use of post-mastectomy radiation would likely indicate a more advanced cancer. Type of surgery (lumpectomy vs. mastectomy) was determined using both SEER data and Medicare claims within 12 months of diagnosis, with the most extensive surgery coded by either source considered to be the definitive surgery. Patients were considered to have received breast reconstruction if any claim for reconstruction was present within 24 months of diagnosis (eTable 2).
Baseline Covariables
Patient characteristics from the SEER data included age at diagnosis, race, sex, and year of diagnosis. Modified Charlson comorbidity index with Klabunde modification was determined from claims spanning an interval of 12 months to one month prior to diagnosis (14,15). Tumor characteristics extracted from SEER included T- and N-stage, grade, histology, estrogen receptor (ER) status, and laterality. Lymphovascular space invasion and margin status are not reported. Area-level characteristics included urban/rural residence, median income, educational attainment, and county-level surgeon and radiation oncologist density determined using the Area Resource File (16).
Determination of cost
Costs for each patient were calculated from a payer perspective using all inpatient, outpatient, and carrier claims within 2 years of diagnosis and were divided by calendar month to evaluate trends over time related to date of diagnosis. Costs were adjusted for geographic variation using the geographic adjustment factor for Part A claims and the geographic practice cost index for Part B claims and for inflation using the Prospective Pricing Index for Part A claims and the Medicare Economic Index for Part B claims (17,18). Costs were also adjusted for differences in use of chemotherapy by normalizing costs of each local therapy to the utilization rate of chemotherapy in patients treated with lumpectomy plus external beam radiation. All costs are reported in 2014 dollars.
Statistical Analysis
Baseline characteristics across treatment strata were compared with Pearson’s χ2 test. Trends in treatment utilization by calendar year quarter were determined using Joinpoint linear regression models (Joinpoint v3.4.3). Adjusted associations between baseline characteristics and treatment strategy were estimated using polychotomous logistic regression. Lumpectomy followed by external beam radiation served as the referent group for this model, as it was the most commonly used strategy and its use was relatively stable over time. Covariables were selected for inclusion in this model a priori based on clinical relevance or if associated with the outcome in univariate analysis at P<0.20. The model was iteratively refined to optimize fit.
To determine trends in costs, total median 2-year costs by treatment strategy and year of diagnosis were calculated. Linear regression was used to determine direction and magnitude of cost growth over time. The trend line for lumpectomy and brachytherapy started at 2002 to ensure adequate numbers for meaningful regression; the Food and Drug Administration first approved balloon brachytherapy for breast cancer in 2002.
All statistical tests were 2-sided with P ≤ 0.05 and conducted using SAS v. 9.3 (Cary, NC). Our institutional review board granted this study exempt status.
Results
Baseline Characteristics
Of 55,327 women, median age was 75 years and 48,792 (88.5%) were white. The number of patients receiving each treatment was as follows: 27,896 (50.3%) lumpectomy with external beam radiation; 18,356 (33.1%) mastectomy alone; 6,159 (11.1%) lumpectomy alone; 1,488 (2.7%) mastectomy with reconstruction; and 1,455 (2.6%) lumpectomy with brachytherapy (Table 1).
Table 1.
Baseline Characteristics
| Lumpectomy & External Radiation |
Mastectomy Alone | Mastectomy & Reconstruction |
Lumpectomy & Brachytherapy |
Lumpectomy Alone |
All Patients | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=27,869 | N=18,356 | N=1488 | N=1455 | N=6159 | N=55,327 | ||||||||
| Patient and Treatment Factors | |||||||||||||
| Age | |||||||||||||
| 66–69 | 7615 | 27.3% | 3460 | 18.8% | 690 | 46.4% | 385 | 26.5% | 545 | 8.8% | 12695 | 22.9% | <0.001 |
| 70–74 | 8214 | 29.5% | 4535 | 24.7% | 469 | 31.5% | 410 | 28.2% | 774 | 12.6% | 14402 | 26.0% | |
| 75–79 | 6919 | 24.8% | 4664 | 25.4% | 236 | 15.9% | 349 | 24.0% | 1159 | 18.8% | 13327 | 24.1% | |
| 80–84 | 3908 | 14.0% | 3543 | 19.3% | 63 | 4.2% | 221 | 15.2% | 1608 | 26.1% | 9343 | 16.9% | |
| 85+ | 1213 | 4.4% | 2154 | 11.7% | 30 | 2.0% | 90 | 6.2% | 2073 | 33.7% | 5560 | 10.0% | |
| Race | |||||||||||||
| White | 25002 | 89.7% | 15778 | 86.0% | 1368 | 91.9% | 1329 | 91.3% | 5495 | 89.2% | 48972 | 88.5% | <0.001 |
| Black | 1515 | 5.4% | 1454 | 7.9% | 74 | 5.0% | 75 | 5.2% | 400 | 6.5% | 3518 | 6.4% | |
| Other/Unknown | 1352 | 4.9% | 1124 | 6.1% | 46 | 3.1% | 51 | 3.5% | 264 | 4.3% | 2837 | 5.1% | |
| Charlson Comorbidity Index | |||||||||||||
| 0 | 18694 | 67.1% | 10612 | 57.8% | 1067 | 71.7% | 971 | 66.7% | 3365 | 54.6% | 34709 | 62.7% | <0.001 |
| 1 | 6110 | 21.9% | 4599 | 25.1% | 298 | 20.0% | 337 | 23.2% | 1542 | 25.0% | 12886 | 23.3% | |
| ≥2 | 2585 | 9.3% | 2529 | 13.8% | 100 | 6.7% | 147 | 10.1% | 1030 | 16.7% | 6391 | 11.6% | |
| Incomplete | 480 | 1.7% | 616 | 3.4% | 23 | 1.5% | 0 | 0.0% | 222 | 3.6% | 1341 | 2.4% | |
| Chemotherapy | |||||||||||||
| No | 23486 | 84.4 | 15365 | 83.8 | 1130 | 75.9 | 1347 | 92.6 | 5947 | 96.6 | 47275 | 85.5% | <0.001 |
| Yes | 4356 | 15.7 | 2978 | 16.2 | 358 | 24.1 | 108 | 7.4 | 209 | 3.4 | 8009 | 14.5% | |
| Year of Diagnosis | |||||||||||||
| 2000 | 2777 | 10.0% | 2262 | 12.3% | <150 | <9% | <20 | <2% | 612 | 9.9% | 5795 | 10.5% | <0.001 |
| 2001 | 2978 | 10.7% | 2502 | 13.6% | <170 | <11% | <11 | <1% | 640 | 10.4% | 6283 | 11.4% | |
| 2002 | 3125 | 11.2% | 2262 | 12.3% | 150 | 10.1% | 38 | 2.6% | 653 | 10.6% | 6228 | 11.3% | |
| 2003 | 3223 | 11.6% | 2082 | 11.3% | 152 | 10.2% | 87 | 6.0% | 577 | 9.4% | 6121 | 11.1% | |
| 2004 | 3233 | 11.6% | 2015 | 11.0% | 166 | 11.2% | 144 | 9.9% | 690 | 11.2% | 6248 | 11.3% | |
| 2005 | 3062 | 11.0% | 1864 | 10.2% | 150 | 10.1% | 187 | 12.9% | 740 | 12.0% | 6003 | 10.9% | |
| 2006 | 3182 | 11.4% | 1763 | 9.6% | 148 | 9.9% | 263 | 18.1% | 779 | 12.6% | 6135 | 11.1% | |
| 2007 | 3123 | 11.2% | 1835 | 10.0% | 209 | 14.0% | 334 | 23.0% | 733 | 11.9% | 6234 | 11.3% | |
| 2008 | 3166 | 11.4% | 1771 | 9.6% | 227 | 15.3% | 381 | 26.2% | 735 | 11.9% | 6280 | 11.4% | |
| Tumor Factors | |||||||||||||
| Tumor Size | |||||||||||||
| T1 (0.0 – 2.0 cm) | 22995 | 82.5% | 11866 | 64.6% | 1026 | 69.0% | 1312 | 90.2% | 4898 | 79.5% | 42097 | 76.1% | <0.001 |
| T2 (2.1 – 5.0 cm) | 4676 | 16.8% | 6326 | 34.5% | 443 | 29.8% | 132 | 9.1% | 1202 | 19.5% | 12779 | 23.1% | |
| Not specified | 198 | 0.7% | 164 | 0.9% | 19 | 1.3% | 11 | 0.8% | 59 | 1.0% | 451 | 0.8% | |
| Nodal Status | |||||||||||||
| Pathological N0 | 21386 | 76.7% | 13493 | 73.5% | 1192 | 80.1% | 1301 | 89.4% | 2737 | 44.4% | 40109 | 72.5% | <0.001 |
| Clinical N0 | 2524 | 9.1% | 1184 | 6.5% | 46 | 3.1% | 95 | 6.5% | 3090 | 50.2% | 6939 | 12.5% | |
| Pathologic N1 | 3959 | 14.2% | 3679 | 20.0% | 250 | 16.8% | 59 | 4.1% | 332 | 5.4% | 8279 | 15.0% | |
| Histology | |||||||||||||
| Ductal, tubular, mucinous | 20920 | 75.1% | 12653 | 68.9% | 884 | 59.4% | 1156 | 79.5% | 4546 | 73.8% | 40159 | 72.6% | <0.001 |
| Lobular | 2282 | 8.2% | 1815 | 9.9% | 184 | 12.4% | 71 | 4.9% | 480 | 7.8% | 4832 | 8.7% | |
| Other invasive | 3959 | 14.2% | 2804 | 15.3% | 260 | 17.5% | 184 | 12.6% | 923 | 15.0% | 8130 | 14.7% | |
| DCIS | 708 | 2.5% | 1084 | 5.9% | 160 | 10.8% | 44 | 3.0% | 210 | 3.4% | 2206 | 4.0% | |
| Grade | |||||||||||||
| Low-intermediate | 19933 | 71.5% | 11438 | 62.3% | 925 | 62.2% | 1175 | 80.8% | 4548 | 73.8% | 38019 | 68.7% | <0.001 |
| High | 6197 | 22.2% | 5249 | 28.6% | 428 | 28.8% | 223 | 15.3% | 1069 | 17.4% | 13166 | 23.8% | |
| Other/unknown | 1739 | 6.2% | 1669 | 9.1% | 135 | 9.1% | 57 | 3.9% | 542 | 8.8% | 4142 | 7.5% | |
| Estrogen Receptor Status | |||||||||||||
| ER+ | 22136 | 79.4% | 12722 | 69.3% | 1086 | 73.0% | 1245 | 85.6% | 4717 | 76.6% | 41906 | 75.7% | <0.001 |
| ER- | 3291 | 11.8% | 2568 | 14.0% | 228 | 15.3% | 108 | 7.4% | 466 | 7.6% | 6661 | 12.0% | |
| Unspecified | 2442 | 8.8% | 3066 | 16.7% | 174 | 11.7% | 102 | 7.0% | 976 | 15.8% | 6760 | 12.2% | |
| Area-level Factors | |||||||||||||
| Type of Patient Residence | |||||||||||||
| Urban | 25823 | 92.7% | 15477 | 84.3% | 1373 | 92.3% | 1369 | 94.1% | 5550 | 90.1% | 49592 | 89.6% | <0.001 |
| Rural | >2025 | >5 | 2879 | 15.7% | 115 | 7.7% | 86 | 5.9% | 609 | 9.9% | >5725 | >5 | |
| Unknown | <11 | <5 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | <11 | <5 | |
| Density of Surgeons | |||||||||||||
| Highest Quartile | 6125 | 22.0% | 5598 | 30.5% | 348 | 23.4% | 351 | 24.1% | 1413 | 22.9% | 13835 | 25.0% | <0.001 |
| Second Quartile | 7222 | 25.9% | 4542 | 24.7% | 364 | 24.5% | 414 | 28.5% | 1711 | 27.8% | 14253 | 25.8% | |
| Third Quartile | 7184 | 25.8% | 3942 | 21.5% | 375 | 25.2% | 323 | 22.2% | 1569 | 25.5% | 13393 | 24.2% | |
| Fourth Quartile | 7338 | 26.3% | 4274 | 23.3% | 401 | 26.9% | 367 | 25.2% | 1466 | 23.8% | 13846 | 25.0% | |
| Density of Radiation Oncologists | |||||||||||||
| Highest Quartile | 5552 | 19.9% | 6088 | 33.2% | 328 | 22.0% | 334 | 23.0% | 1495 | 24.3% | 13797 | 24.9% | <0.001 |
| Second Quartile | 7416 | 26.6% | 4167 | 22.7% | 393 | 26.4% | 458 | 31.5% | 1582 | 25.7% | 14016 | 25.3% | |
| Third Quartile | 7600 | 27.3% | 3764 | 20.5% | 382 | 25.7% | 298 | 20.5% | 1642 | 26.7% | 13686 | 24.7% | |
| Fourth Quartile | 7301 | 26.2% | 4337 | 23.6% | 385 | 25.9% | 365 | 25.1% | 1440 | 23.4% | 13828 | 25.0% | |
| Residents with Some College Education | |||||||||||||
| 1st Quartile (0 – 8.1%) | 8117 | 29.1% | 3409 | 18.6% | 517 | 34.7% | 490 | 33.7% | 1545 | 25.1% | 14078 | 25.4% | <0.001 |
| 2nd Quartile (8.2% – 14.4%) | 7190 | 25.8% | 4108 | 22.4% | 387 | 26.0% | 375 | 25.8% | 1527 | 24.8% | 13587 | 24.6% | |
| 3rd Quartile (14.5 – 24.0%) | 6755 | 24.2% | 4979 | 27.1% | 294 | 19.8% | 308 | 21.2% | 1500 | 24.4% | 13836 | 25.0% | |
| 4th Quartile (>24.1%) | 5807 | 20.8% | 5860 | 31.9% | 290 | 19.5% | 282 | 19.4% | 1587 | 25.8% | 13826 | 25.0% | |
| Income Level | |||||||||||||
| 1st Quartile ($0 – $35,453) | 5672 | 20.4% | 6069 | 33.1% | 279 | 18.8% | 258 | 17.7% | 1556 | 25.3% | 13834 | 25.0% | <0.001 |
| 2nd Quartile ($35,454 – $46,559) | 6619 | 23.8% | 4967 | 27.1% | 317 | 21.3% | 370 | 25.4% | 1557 | 25.3% | 13830 | 25.0% | |
| 3rd Quartile ($46,560 – $62,311) | 7451 | 26.7% | 4135 | 22.5% | 371 | 24.9% | 369 | 25.4% | 1505 | 24.4% | 13831 | 25.0% | |
| 4th Quartile (>$62,312) | 8127 | 29.2% | 3185 | 17.4% | 521 | 35.0% | 458 | 31.5% | 1541 | 25.0% | 13832 | 25.0% | |
Abbreviations: DCIS (ductal carcinoma in situ), ER (estrogen receptor). Cell sizes < 11 have been suppressed in accordance with guidelines from SEER-Medicare.
Trends in Local Therapy
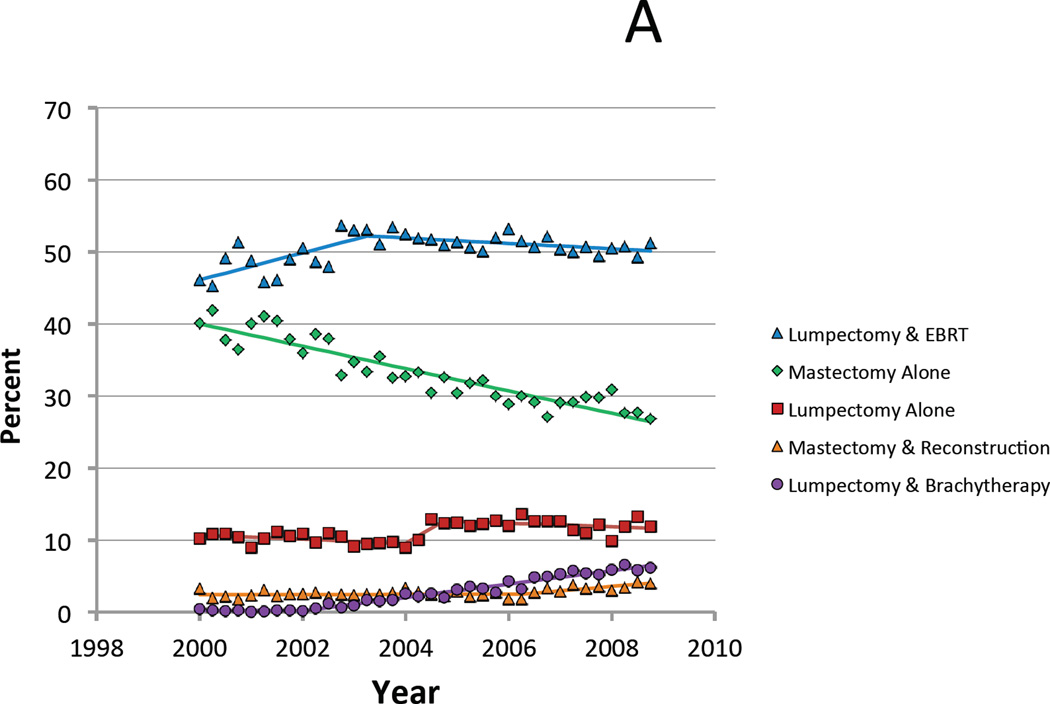
During the study interval, the proportion of patients undergoing mastectomy alone declined from 39.0% in 2000 to 28.2% in 2008 while use of breast conserving local therapies rose from 58.7% to 68.2% (Figure 1A). Specifically, lumpectomy plus external radiation rose from 47.9% in 2000 to a peak of 52.6% in 2003 before declining modestly to 50.4% of cases in 2008. This later decline was accompanied by a rise in breast conservation utilizing brachytherapy. This strategy increased from less than 0.3% of cases in 2000 and 2001 to 6.1% of cases in 2008, which represented the fastest growth among all treatment options. Mastectomy followed by reconstruction accounted for 2.2% of cases in 2000 and 3.6% of cases in 2008, with most of this increase occurring during the final two years of the interval. Finally, utilization of lumpectomy alone rose slightly, from 10.6% to 11.7% of cases during the study period.
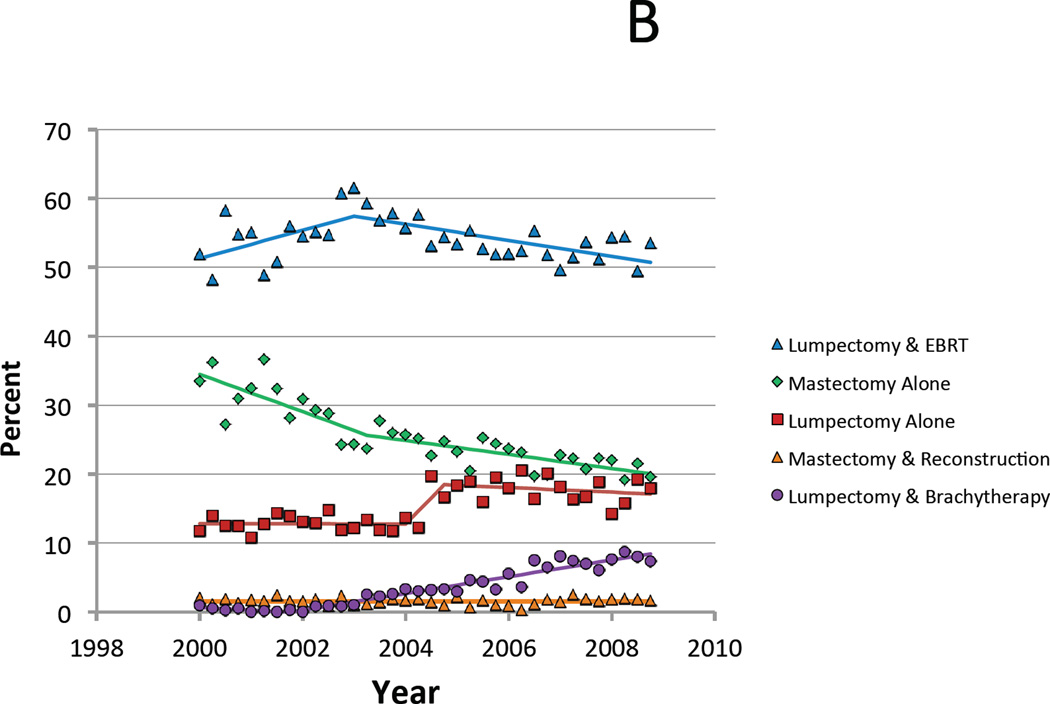
Figure 1.
A. Proportion of Medicare patients who received each treatment for each annual quarter between 2000 and 2008. B. Limited to favorable risk patients with T1 N0 estrogen receptor positive breast cancer age 70 and older at diagnosis.
When limited to only those patients for whom all of these treatments are considered guideline-concordant (i.e. age 70 and older, stage T1N0, ER+), similar trends in local therapy strategies were observed, with the exception that there was a much more pronounced increase in use of lumpectomy alone, with the percent of patients treated with this strategy stable between 2000 to 2003 at 12.9%, then increasing to a high of 18.7% in 2006, and subsequently falling slightly to 16.8% in 2008 (Figure 1B).
Predictors of Treatment
Polychotomous logistic regression employing lumpectomy plus external radiation as the referent was used to identify predictors for use of the other four treatment strategies (Table 2). The youngest patients and those with minimal comorbidities were more likely to undergo mastectomy with reconstruction. In contrast, older patients and those with more comorbidities were more likely to undergo shorter treatment strategies such as mastectomy alone, lumpectomy alone, or lumpectomy with brachytherapy. Additionally, lumpectomy with brachytherapy was strongly associated with a later year of diagnosis as well as tumor features including smaller size, lower grade, ER-positivity, node negativity, and ductal, rather than lobular, histology. Socioeconomic factors also correlated with treatment (Table 2). One notable finding was a correlation between regions with low incomes or rural settings and the use of mastectomy alone.
Table 2.
Predictors of Treatment Strategy using Polychotomous Logistic Regression with Lumpectomy plus External Beam Radiation Designated as Referent Group.
| Mastectomy Alone | Mastectomy & Reconstruction |
Lumpectomy & Brachytherapy | Lumpectomy Alone | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Patient Factors | ||||||||||||
| Age | ||||||||||||
| 66–69 | 1 | 1 | 1 | 1 | ||||||||
| 70–74 | 1.23 | 1.17–1.31 | <.0001 | 0.65 | 0.57–0.73 | <.0001 | 1.02 | 0.89–1.18 | 0.7611 | 1.28 | 1.14–1.44 | <.0001 |
| 75–79 | 1.57 | 1.49–1.67 | <.0001 | 0.4 | 0.34–0.46 | <.0001 | 1.07 | 0.92–1.25 | 0.3754 | 2.05 | 1.83–2.29 | <.0001 |
| 80–84 | 2.22 | 2.08–2.37 | <.0001 | 0.19 | 0.15–0.25 | <.0001 | 1.14 | 0.96–1.36 | 0.1338 | 4.21 | 3.77–4.70 | <.0001 |
| 85+ | 4.4 | 4.04–4.81 | <.0001 | 0.31 | 0.21–0.45 | <.0001 | 1.56 | 1.22–2.00 | 0.0003 | 12.69 | 11.3–14.3 | <.0001 |
| Race | ||||||||||||
| White | 1 | 1 | 1 | 1 | ||||||||
| Black | 1.18 | 1.09–1.29 | <.0001 | 0.85 | 0.66–1.1 | 0.22 | 1.12 | 0.87–1.44 | 0.38 | 1.12 | 0.98–1.29 | 0.089 |
| Other/Unknown | 1.57 | 1.44–1.72 | <.0001 | 0.6 | 0.44–0.81 | 0.0009 | 0.67 | 0.5–0.9 | 0.0069 | 0.99 | 0.85–1.15 | 0.86 |
| Charlson Comorbidity Index | ||||||||||||
| 0 | 1 | 1 | 1 | 1 | ||||||||
| 1 | 1.25 | 1.19–1.31 | <.0001 | 0.92 | 0.81–1.06 | 0.25 | 1.03 | 0.91–1.18 | 0.6307 | 1.63 | 1.48–1.78 | <.0001 |
| ≥2 | 1.52 | 1.42–1.62 | <.0001 | 0.78 | 0.63–0.96 | 0.021 | 1.04 | 0.86–1.25 | 0.7006 | NA | NA | NA |
| Incomplete | 1.54 | 1.34–1.76 | <.0001 | 0.85 | 0.55–1.32 | 0.47 | 1.19 | 1.1–1.28 | 0.8575 | 3.43 | 2.84–4.15 | <.0001 |
| Year of Diagnosis | ||||||||||||
| 2000 | 1 | 1 | 1 | 1 | ||||||||
| 2001 | 1.04 | 0.96–1.13 | 0.37 | 1.09 | 0.86–1.40 | 0.47 | 0.46 | 0.19–1.15 | 0.098 | 1.05 | 0.91–1.20 | 0.52 |
| 2002 | 0.92 | 0.84–0.99 | 0.042 | 0.98 | 0.77–1.25 | 0.87 | 2.25 | 1.21–4.17 | 0.0098 | 1.21 | 1.06–1.40 | 0.0068 |
| 2003 | 0.78 | 0.72–0.85 | <.0001 | 0.98 | 0.77–1.25 | 0.87 | 5.02 | 2.85–8.85 | <.0001 | 1 | 0.86–1.15 | 0.95 |
| 2004 | 0.78 | 0.71–0.85 | <.0001 | 1.05 | 0.82–1.33 | 0.73 | 8.55 | 4.92–14.85 | <.0001 | 1.41 | 1.23–1.62 | <.0001 |
| 2005 | 0.75 | 0.69–0.82 | <.0001 | 0.95 | 0.75–1.22 | 0.70 | 12.09 | 7.00–20.90 | <.0001 | 1.67 | 1.45–1.91 | <.0001 |
| 2006 | 0.69 | 0.63–0.75 | <.0001 | 0.92 | 0.72–1.18 | 0.50 | 16.44 | 9.56–28.26 | <.0001 | 1.76 | 1.54–2.02 | <.0001 |
| 2007 | 0.71 | 0.65–0.78 | <.0001 | 1.31 | 1.04–1.65 | 0.023 | 21.25 | 12.4–36.4 | <.0001 | 1.67 | 1.45–1.92 | <.0001 |
| 2008 | 0.68 | 0.62–0.74 | <.0001 | 1.37 | 1.09–1.73 | 0.0081 | 23.58 | 13.8–40.4 | <.0001 | 1.78 | 1.55–2.05 | <.0001 |
| Tumor Factors | ||||||||||||
| Tumor Size | ||||||||||||
| T1 (0.0 – 2.0 cm) | 1 | 1 | 1 | 1 | ||||||||
| T2 (2.1 – 5.0 cm) | 2.27 | 2.17–2.38 | <.0001 | 2.01 | 1.78–2.27 | <.0001 | 0.58 | 0.48–0.7 | <.0001 | 1.04 | 0.96–1.13 | 0.33 |
| Not specified | 1.71 | 1.37–2.14 | <.0001 | 1.71 | 1.05–2.78 | 0.032 | 0.65 | 0.35–1.2 | 0.168 | 0.91 | 0.65–1.27 | 0.58 |
| Nodal Status | ||||||||||||
| Pathological N0 | 1 | 1 | 1 | 1 | ||||||||
| Clinical N0 | 0.49 | 0.45–0.53 | <.0001 | 0.48 | 0.35–0.65 | <.0001 | 0.77 | 0.62–0.96 | 0.018 | 5.66 | 5.25–6.1 | <.0001 |
| Pathological N+ | 1.32 | 1.25–1.40 | <.0001 | 1.1 | 0.95–1.27 | 0.21 | 0.28 | 0.21–0.36 | <.0001 | 0.7 | 0.62–0.79 | <.0001 |
| Histology | ||||||||||||
| Ductal, tubular, mucinous | 1 | 1 | 1 | 1 | ||||||||
| Lobular | 1.27 | 1.18–1.36 | <.0001 | 1.79 | 1.5–2.13 | <.0001 | 0.54 | 0.42–0.69 | <.0001 | 0.83 | 0.74–0.94 | 0.0034 |
| Other invasive | 1.17 | 1.11–1.24 | <.0001 | 1.53 | 1.32–1.77 | <.0001 | 0.9 | 0.76–1.05 | 0.1844 | 1.02 | 0.94–1.12 | 0.62 |
| DCIS | 2.27 | 2.04–2.53 | <.0001 | 4.06 | 3.33–4.96 | <.0001 | 0.9 | 0.65–1.23 | 0.5041 | 2.47 | 2.08–2.92 | <.0001 |
| Grade | ||||||||||||
| Low-intermediate | 1 | 1 | 1 | 1 | ||||||||
| High | 1.16 | 1.10–1.22 | <.0001 | 1.16 | 1.02–1.33 | 0.027 | 0.75 | 0.64–0.88 | 0.0005 | 0.79 | 0.72–0.86 | <.0001 |
| Other/unknown | 1.23 | 1.13–1.33 | <.0001 | 1.29 | 1.06–1.58 | 0.012 | 0.82 | 0.62–1.09 | 0.1678 | 1.14 | 1.01–1.28 | 0.039 |
| Estrogen Receptor Status | ||||||||||||
| ER+ | 1 | 1 | 1 | 1 | ||||||||
| ER− | 1.19 | 1.12–1.27 | <.0001 | 1.2 | 1.02–1.41 | 0.032 | 0.65 | 0.52–0.8 | <.0001 | 0.77 | 0.69–0.87 | <.0001 |
| Unspecified | 1.82 | 1.71–1.94 | <.0001 | 1.26 | 1.06–1.51 | 0.011 | 1.34 | 1.08–1.66 | 0.0076 | 1.64 | 1.49–1.8 | <.0001 |
| Area-Level Factors | ||||||||||||
| Type of Patient Residence | ||||||||||||
| Metropolitan | 1 | 1 | 1 | 1 | ||||||||
| Non-metropolitan | 1.4 | 1.3–1.52 | <.0001 | 0.96 | 0.76–1.22 | 0.73 | 0.62 | 0.48–0.8 | 0.0003 | 1.14 | 1–1.3 | 0.053 |
| Density of Surgeons | ||||||||||||
| Highest Quartile | 1 | 1 | 1 | 1 | ||||||||
| Second Quartile | 0.9 | 0.85–0.95 | <.0001 | 0.95 | 0.8–1.12 | 0.031 | 1.19 | 1.01–1.41 | 0.0331 | 1.16 | 1.06–1.28 | 0.002 |
| Third Quartile | 0.9 | 0.84–0.96 | 0.51 | 1.03 | 0.86–1.24 | 0.74 | 1.13 | 0.94–1.35 | 0.2116 | 1.09 | 0.98–1.21 | 0.13 |
| Fourth Quartile | 0.92 | 0.86–0.99 | 0.002 | 1.12 | 0.92–1.37 | 0.2631 | 1.42 | 1.16–1.74 | 0.0008 | 1.03 | 0.91–1.16 | 0.64 |
| Density of Radiation Oncologists | ||||||||||||
| Highest Quartile | 1 | 1 | 1 | 1 | ||||||||
| Second Quartile | 0.68 | 0.63–0.73 | <.0001 | 0.85 | 0.71–1.03 | 0.093 | 0.86 | 0.72–1.02 | 0.083 | 0.86 | 0.77–0.95 | 0.0051 |
| Third Quartile | 0.58 | 0.54–0.62 | <.0001 | 0.76 | 0.62–0.92 | 0.0064 | 0.53 | 0.43–0.65 | <.0001 | 0.85 | 0.76–0.96 | 0.0082 |
| Fourth Quartile | 0.75 | 0.7–0.81 | <.0001 | 0.76 | 0.62–0.95 | 0.015 | 0.55 | 0.44–0.68 | <.0001 | 0.73 | 0.64–0.83 | <.0001 |
| Residents with Some College Education | ||||||||||||
| 1st Quartile (0 – 8.1%) | 1 | 1 | 1 | 1 | ||||||||
| 2nd Quartile (8.2% – 14.4%) | 1.04 | 0.97–1.1 | 0.28 | 0.88 | 0.76–1.03 | 0.104 | 0.83 | 0.71–0.97 | 0.018 | 0.97 | 0.88–1.07 | 0.58 |
| 3rd Quartile (14.5 – 24.0%) | 1.12 | 1.05–1.21 | 0.001 | 0.73 | 0.61–0.88 | 0.001 | 0.69 | 0.57–0.83 | <.0001 | 0.94 | 0.84–1.05 | 0.24 |
| 4th Quartile (>24.1%) | 1.2 | 1.11–1.3 | <.0001 | 0.82 | 0.66–1.02 | 0.067 | 0.74 | 0.59–0.91 | 0.0057 | 1.07 | 0.95–1.21 | 0.28 |
| Income Level | ||||||||||||
| 1st Quartile ($0 – $35,453) | 1 | 1 | 1 | 1 | ||||||||
| 2nd Quartile ($35,454 – $46,559) | 0.87 | 0.82–0.92 | <.0001 | 0.97 | 0.8–1.16 | 0.72 | 1.15 | 0.96–1.38 | 0.1371 | 0.98 | 0.89–1.08 | 0.70 |
| 3rd Quartile ($46,560 – $62,311) | 0.74 | 0.69–0.79 | <.0001 | 0.98 | 0.8–1.21 | 0.86 | 0.91 | 0.74–1.12 | 0.3808 | 0.86 | 0.77–0.96 | 0.0084 |
| 4th Quartile (>$62,312) | 0.59 | 0.54–0.64 | <.0001 | 1.1 | 0.88–1.38 | 0.40 | 0.94 | 0.75–1.18 | 0.5801 | 0.86 | 0.76–0.98 | 0.024 |
Abbreviations: CI (confidence interval), DCIS (ductal carcinoma in situ), ER (estrogen receptor), HR (hazard ratio
Cost of Treatment
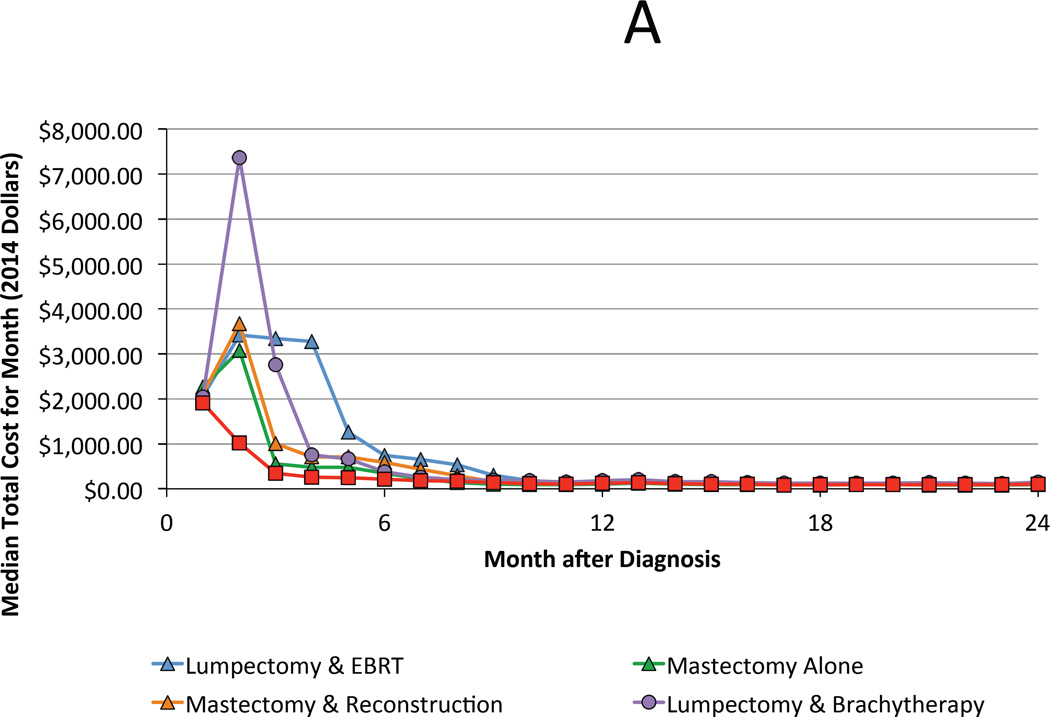
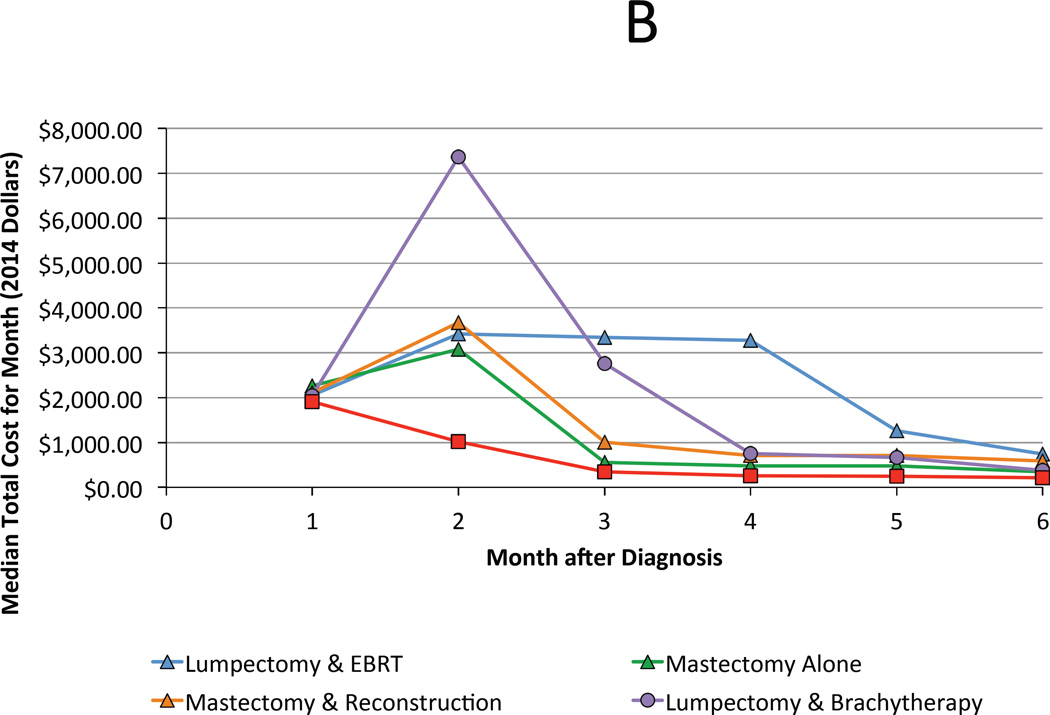
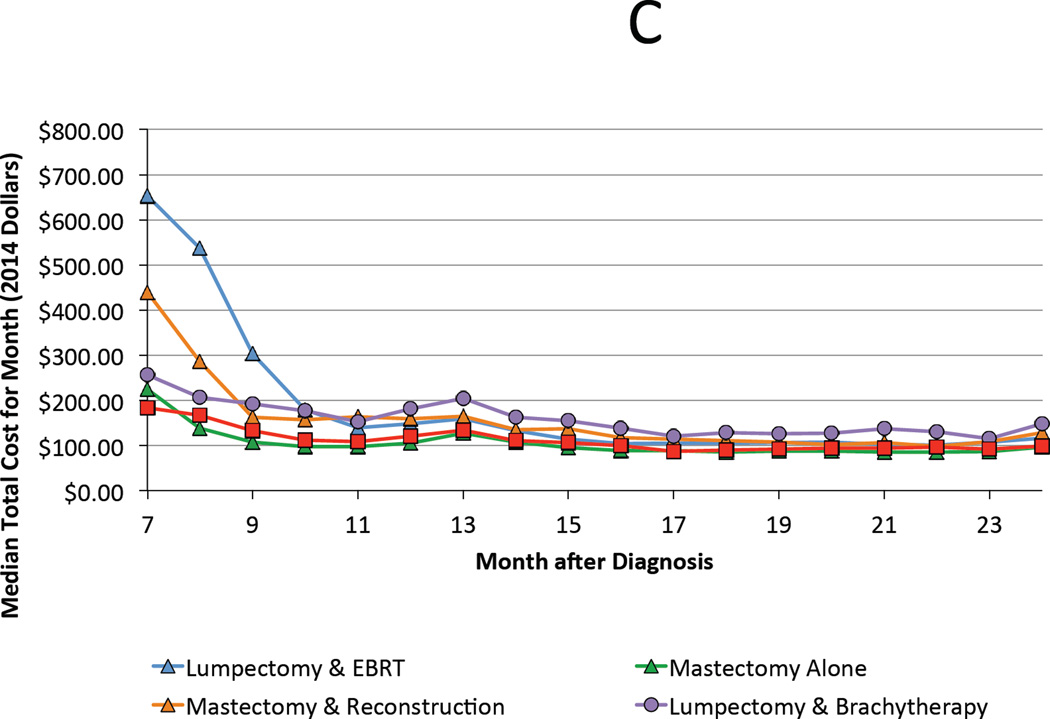
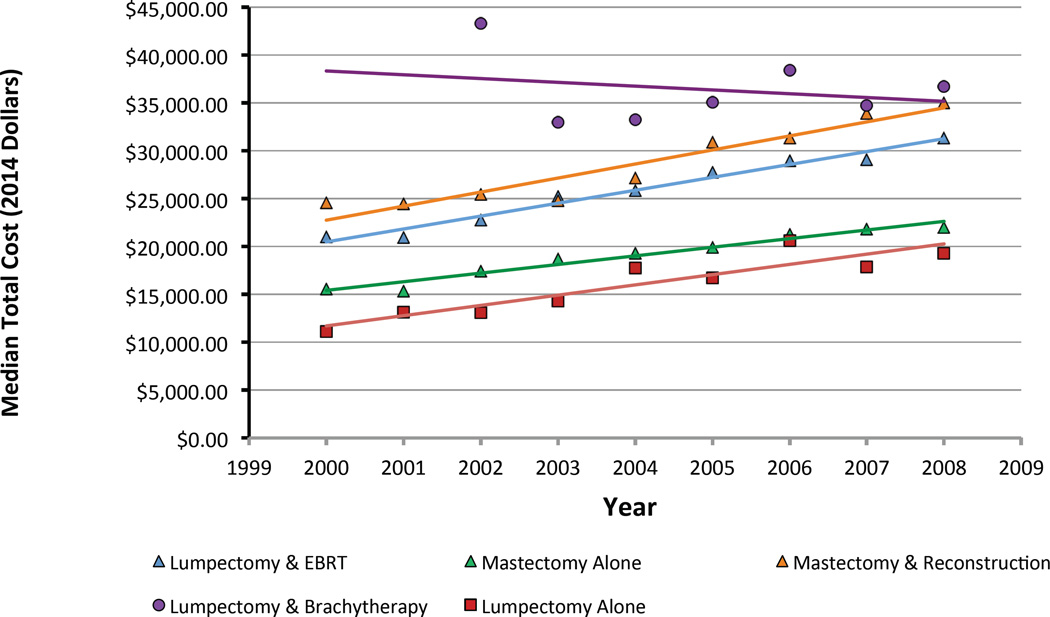
For the year 2008, median total costs for each treatment strategy, from highest to lowest, are ranked as follows: lumpectomy with brachytherapy ($36,749), mastectomy with reconstruction ($35,030), lumpectomy with external beam radiation ($31,388), mastectomy alone ($21,993), and lumpectomy alone ($19,287). The majority of costs were accrued during the treatment phase (0–6 months following diagnosis) regardless of the chosen therapy (Figure 2A & B). However, qualitative differences were preserved during the period associated with managing complications (6–24 months), with combination therapies attendant with higher costs (Figure 2C). Cost of all treatment strategies grew at a rate faster than inflation with the exception of lumpectomy with brachytherapy, whose cost was stable over time (Figure 3).
Figure 2.
A. Median total cost by month of each treatment strategy during the first 24 months following diagnosis. B and C highlight trends during the first 6 months and between months 7 and 24, respectively.
Figure 3.
Trends in median total cost for each treatment during the study interval. The lumpectomy and brachytherapy trend line starts in 2002 when FDA approval of a balloon-based breast brachytherapy occurred.
Discussion
We utilized the SEER-Medicare database to characterize trends in local therapy and associated costs for older women with early-stage breast cancer diagnosed between 2000 and 2008 in the SEER-Medicare cohort. The main trend we observed was a steady decline in the use of mastectomy alone during this time frame, with increasing utilization of breast conserving strategies, particularly driven by increasing utilization of lumpectomy with brachytherapy and lumpectomy alone. Though lumpectomy with brachytherapy was the most costly intervention prior to 2007, its inflation-adjusted cost was roughly stable over time in contrast to the other strategies whose growth in cost regularly exceeded inflation.
The groundbreaking National Surgical Adjuvant Breast and Bowel Project B-6 study demonstrated that patients diagnosed with early-stage breast cancers had statistically equivalent survival whether they were treated with mastectomy or lumpectomy followed by adjuvant radiation (19). After the study’s publication in 1985, a steady rise in breast conservation was observed in the United States (20–22). Patients benefited from less extensive surgeries requiring shorter hospital stays, fewer operative complications, and likely better cosmetic outcomes. Costs during this era were comparable between the two approaches as savings from reduced length of hospitalization and faster surgical recovery were offset by the cost of radiotherapy among those who underwent breast conservation (23,24).
In 2004, two landmark randomized clinical trials were published that sought to evaluate the need for whole breast irradiation specifically in older women with stage I, estrogen receptor positive breast cancer (25,26). These studies found that whole breast irradiation conferred a small (<5%) absolute reduction in risk of local recurrence at 5 years for older women, without an accompanying benefit in overall breast preservation or survival. Entry criteria for one of these trials, the Cancer and Leukemia Group B (CALGB) 9343, were subsequently incorporated into the National Comprehensive Cancer Network guidelines to define a group of older patients for whom radiation could be omitted (27), specifically women age 70 and older with clinical T1 N0, estrogen receptor positive disease resected with negative margins. Omission of radiation continues to be debated, however, with some experts arguing that the modest local control benefit conferred by radiation may justify its use for older patients with longer life expectancy (28).
Yet despite the research demonstrating the safety of breast conservation, by the 2000s, the trend favoring adoption of breast conservation reversed in several single-institution and population-based studies (5–9). The reasons for the renewed popularity of mastectomy were unclear, but possibilities included better techniques for breast reconstruction and improved access to reconstruction after the passage of the Women’s Health and Cancer Rights Act in 1998 (29). Psychological factors favoring mastectomy may have included patient anxieties over the malignant potential of residual breast tissue and the carcinogenicity of radiation. Advances in breast imaging, including the widespread use of breast MRI, may also have contributed to these concerns (30). Finally, the logistics of conventional radiotherapy, which requires 6 weeks of therapy, may have steered some patients to shorter interventions (31).
In contrast, among the older Medicare population, we identified a trend in the opposite direction, with an 11% decline in the proportion of patients opting for mastectomy alone accompanied by a 10% rise in use of breast conserving strategies. This finding is similar to recent analyses of the National Cancer Database (32) and the SEER-Medicare database (33). A unique contribution of this manuscript, however, is the incorporation of data about use of breast reconstruction following mastectomy and the accompanying cost data. For example, our data indicate that for each patient who chooses lumpectomy plus external beam radiation over mastectomy plus reconstruction, approximately $3,600 is saved. This vital information serves as an important reminder of the practical benefits of organ preservation facilitated by radiation.
Notably we did observe shifts over time in the relative proportions of the different breast conservation strategies used in the community. Breast conservation utilizing external beam radiation declined modestly after 2003 in favor of strategies employing lumpectomy alone or brachytherapy. The use of lumpectomy alone rose markedly after 2004, especially among favorable risk patients who fit CALGB 9343 entry criteria (25) . This observation is in accordance with a prior study by Soulos et al, which also reported a modest trend towards the omission of radiation after the CALGB trial was published (34). However, another unique finding of the current study is that brachytherapy also rose significantly after 2004 among favorable risk patients (Figure 1B). This suggests that some practitioners, rather than omit radiation, may have instead selected brachytherapy, perhaps in an effort to garner the local control benefits of radiation while avoiding the toxicities and inconvenience of whole breast treatment.
In the larger cohort of patients not limited by CALGB criteria, there was also observed a movement in favor of breast brachytherapy in later years which appeared to be at the expense of external radiation (Figure 1A). The time and effort required of the patient for several weeks of traditional external radiotherapy as well as its inferior reimbursement relative to brachytherapy may have influenced the adoption of the latter after its approval for use in the United States by the FDA.
Our second objective was to determine predictors of treatment strategy. We found that the youngest patients and those with the least comorbidities were more likely to receive mastectomy with reconstruction, echoing the findings of previously published studies that examined younger cohorts (5–9). We also found that patients with the least aggressive tumors (smaller size, lower grade, ER-positive, node-negative) were the most likely to undergo brachytherapy instead of conventional radiation, which may reflect published consensus statements and general caution employing a new technology during its early-adoption phase (3,35,36). A later year of diagnosis was very strongly correlated with brachytherapy, which, in similar fashion, implies improving physician comfort with this newer technique. Another important observation is that factors signifying lower socioeconomic status such as low area-level income and rural residence were associated with the use of mastectomy alone in lieu of combination strategies employing radiation or reconstruction. This finding reiterates an oft-described failure in the United States to diffuse innovations in breast cancer care to the least advantaged (37–39).
Our third objective was to examine costs of these treatments. Lumpectomy plus brachytherapy was associated with the highest cost. However, examination of trends revealed that brachytherapy exhibited stable cost during the study period in contrast to other treatments whose cost curves consistently exceeded inflation. The relative stability in the cost of brachytherapy may be attributable to predictable Medicare fee schedules and a limited number of procedure codes. Declines in reimbursement for brachytherapy may have also offset inflationary trends. In contrast, costs associated with lumpectomy and conventionally delivered radiation grew at a rate exceeding inflation. This trend is likely due to adoption of 3-dimensional conformal and intensity modulated radiation techniques over the study period (40,41). In the future, the cost curve for external beam radiation may more closely track with inflation as a result of bundled payments prompted by the Accountable Care Act and the publication of convincing studies supporting the use of hypofractionation in postmenopausal women (41–43).
This highlights a larger point: policymakers, payers, and physicians can control costs by promoting payment structures that encourage high value interventions such as hypofractionation (44). For example, a potential policy intervention based on this data would be to develop a bundle for local therapy for older women with early breast cancer amenable to breast conserving therapy. Setting a price point comparable to the cost of lumpectomy plus hypofractionated whole breast irradiation in patients with life expectancy greater than ten years could incentivize this high value treatment while discouraging more expensive treatment. Setting a price point comparable to lumpectomy alone in patients with life expectancy less than 10 years and estrogen receptor positive disease could incentivize this high value treatment in patients unlikely to benefit from radiation. Importantly, the use of qualifiers such as life expectancy or other pertinent characteristics can help ensure that bundled payments do not fail to account for meaningful differences among individual patients.
We used a combination of SEER data plus Medicare billing claims to classify treatment, thereby reducing the likelihood of misclassification bias when compared to studies that rely only on SEER coding (45,46). Nevertheless, our study has certain limitations. First, the study cohort is limited to fee-for-service Medicare patients and may not generalize to younger patients or those covered by private insurance. Second, our cost analysis only measures expenses for which claims data are available. It does not include lost work time or discretionary health care expenses. Third, the period of time studied for cost calculations captures treatment-related costs as well as the costs of complications over the medium term. Since differences in disease-free survival emerge later in the course of treated early-stage breast cancer, it is possible that expenses attributable to salvage therapy could change the relative cost profiles observed in our study. Cost of salvage therapy is expected to approximate initial costs of therapy, for example cost of salvage mastectomy is likely to be similar to the cost of mastectomy alone. However, as risk of local recurrence is low, and difference in local recurrence risk between treatments is small (4,26), the overall impact of salvage therapies on cost differences is expected to be minimal. Fourth, claims for external beam partial breast irradiation are indistinguishable from claims for whole breast irradiation, and thus we did not attempt to distinguish between these two treatments. However, other studies indicate that utilization of external beam partial breast irradiation was quite low during this time interval, and thus our findings regarding lumpectomy followed by external beam radiation likely primarily reflect the experience with delivering whole breast irradiation. Fifth, costs of endocrine therapy were not included and can vary widely, from as little as $600 for five years of tamoxifen to as much as approximately $36,000 for five years of letrozole (47). Notably, given the expense of aromatase inhibitors, hypofractionated radiation may be a higher value alternative for those at very low risk of distant recurrence, for whom the primary intent of adjuvant therapy is to improve local disease control in the breast (41,48). Finally, our discussion of value and bundled payments assumes that costs are defined by reimbursement dollars. Other models for calculating costs, such as time-driven activity-based costing, are also under investigation. Such diligence by health economists and policymakers is necessary when creating value-based payment models in order to ensure that those models benefit patients rather than deny needed care.
Conclusion
In this population-based cohort of older women with early breast cancer, use of mastectomy decreased, accompanied by increases in breast conserving approaches, including both standard external beam radiation and newer treatment strategies such as lumpectomy with brachytherapy or lumpectomy alone. Although mastectomy with reconstruction has become more popular in younger women, it has not yet gained significant traction among the population studied here. Using the cost estimates provided in this manuscript, price points for local therapy bundles can be constructed to incentivize treatment strategies which confer the highest value to patients.
Supplementary Material
Summary.
A population-based database of 55,327 older women with early-stage breast cancer treated during 2000–2008 was utilized to determine trends in local therapies and their associated costs. During this interval, the use of mastectomy in the elderly declined in favor of breast conserving strategies. Mastectomy with reconstruction was infrequently utilized. Costs generally grew faster than inflation and varied substantially by chosen local therapy, suggesting that policies encouraging high value care are needed.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Dr. Benjamin Smith had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported in part by a research grant from Varian Medical Systems to Dr. Smith. Drs. Smith and Giordano are supported by grants from the Cancer Prevention & Research Institute of Texas [Grant RP140020]. Dr. Likhacheva and Dr. Shaitelman report research funding from Elekta Incorporated. This work was also supported by the Department of Health and Human Services National Cancer Institute [Grant P30CA016672], The Duncan Family Institute, and a philanthropic gift from Ann and Clarence Cazalot. These entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Shaitelman is a consultant to the MD Anderson Physician’s Network.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no other conflicts of interest to report.
Contributor Information
Shervin M Shirvani, Email: smshirvani@mdanderson.org.
Jing Jiang, Email: jjiang@mdanderson.org.
Anna Likhacheva, Email: alikhacheva@mdanderson.org.
Abigail Caudle, Email: ascaudle@mdanderson.org.
Thomas A Buchholz, Email: tbuchhol@mdanderson.org.
Sharon H Giordano, Email: sgiordan@mdanderson.org.
References
- 1.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Polgar C, Fodor J, Major T, et al. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol. 2013;108:197–202. doi: 10.1016/j.radonc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy Plus Tamoxifen With or Without Irradiation in Women Age 70 Years or Older With Early Breast Cancer: Long-Term Follow-Up of CALGB 9343. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragun AE, Huang B, Tucker TC, et al. Increasing mastectomy rates among all age groups for early stage breast cancer: a 10-year study of surgical choice. Breast J. 2012;18:318–325. doi: 10.1111/j.1524-4741.2012.01245.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol. 2013;20:1436–1443. doi: 10.1245/s10434-012-2732-5. [DOI] [PubMed] [Google Scholar]
- 7.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150:9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 9.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16:2682–2690. doi: 10.1245/s10434-009-0635-x. [DOI] [PubMed] [Google Scholar]
- 10.Porter ME, Lee TH. Why strategy matters now. N Engl J Med. 2015;372:1681–1684. doi: 10.1056/NEJMp1502419. [DOI] [PubMed] [Google Scholar]
- 11.Newcomer LN, Gould B, Page RD, et al. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10:322–326. doi: 10.1200/JOP.2014.001488. [DOI] [PubMed] [Google Scholar]
- 12.Dieng M, Trevena L, Turner RM, et al. What Australian women want and when they want it: cervical screening testing preferences, decision-making styles and information needs. Health Expect. 2013;16:177–188. doi: 10.1111/j.1369-7625.2011.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40:IV-26–IV-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 16.Smith GL, Xu Y, Buchholz TA, et al. Brachytherapy for accelerated partial-breast irradiation: a rapidly emerging technology in breast cancer care. J Clin Oncol. 2011;29:157–165. doi: 10.1200/JCO.2009.27.0942. [DOI] [PubMed] [Google Scholar]
- 17.Brown ML, Riley GF, Schussler N, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV-104–IV-117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 18.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 20.Freedman RA, He Y, Winer EP, et al. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 21.Lazovich D, Solomon CC, Thomas DB, et al. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999;86:628–637. [PubMed] [Google Scholar]
- 22.Foote RL, Johnson RE, Donohue JH, et al. Trends in surgical treatment of breast cancer at Mayo Clinic 1980–2004. Breast. 2008;17:555–562. doi: 10.1016/j.breast.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Barlow WE, Taplin SH, Yoshida CK, et al. Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. J Natl Cancer Inst. 2001;93:447–455. doi: 10.1093/jnci/93.6.447. [DOI] [PubMed] [Google Scholar]
- 24.Palit TK, Miltenburg DM, Brunicardi FC. Cost analysis of breast conservation surgery compared with modified radical mastectomy with and without reconstruction. Am J Surg. 2000;179:441–445. doi: 10.1016/s0002-9610(00)00383-4. [DOI] [PubMed] [Google Scholar]
- 25.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 26.Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351:963–970. doi: 10.1056/NEJMoa040595. [DOI] [PubMed] [Google Scholar]
- 27.Carlson RW, Anderson BO, Burstein HJ, et al. Breast cancer. J Natl Compr Canc Netw. 2005;3:238–289. doi: 10.6004/jnccn.2005.0015. [DOI] [PubMed] [Google Scholar]
- 28.Scalliet PG, Kirkove C. Breast cancer in elderly women: can radiotherapy be omitted? Eur J Cancer. 2007;43:2264–2269. doi: 10.1016/j.ejca.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Yang RL, Newman AS, Lin IC, et al. Trends in immediate breast reconstruction across insurance groups after enactment of breast cancer legislation. Cancer. 2013;119:2462–2468. doi: 10.1002/cncr.28050. [DOI] [PubMed] [Google Scholar]
- 30.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013;257:249–255. doi: 10.1097/SLA.0b013e31827a8d17. [DOI] [PubMed] [Google Scholar]
- 31.Goyal S, Chandwani S, Haffty BG, et al. Effect of travel distance and time to radiotherapy on likelihood of receiving mastectomy. Ann Surg Oncol. 2015;22:1095–1101. doi: 10.1245/s10434-014-4093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lautner M, Lin H, Shen Y, et al. Disparities in the Use of Breast-Conserving Therapy Among Patients With Early-Stage Breast Cancer. JAMA Surg. 2015;150:778–786. doi: 10.1001/jamasurg.2015.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Showalter SL, Grover S, Sharma S, et al. Factors influencing surgical and adjuvant therapy in stage I breast cancer: a SEER 18 database analysis. Ann Surg Oncol. 2013;20:1287–1294. doi: 10.1245/s10434-012-2693-8. [DOI] [PubMed] [Google Scholar]
- 34.Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012;30:1601–1607. doi: 10.1200/JCO.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arthur DW, Koo D, Zwicker RD, et al. Partial breast brachytherapy after lumpectomy: low-dose-rate and high-dose-rate experience. Int J Radiat Oncol Biol Phys. 2003;56:681–689. doi: 10.1016/s0360-3016(03)00120-2. [DOI] [PubMed] [Google Scholar]
- 36.Dickler A, Kirk MC, Chu J, et al. The MammoSite breast brachytherapy applicator: a review of technique and outcomes. Brachytherapy. 2005;4:130–136. doi: 10.1016/j.brachy.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Albain KS, Green SR, Lichter AS, et al. Influence of patient characteristics, socioeconomic factors, geography, and systemic risk on the use of breast-sparing treatment in women enrolled in adjuvant breast cancer studies: an analysis of two intergroup trials. J Clin Oncol. 1996;14:3009–3017. doi: 10.1200/JCO.1996.14.11.3009. [DOI] [PubMed] [Google Scholar]
- 38.Black DM, Jiang J, Kuerer HM, et al. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149:788–796. doi: 10.1001/jamasurg.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 40.Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103:798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 41.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 42.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 43.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and Short-term Toxic Effects of Conventionally Fractionated vs Hypofractionated Whole-Breast Irradiation: A Randomized Clinical Trial. JAMA Oncol. 2015;1:931–941. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaby V, Tawk R, Sanogo V, et al. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res Treat. 2015;151:27–40. doi: 10.1007/s10549-015-3383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker GV, Giordano SH, Williams M, et al. Muddy water? Variation in reporting receipt of breast cancer radiation therapy by population-based tumor registries. Int J Radiat Oncol Biol Phys. 2013;86:686–693. doi: 10.1016/j.ijrobp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagsi R, Abrahamse P, Hawley ST, et al. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118:333–341. doi: 10.1002/cncr.26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drug Pricing. [Accessed October 17, 2015]; www.goodrx.com.
- 48.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.