Abstract
Addiction is characterised by a persistent loss of behavioural control resulting in insensitivity to negative feedback and abnormal decision-making. Here we investigated the influence of methamphetamine (METH)-paired contextual cues on decision-making in rats. Choice between goal-directed actions was sensitive to outcome devaluation in a saline-paired context but was impaired in the METH-paired context, a deficit that was also found when negative feedback was provided. Reductions in c-Fos-related immunoreactivity were found in dorsomedial but not dorsolateral striatum after exposure to the METH context suggesting this effect reflected a loss specifically in goal-directed control in the METH context. This reduction in c-Fos was localized to non-enkephalin expressing neurons in the DMS, likely dopamine D1-expressing direct pathway neurons, suggesting a relative change in control by the D1-direct vs. D2-indirect pathways originating in the DMS may have been induced by METH context exposure. To test this suggestion we infused the adenosine 2A receptor antagonist ZM241385 into the dorsomedial striatum prior to test to reduce activity in D2 neurons relative to D1 neurons in the hope of reducing the inhibitory output from this region of the striatum. We found that this treatment fully restored sensitivity to negative feedback in a test conducted in the METH-paired context. These results suggest that drug-exposure alters decision-making by down-regulation of the circuitry mediating goal-directed action, an effect that can be ameliorated by acute A2A receptor inhibition in this circuit.
Keywords: Drug seeking, goal-directed action, habit, dorsomedial striatum, dorsolateral striatum, methamphetamine, adenosine 2A receptor
Introduction
Understanding how the compulsive pursuit of drugs develops from volitional drug-taking is key to treating addictive behaviour. Environmental cues that predict the availability of drugs play a well-recognised role in influencing motivational processes that drive persistent drug-use including craving, withdrawal and relapse (Garavan et al., 2000; Shaham et al., 2003; Crombag et al., 2008). As such, drug cues are important targets of current psycho- and pharmaco-therapies treating drug addiction (Conklin & Tiffany, 2002; Koob et al., 2009). Accumulating evidence suggests, however, that compulsive reward-seeking is not only driven by disordered motivation but by changes in the goal-directed control of reward seeking actions (Ostlund & Balleine, 2008; Ostlund et al., 2010), an effect interpreted in terms of the propensity of drugs to promote habits (Everitt & Robbins, 2005; Everitt et al., 2008).
It is important to recognise, however, that the development of habits is not itself pathological; although impulsive, normal habits are not unregulated and negative feedback can result in the rapid suppression of even well learned habitual responses (Ostlund & Balleine, 2008). This is not true of all habits, however; pathological or ‘compulsive’ habits are relatively dysregulated and are generated when the circuitry that mediates goal-directed action, particularly the prelimbic prefrontal cortex and posterior dorsomedial striatum (pDMS), is damaged or pharmacologically inactivated (Yin et al., 2005b; Ostlund & Balleine, 2008; Balleine et al., 2009; Balleine & O’Doherty, 2010). Drug exposure can clearly trigger important changes in this circuitry (Jedynak et al., 2007) and it is possible that drug-induced habits are compulsive for much the same reason as habits in animals with damage to the corticolimbic-basal ganglia circuit.
To assess this hypothesis we took advantage of a previous finding that drug–paired contexts can render otherwise goal-directed actions habitual (Ostlund et al., 2010), pairing one context with methamphetamine (METH) and another with saline in rats. The rats were then trained on two actions to earn distinct food outcomes in a third context before assessing goal-directed control in both the METH and saline paired contexts using an outcome devaluation test and, subsequently, a negative feedback test. We also assessed regional changes in neuronal activity after exposure to the METH- and saline-paired contexts using c-Fos immunohistochemistry. We found that the METH-paired context generated both a profound deficit in goal-directed action and a reduction in c-Fos activity in the DMS. Half of the projection neurons in the striatum express dopamine D1 receptors and dynorphin and the remainder D2 receptors and enkephalin (Gerfen & Surmeier, 2011), and, using a counterstain for enkephalin, we found that the reduction in c-Fos was localized to non-D2, likely D1, neurons. As D1 and D2 projection pathways exert opposing influences on performance (Surmeier et al., 2007; Gerfen & Surmeier, 2011), we hypothesized that, by correcting this relative reduction in activity in the D1-compared to the D2 pathway, we should be able to rescue goal-directed control. We sought to achieve this by infusing the adenosine 2A (A2A) antagonist ZM241385, reported to reduce the activity of D2 neurons (Lovinger, 2010), into the DMS.
Materials and Methods
Overview
We conducted 3 experiments each with 3 stages: context-conditioning, instrumental training and outcome devaluation testing. Following methods previously described (Ostlund et al., 2010), all rats were first given alternating exposure to two distinct contexts, one after an injection of methamphetamine (METH) and the other after an injection of saline, and then trained to press two levers for two food outcomes in a third context. Tests of goal-directed action control were then given in both the METH- and saline-paired contexts using a specific-satiety outcome devaluation procedure. In Experiment 1 the tests in the two contexts were conducted in extinction. Experiment 2 replicated Experiment 1 except that c-Fos-related neuronal activity was examined after the test using immunohistochemistry. Given that goal-directed and habitual actions are mediated by parallel corticostriatal circuits centered on the dorsomedial and dorsolateral striatum, respectively, we examined c-Fos-IR in these regions. For one group of rats, testing was conducted in extinction (Experiment 2A) whereas another group was given a negative feedback test in which both the devalued and non-devalued outcomes were delivered following the appropriate actions as a test of behavioral control over habits or compulsivity (Experiment 2B). Based on the changes in DMS activity identified in Experiment 2, in Experiment 3 the A2A receptor antagonist, ZM241385 was microinfused after cannulation of the posterior DMS prior to the outcome devaluation test in an attempt to reduce the impact of methamphetamine cues on goal-directed action.
Subjects
We used experimentally naive Long-Evans rats (300–400 g) obtained from Monash Animal Services (Gippsland, VIC, Australia). Rats were housed in pairs in temperature- and humidity-controlled semi-transparent plastic boxes on a 12:12-h light/dark cycle (lights on at 7 am). Standard chow and water were freely available, except during instrumental conditioning and testing, when rats were food restricted to 10–15 g of chow per day to maintain bodyweight at approximately 90% of ad libitum bodyweight. All procedures were approved by the Animal Care and Ethics Committee at the University of Sydney.
Apparatus
Behavioural procedures were conducted in 16 identical MED Associates operant chambers (30 cm [width] × 24 cm [length] and 20 cm [height]), enclosed in sound and light-attenuated cabinets with fans for constant ventilation. The hinged-front, top and rear walls of the chambers were composed of transparent Plexiglas, and the remaining two side-walls of stainless steel. The floor consisted of 19 stainless steel rods over a tray of paper bedding. Chambers were illuminated by individual houselights during all procedures. On one wall of the chambers, two retractable levers were positioned to the left and right of a recessed food magazine. When appropriate, food outcomes were dispensed into the magazine, either 0.1 ml of 20 % sucrose solution via a pump or a 45 mg grain pellet (Bio Serve) from a pellet dispenser. An infrared photobeam that crossed the magazine detected head entry responses. The bare, unadorned operant chamber was used during initial instrumental training sessions whereas two distinct contexts were used during context conditioning by varying the visual, olfactory and tactile components of the chambers. For one context, Context A, laminated sheets of black-and-white vertical stripes were positioned on the transparent walls of the chambers, Plexiglas sheets covered with rough black grip tape were placed on the floor, and 0.5 ml of 10 % artificial vanilla essence was added (Queen Fine Foods, Alderly, QLD, Australia) on paper towel positioned beneath the floor. For the other context, Context B, laminated white panels with black filled circles were positioned on the walls, smooth white acrylic sheets covered the floor, and 0.5 ml of 10 % peppermint essence (Queen Fine Foods, Alderly, QLD, Australia) was added beneath the floor.
Context conditioning
All rats were exposed to both contexts. For half the rats Context A was paired with an intraperitoneal injection of (±)-methamphetamine hydrochloride (METH - Australian Government National Measurement Institute, 1 mg/kg in Experiments 1 and 2, and 2 mg/kg in Experiment 3, dissolved in 0.9 % saline) and Context B was paired with injection of 0.9 % saline solution of equivalent volume. For the other rats, Context B was paired with METH and Context A with saline. In Experiments 1 and 2, there were 7 exposure sessions to each context that alternated daily for a period of 14 days (i.e. 14 sessions in total, 7 METH-context pairings and 7 saline-context pairings for each rat). In Experiment 3, there were 8 exposure sessions to each context prior to DMS cannulation and 4 exposure sessions post-cannulation (i.e. 24 sessions in total, 12 METH-context pairings and 12 saline-context pairings for each rat). The larger number of context exposures, combined with the higher dose of METH (1 mg/kg versus 2 mg/kg) were used to increase the salience of the context conditioning as the cannulation procedure required a 2 week interval between context conditioning and instrumental training due to surgery and recovery. During each session, rats were placed in the context for 5–10 min, removed to administer the appropriate injection, and then returned to the context for a further 30–35 min. Importantly, all of the rats in each experiment had the same history of exposure to METH.
Cannulation
In Experiment 3, after the initial context conditioning, rats were implanted with bilateral guide cannulae targeted at the posterior dorsomedial striatum prior to instrumental training. Surgery was conducted under ketamine anesthesia (100 mg/kg with Xylazine, 20 mg/kg, i.p) combined with pre-surgery analgesic (Carprofen, 5 mg/kg, s.c and Bupivicane 3 mg/kg) and post-surgery antibiotics (procaine penicillin, 50 mg/kg, i.p). Rats were placed in a flat-skull position in a stereotaxic frame and two 6 mm guide cannulas (Plastics One, Roanoke, VA) were targeted 1 mm above the DMS. Co-ordinates relative to bregma were −0.6 mm anteroposterior (AP), ± 2.4 mm mediolateral (ML) and −3.6 mm dorsoventral (DV) according to the atlas of Paxinos and Watson (2005). Cannulas were anchored to the skull with stainless steel screws and acrylic dental cement. After surgery, dustcaps were fitted to guide cannulas, and animals monitored daily for 5–7 days before recommencement of the context conditioning sessions.
Instrumental conditioning
Food deprivation was initiated 24 hr after the final context conditioning session. After 3–4 days, two sessions of magazine training were given across two days in which 15 pellet and 15 sucrose outcomes were delivered into the magazine on a random time 60 sec schedule. Over the next 7 days, rats received instrumental training on the two levers. During a session each lever was trained separately in alternation in two blocks of 10 min with 2.5 min between each block. The first lever inserted, whether left or right, was alternated across days. Half the rats earned pellets by pressing the left lever and sucrose by pressing the right lever, and the other half earned sucrose on the left lever and pellets on the right lever. Lever-outcome pairings were also counterbalanced with respect to prior context conditioning. The reinforcement schedule changed across days: Days 1–2 used a continuous reinforcement schedule in which the appropriate outcome was delivered with each press; days 3–4 used a random-ratio 5 schedule (RR-5) and days 5–7 a RR-10 schedule with outcomes delivered, on average, every 5th and 10th press, respectively.
Outcome devaluation test
Goal-directed action control was assessed starting the day after the completion of training using an outcome devaluation test conducted in the METH- and saline-paired contexts. For the 60 min prior to the test, rats were placed in semi-transparent feeding cages and allowed unrestricted access to one of the two outcomes. Half the rats received pellets and the other half sucrose solution, counterbalanced with respect to both the context and response-outcome counterbalancing conditions. Rats were then immediately placed in either the METH- or saline-paired context. After 2 min, both levers were inserted into the chambers and lever pressing recorded. Responding was assessed in extinction for either 3 min, in Experiments 1, 2B and 3, or for 10 min in Experiment 2A.
Negative feedback test
In Experiments 2B and 3, the 3 min extinction period was followed immediately by a 15 min period where responses again earned the outcomes as in training but on concurrent ratio schedules. The first press on each lever within this period earned the appropriate outcome and then every 10th press on the specific lever thereafter delivered the outcome to provide immediate feedback of the outcome associated with an action.
Test order
In Experiment 1, all rats were then given one session of instrumental re-training (RR=10) and then, the next day, tested in the other context (either saline, if the first test was conducted in the METH-paired context or vice versa) with the same outcome devalued as in the first test. In Experiments 2A and 2B, rats were tested once only in one context before the processing of the brains for c-Fos immunoreactivity (IR). Half the rats where tested for 10 min in extinction (Experiment 2A), whereas the others received 3 min in extinction followed by 15 min with the outcomes delivered in a negative feedback test (Experiment 2B).
Rescuing goal-directed control
In Experiment 3, bilateral infusions of ZM241385 into the DMS were given 12 min prior to the outcome devaluation and subsequent negative feedback test. Each rat was tested twice in the same context, once after infusion of ZM241385 and once after an infusion of saline, such that, for half the rats, testing occurred in the METH-paired context and for half the rats in the saline-paired context. Rats were removed from the feeding chambers and immediately infused with either the selective A2A receptor antagonist ZM241385 (4 ng/ul, Tocris Cookson Ltd., Bristol U.K., dissolved in 0.9 % saline and stored at −20°C until use) or vehicle (0.9 % saline). This dose of ZM241385 has previously been shown to modify instrumental performance in mice when infused into DMS (Nam et al., 2013) and to inhibit A2A receptors (Stella et al., 2003). The order of drug and saline administration was counterbalanced across the two tests, which were separated by one day of recovery and one day for instrumental re-training (RR=10). The infusions were made using 33 gauge cannula injectors (Plastics One, Roanoke, VA), which projected 1 mm ventral to the tip of the guide cannula. Cannulas were connected to 25 μL Hamilton syringes, via polyethylene-50 tubing, which were mounted on an infusion pump. Infusions were made over 2 min, with 1 min for diffusion at a volume of 0.5 μL per side. A delay of 12 min was given before rats were tested to allow for time for the drug to act.
Histology – Placements
To confirm cannula placements at the end of the experiment, rats were overdosed (pentobarbitone, 120 mg/kg, i.p) and the brains extracted, frozen and sectioned using a cryostat (40 μm, Leica microsystems). Every second section containing the striatum was collected onto glass slides, allowed to dry and then stained with cresyl violet. After coverslipping with Entellan, sections were examined under a light microscope (Olympus BX50), and the most ventral scarring from the cannula insertion plotted on Adobe Illustrator templates (modified from Paxinos & Watson, 2007).
Immunohistochemistry
In Experiment 2, immunohistochemistry for c-Fos-IR was conducted to compare neuronal activity during testing in the METH- and saline-paired contexts. Two hours after commencement of the test, rats were deeply anesthetised (pentobarbitone, 120 mg/kg) and perfused transcardially with heparinised saline (0.9 %) and paraformaldehyhde (4 %). Brains were then removed and post-fixed (1 hr) and cytoprotected in sucrose (48 hr). The forebrain was then sectioned at 40 μm on a cryostat. Four serially adjacent sections were collected (160 μm apart) and stored in 0.1 % sodium azide in phosphate buffer (PB, pH=7.4, at 4 °C). For one series, the striatum was processed for c-Fos-IR. For another series, the DMS was double stained to reveal enkephalin positive neurons to distinguish neurons of the indirect from the indirect striatal output pathways. We also verified that our ENK antibody labeled approximately half of the neurons in dorsal striatum in two serial sections through striatum from 3 untreated rats by labelling neurons with the NeuN antibody [mouse-anti-NeuN primary antibody (1: 5000, Millipore), followed by a goat-anti-mouse secondary (1:1000, Jackson ImmunoResearch Laboratories)].
Sections were first rinsed in 50 % ethanol, then 3 % hydrogen peroxide in 50 % ethanol and then blocked in 5 % normal horse serum (NHS) in PB (30 min each). All series were incubated in rabbit anti-c-Fos (4): sc-52 (1:1000, Santa-Cruz Biotechnology) in 2 % NHS with 0.2 % triton X-10 and 0.1 % sodium azide for 48 hr at room temperature (RT). After rinsing in PB, sections were then incubated in biotinylated donkey anti-rabbit IgG (1:1000, Jackson ImmunoResearch Laboratories, 24 hr, RT), and then ABC reagent (1:170, Vectastain Elite, Vector Laboratories, 2 hr, RT). Black c-Fos-IR was revealed using a nickel-intensified diaminobenzidine (DAB) solution (0.5 % DAB, 0.04 % ammonium chloride, 0.2 % D-glucose and 1 % nickel sulfate in sodium acetate buffer, pH=6) with the addition of glucose oxidase (735 units/ml, Sigma-Aldrich).
For c-Fos-IR double staining with enkephalin-IR, sections were reacted serially, so that after black c-Fos-IR was revealed, sections were incubated in rabbit anti-pre-pro enkephalin (1:1000, Neuromics), and then again in donkey anti-rabbit IgG with DAB reaction as described above but without nickel intensification in order to reveal brown cytoplasmic reaction product. Serial-staining with two antibodies raised in the same animal (rabbit) was necessary because of the low numbers of c-Fos-IR revealed by goat-anti-c-Fos antibody (1:50 to 1:1000, Santa-Cruz Biotechnology), and lack of a suitable commercially available alternative rabbit anti-c-Fos antibody. Finally, sections were mounted on gelatin coated-slides, and cover slipped.
c-Fos-IR quantification
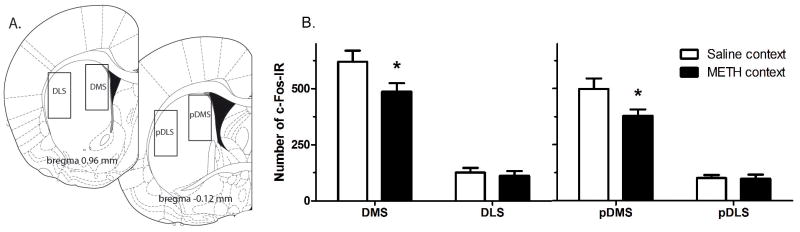
Single and double c-Fos-IR was manually counted using a transmitted light microscope at 20x magnification. Counts were made at the microscope on consecutive sections on one side of the brain (right and left sides equivalent for both groups) by an experimenter blind to group identity. The striatum was delineated using clearly visible landmarks and a grid reticule located in the right eyepiece of the microscope. The subregions quantified included 4 sections from the anterior part of DMS and DLS (between 1.30 and 1.95 mm from bregma, 1 mm × 2 mm area quantified per section) and 2 sections for posterior striatum (pDMS and pDLS, between 0.00 and −0.32 mm from bregma, 1 mm × 1 mm area quantified), which was repeated with sections double labeled with enkephalin (refer Figure 2, left panel). Example images were captured using a digital camera (Olympus, Japan).
Figure 2.
c-FOS-IR cell counts in the striatum in Experiment 2. (A) Coronal sections showing regions where c-Fos-IR counts were made (left side of figure - adapted from Paxino & Watson, 2005). (B) c-Fos-IR counts in anterior (left panel) and posterior (right panel) DMS and DLS in rats exposed either to the saline-paired context (white bars) or the METH-paired context (dark bars).
Data analysis
Lever response rates from Experiment 1 were analyzed using 2 × 2 within subjects ANOVA, where test context and lever choice served as factors. Lever response rates from Experiment 2 were analyzed as mixed 2 × (2) ANOVA where test context served as the between factor and lever choice as the within factor, and Experiment 3 as a mixed 2 × (2) × (2) ANOVA where infusant served as the additional factor. Simple main effects were then carried out to determine the source of any interaction. Rates of magazine entry across the two test contexts were analyzed using a within subjects ANOVA in Experiment 1 and between subjects ANOVA in Experiment 2. For c-Fos-IR, total single and double labeled Fos-IR was summed for each region examined, and analyzed using mixed ANOVA with striatal area as a between subjects factor and context as a between subjects factor.
Results
Experiment 1. Effect of a METH-paired context on goal-directed action
All animals acquired the instrumental actions with responding increasing over sessions from an average rate of 4.4 ± 0.1 presses per min on CRF to a rate of 28.6 ± 1.3 presses per min on the RR-10 schedule. The results of the outcome devaluation tests are presented in Figure 1 panel A. The extinction test in the saline context revealed a clear outcome devaluation effect: the rats pressed the lever that, in training, was associated with the now devalued outcome less than the other lever. In contrast, when tested in the METH-paired context there was no such preference: the rats pressed both levers at a similar rate. Statistical analysis revealed neither a main effect of context (F<1) or of devaluation (F(1, 16) =2.206, p=0.156) but found a significant context x devaluation interaction (F(1, 16)=9.474, p=0.006). Simple effects analysis comparing lever pressing in each context confirmed that a significant outcome devaluation effect emerged in the saline-paired context (F(1, 16)=17.709, p=0.001) and not the METH-paired context (F<1). These results demonstrate that methamphetamine-paired contextual cues interfere with the expression of goal-directed action selection. There was, however, no difference in the number of head entries per minute into the magazine across contexts (4.16 ± 0.58 saline context and 4.53 ± 0.51 METH context, F<1).
Figure 1.
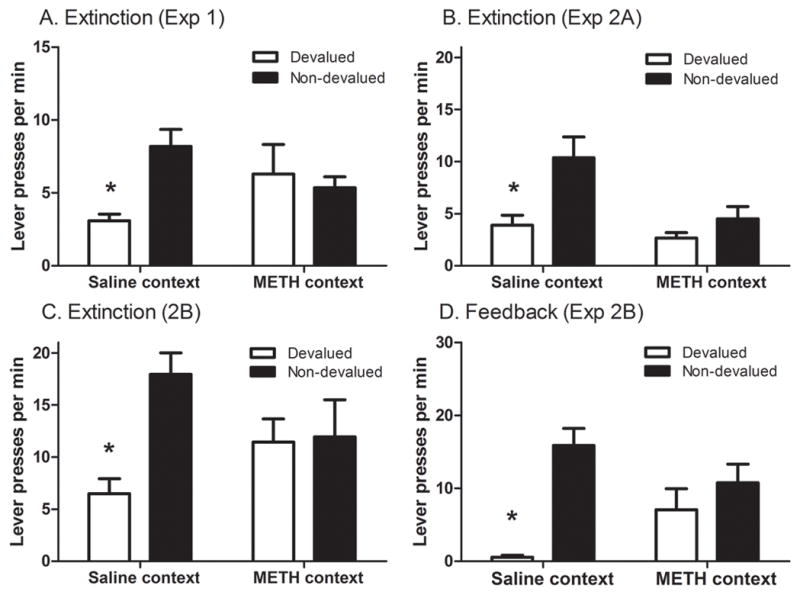
(A) Experiment 1. Mean (± SEM) number of lever presses per min during 3 min choice extinction tests conducted in saline and METH associated contexts. All rats were tested in both contexts and showed a significant preference for the lever previously associated with the non-devalued reward (non-devalued) over that previously associated with the devalued reward (devalued) when tested in the saline context, but not when tested in the METH context. (B) Mean (± SEM) number of lever presses per min in the outcome devaluation extinction test in Experiment 2A (C) Outcome devaluation extinction test in Experiment 2B after which c-Fos immunohistochemistry was conducted to examine neuronal activity during goal-directed responding. In both experiments a significant preference for the non-devalued lever was observed in rats tested in the saline-paired, but not in the METH-paired context. (D). Mean (± SEM) number of lever presses during the negative feedback test in Experiment 2B. As when tested in extinction, a preference for the non-devalued lever was found only in the saline-paired but not the METH-paired context. Hence, rats tested in the METH context were unable to modify their actions even in the face of negative feedback.
Experiments 2A and 2B: Effect of a METH-paired context on goal-directed action, negative feedback and c-Fos-related activity in the corticolimbic basal ganglia network
Outcome devaluation test
Experiments 2A and 2B examined the impact of the METH-context on goal-directed action in a choice extinction test and also, in Experiment 2B, the effect of negative feedback produced by delivery of the devalued outcome. Furthermore, after both kinds of test, c-Fos-IR was examined to assess changes in the neuronal circuits associated with performance in the drug-paired context. As in Experiment 1, the instrumental actions were acquired during training with average response rates increasing from 4.2±0.6 to 29.8±1.7 presses per min in Experiment 2A, and from 4.4± 0.5to 31.5±2.2 presses per min in Experiment 2B. Performance during the outcome devaluation tests in Experiments 2A and 2B are presented in Figure 1, panels B and C. As can be seen from these figures outcome devaluation effects emerged in the extinction tests conducted in both Experiments 2A and 2B but only in the saline paired context; outcome devaluation effects again failed to emerge in the METH-paired context. In both replications there was a significant context x devaluation interaction (F(1, 13) = 8.319; p=0.013, and F(1,12) =4.706; p=0.05, respectively), confirming that preference for the non-devalued lever was selective to the saline context. Simple effects confirmed significant devaluation effects in the saline context (F(1, 13) = 34.940, p=0.001 and F(1,12) =10.333; p=0.007, respectively) but not the METH-context (F(1, 13) =2.496; p=0.138 and F<1, respectively) in both replications. Magazine entries per min were again similar across contexts in both replications (4.78 ± 0.67 saline context and 5.4 ± 2.96 meth context, (F<1), for Experiment 2A, and 3.00 ± 0.39 saline context and 3.71 ± 0.70 meth context, (F<1), for Experiment 2B).
Negative feedback test
Performance in the negative feedback test in Experiment 2B, in which both the valued and devalued outcomes were delivered contingent on pressing one or other lever, is shown in Figure 1 panel D. As in the extinction tests, a clear preference for the non-devalued lever emerged only in the saline-paired context and was not found in the METH-paired context. Statistically, there was no main effect of context (F<1) indicating no difference in total response rate across contexts. There was, however, a significant main effect of lever (F(1,12) =14.636; p=0.002), and, more critically, a significant Context x Feedback interaction (F(1,12) =5.468; p=0.037), demonstrating that sensitivity to negative feedback produced by delivering the devalued outcome emerged in the saline paired but not in the METH–paired context. Simple effects revealed that this difference was significant in the saline-paired context (F(1,12) =18.998; p=0.01) but not in the METH-paired context (F(1,12) =1.106, p=0.314). Again, rate of magazine entry did not differ across contexts (6.78 ± 1.03 saline context and 7.24 ± 0.75 meth context (F<1)), confirming that drug-cues did not alter overall motivation for the outcomes.
c-Fos immunohistochemistry
Based on evidence that the DMS and the DLS subregions of the dorsal striatum play a differential role in goal-directed and habitual action, we focused c-Fos-IR counts on these regions of interest examining c-Fos-IR across the anterior-posterior extent of the dorsal striatum. Total c-Fos-IR for the anterior and posterior regions of the DMS and DLS for the saline and METH groups, respectively, are presented in Figure 2. To analyse the data we conducted a three-way analysis of variance with factors comparing Groups (METH vs. saline), Striatal area (DMS vs. DLS) and Anterior vs. posterior subregions. This analysis found no main effect of group, F(1,27)=3.6, p>0.05, but found a main effect of striatal area, F(1,27)=292.7, p<0.001, and, importantly, a significant Group x Area interaction, F(1,27)=6.8, p=0.015. Simple effects analyses confirm that c-Fos-IR was reduced in the DMS in both anterior and posterior subregions, F(1,27)=4.7, p=0.039, F(1,27)=4.5, p=0.04, respectively. In contrast, there were no differences in DLS in either anterior or posterior subregions, F(1,27)=0.02, p=0.89, F(1,27)=0.23, p=0.64, respectively. Although there was main effect of subregion, F(1,27)=18.5, p<0.001, with more c-Fos-IR overall in anterior regions, neither the two-way nor the three-way interactions with subregion were significant (Fs<1).
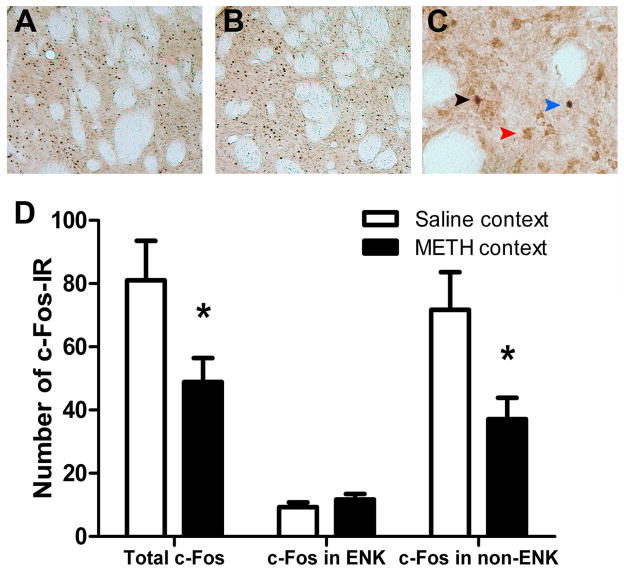
Taken together these results suggest that: (i) METH context exposure produced a regionally consistent and significant reduction in c-Fos activity in the DMS, and (ii) that this effect was specific to the DMS and did not extend to the DLS. For this reason we focussed our double staining for c-Fos-IR and enkephalin, known to be expressed selectively by D2-indirect pathway MSNs (Gerfen, 1992), on the DMS. c-Fos-IR was compared in rats sacrificed after testing in the saline- and METH-paired contexts. Figures 3A and 3B shows representative black nuclear c-Fos-IR in the DMS for each group and an example of double, brown cytoplasmic staining for c-Fos-enkephalin-IR is shown in Figure 3C. Although there was high background for c-Fos-enkephalin-IR, where the same secondary Ig was used for each primary antibody, serial staining did not result in any staining that could be misinterpreted, i.e. there was no black cytoplasmic staining (Bossert et al., 2012). As shown in Figure 3D, we found a greater number of total c-Fos-IR neurons for the saline vs. METH group (F(1,12)=4.804, p=0.049). There was no difference in total number of ENK neurons counted (F<1) nor was there a difference in the total percentage of ENK double labelled with c-Fos, which was low for both groups (F(1,12)=1.112, p=0.312). In contrast, the number of non-ENK labelled neurons was significantly greater for the saline group compared to METH group (F(1,12)=6.399, p=0.026) suggesting that the effect of METH context exposure was restricted to non-enkephalin expressing MSNs; i.e., putatively, D1-expressing MSNs (see Figure 3D). Although c-Fos activity in the pDMS was assessed after the outcome devaluation (Experiment 2A) or negative feedback (Experiment 2B) test, it is important to note that these counts are similar to counts taken after exposure to the context alone: See Supplemental Methods and Results and Supplemental Figure S1. Similarly, we confirmed that our ENK antibody labelled approximately half of all neurons in the dorsal striatum. We found that, on average, the DMS contained 404±16 ENK-IR and 926±50 NeuN-IR, and the DLS contained 388±32 ENK-IR and 808±82 NeuN-IR, per 1 mm2 of tissue. Therefore, as expected, there were approximately half as many ENK as total neurons labeled in this sample (44±3% for DMS and 49±3% for DLS).
Figure 3.
c-FOS and ENK-IR photomicrographs and cell counts in the pDMS for rats that underwent the negative feedback test in Experiment 2B. Panels A–C show representative c-Fos-IR in the DMS in: (A) the saline group (B) the METH group and (C) in a section double labelled for c-Fos-IR and ENK-IR; c-Fos+ENK = black arrow; ENK only = red arrow; c-Fos only = blue arrow. (D) Counts revealed that differences in c-Fos-IR between the saline and METH contexts occurred in the single rather than double-labelled c-Fos-IR+ENK neurons (i.e. non-ENK neurons, likely D1 neurons). It can be seen that there was less total c-Fos for rats exposed to the METH context, as well as less c-Fos-IR in non-ENK neurons rather than c-Fos-IR double stained with ENK, than rats exposed to the saline context.
These results suggest, therefore, that the reduction in c-Fos activity was specific to D1 receptor-expressing neurons in the DMS and that the changes due to exposure to the METH-paired context were localised to those neurons and to the D1-direct striatonigral pathway.
Experiment 3: Effect of adenosine A2A antagonism in DMS on responding in the METH-paired context
Our c-Fos-IR data implicated a neural circuit centered on the DMS, rather than on the DLS, in the altered decision-making observed in the METH-paired context. Furthermore, these data could be taken to suggest that, in the METH context, control over behavior by the D1-direct striatonigral pathway was reduced in the METH-paired context compared to D2-indirect striatopallidal pathway. As the former pathway is known to be excitatory and the latter inhibitory for the specific circuit functions in which they are engaged (Gerfen & Surmeier, 2011), any such imbalance could have resulted in reduced functional activity; in this case in the circuitry associated with goal-directed action. However, as our c-Fos-IR provides only indirect evidence for this claim, the aim of Experiment 3 was to target the DMS pharmacologically to assess its role directly. Specifically, we aimed to reverse the deficit in goal-directed responding in the METH context by reducing the inhibitory output of the DMS that is provided when the indirect pathway is activated (Gerfen & Surmeier, 2011). We did this by antagonizing A2A receptors, which are located predominately on indirect pathway neurons (Ferre, et al, 1997; Svenningsson et al, 1999), a treatment that has been shown to inhibit the ongoing activity of D2 neurons (Tozzi et al., 2007). As such this treatment should produce a relative increase in control by the D1 striato-nigral direct pathway in the DMS, which should be predicted to restore goal-directed responding when given prior to test. This hypothesis is illustrated in Figure 4 (left and center panels).
Figure 4.
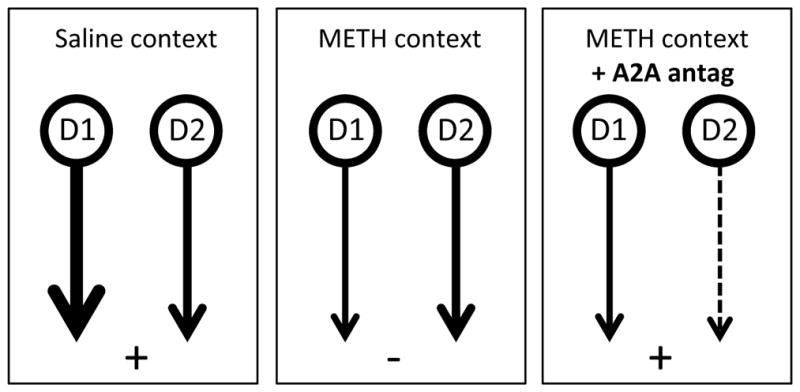
Illustration of hypothetical activity in the D1 and D2 output pathways from DMS during the negative feedback tests in the current study. When tested in the saline context (left panel) activation of the D1 neurons coincides with goal-directed performance, whereas in the METH paired context (center panel), a reduction in activity in D1 neurons results in a relative increase in activation of the D2 pathway and habitual performance. Administration of the A2A antagonist (right panel) is predicted to reduce activity in the D2 pathway, and to result in a relative increase in activity in the D1 pathway. By rebalancing the relative output of the D1 and D2 pathways this treatment is hypothesised to restore goal-directed action.
Devaluation Test
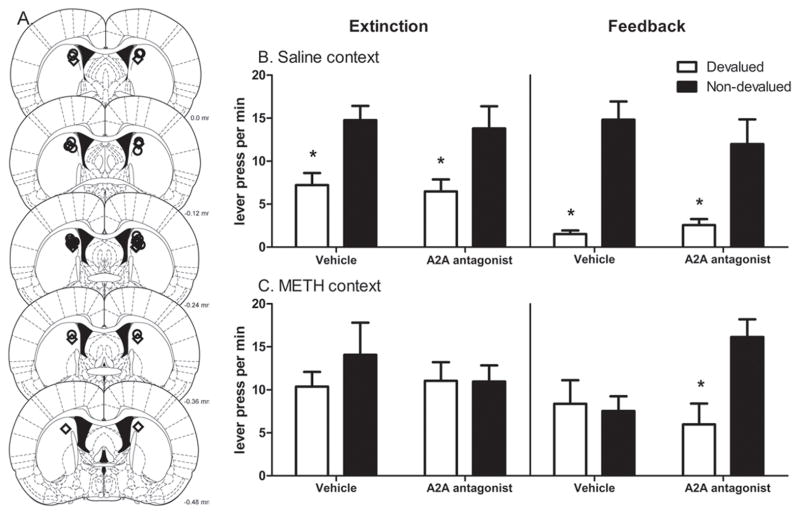
Cannula placements in the posterior portion of the pDMS are shown in Figure 5A. Two rats were eliminated for placement outside of DMS (and into the lateral ventricle) making n=11 and n=9 rats tested in saline and METH-context, respectively. Results of the outcome devaluation and negative feedback tests conducted after the infusion of vehicle or ZM241385 are shown for the saline-paired and METH-paired contexts in Figures 5B and 5C respectively. It can be seen that, when tested in extinction, there was a preference for the non-devalued lever when either vehicle or ZM241385 was infused prior to testing in the saline context (top left panel). In contrast, there was no such preference when tested in the METH-paired context in extinction with either infusion (top right panel). Statistically, there was no main effect of context (F(1,18)<1, p=0.599) or infusion (F(1,18)= 0.941, p=0.345), but a main effect of devaluation (F(1,18)=11.348, p=0.003). There was also no infusion x context interaction (F<1) but there was a context x devaluation interaction (F(1,18)=4.188, p=0.05) suggesting that the context of testing influenced the choice of lever overall. Analysis of lever pressing within each context confirmed effect of devaluation in the saline context (F(1,18)=15.477, p=0.001) but not in the METH context (F<1) and, in the saline context after both infusions (vehicle: F(1,18)=6.008, p=0.025; ZM241385: F (1,18)=7.401, p=0.025) but not in the METH context (vehicle: F(1,18)=1.307, p=0.268; ZM241385: F<1).
Figure 5.
Experiment 3. (A) Schematic representation of injection sites in the DMS (from 0.0 mm to −4.8 mm AP from bregma, adapted from Paxinos and Watson, 2005). The location of the most ventral point of the cannula tract is depicted for rats exposed to the saline (circles) and METH (diamonds) paired-contexts. (B) Mean (± SEM) number of lever presses during extinction and negative feedback tests when rats were tested in the Saline Context after infusion of either vehicle or the A2A antagonist ZM241385 in Experiment 3. Preference for the non-devalued lever is shown in these tests and this preference was unaffected by ZM241385. (C) Mean (± SEM) number of lever presses during extinction and negative feedback tests when rats were tested in the METH Context after infusion of either vehicle or the A2A antagonist ZM241385. No effect of outcome devaluation was observed in the Meth context except when ZM241385 was infused during the negative feedback test indicating that sensitivity to negative feedback was restored in this condition.
Negative feedback test
In contrast to the test in extinction, ZM241385 produced a clear reversal of the effect of the METH context on sensitivity to negative feedback. As shown in Figure 5C, whether injected with vehicle or ZM241385 a clear negative feedback effect emerged in the saline context. In contrast, whereas rats tested in the METH context after infusion of vehicle were insensitive to negative feedback this deficit was fully reversed by the infusion of ZM241385. Statistically, there was no main effect of context (F(1,18)=1.865, p=0.189) or infusion (F(1,18)=1.680, p=0.526) but a main effect of feedback (F(1,18)=18.417, p=0.00), and, although the infusion x context interaction and feedback x context interactions were also not significant (F(1,18)=1.173, p=0.293 and F(1,18)=2.342, p=0.143, respectively), there was a significant three-way feedback x context x infusion interaction indicating that the choice of lever was not only dependent on test context but also on the infusion (F(1,18)=5.248, p=0.034). Examination of the infusion x lever interactions within each context revealed that, although not significant in the saline context (F(1,18)=1.257, p=0.277), this interaction was significant in the METH context (F(1,18)=4.306, p=0.05). Simple effects confirmed that within the METH context, there was a significant negative feedback effect after ZM241385 infusion (F=(1,18)=5.897, p=0.026) but not after vehicle infusion (F<1), indicating that A2A antagonism was able to rescue goal-directed control sufficiently to exert significant regulation over drug-induced habitual actions.
Discussion
Perhaps the most striking characteristic of exposure to addictive drugs is their influence on decision-making, particularly the willingness of addicts to continue drug seeking in the face of the often extreme negative consequences drugs have on long-term health and wellbeing. Although commonly characterized as a form of habit (Everitt & Robbins, 2005), this persistent insensitivity to negative feedback marks addictive behavior as quite distinct from normal, adaptive, habitual actions which are suppressed in the face of negative feedback, with actions reverting to goal-directed control. Here we establish the rapidly acquired and pervasively debilitating effect of drug-associated contextual cues on non-drug related decision-making in rats working for food rewards and, in particular, on their ability to withhold maladaptive choices even when those choices generate negative feedback.
To demonstrate these effects we used three distinct contexts, one paired with methamphetamine (METH), one paired with saline and a third context used during the acquisition of two distinct goal-directed actions. These experiments found that, after outcome devaluation, when the rats were placed in a context in which they had received only exposure to saline and were asked to choose between two actions previously trained with either the devalued or another, currently non-devalued, outcome, they were able to choose appropriately; i.e. they reduced their performance of the action that in training had delivered the now devalued outcome. When the same rats were placed in the METH-paired context, however, they developed an immediate deficit in goal-directed action control and were unable to choose between these distinct courses of action. This was not only true of the ability of the rats to make adaptive decisions but also their ability to adjust those decisions in the face of negative feedback. When punished for making maladaptive choices in the saline context they, if anything, further reduced the performance of an action associated with a devalued outcome. When placed in the METH context, however, the same rats were insensitive to negative feedback and continued to respond on the levers as if they were delivering a still valued outcome.
It should be noted that, in these studies, these effects of a METH paired context on decision-making were established using rewards other than drugs so as to assess the effect of the drug context on choice in the absence of other effects of the drug. Although self-administration studies demonstrate drug-induced changes in drug-seeking (Belin et al., 2009), any changes in decision-making in such studies are necessarily confounded with the effects of the drug on both learning and performance factors and so cannot establish the causal relationship between drug exposure and deficits specifically in decision-making per se. Nevertheless, there is every reason to suspect that drug-paired contexts will render goal-directed drug self-administration habitual in much the same way that affects actions earning other rewards given previous demonstrations of contextual control of drug-seeking and the generally similar effects on drug- and food self-administration in related paradigms (e.g., renewal; Marchant et al., 2014).
Our results also demonstrate that METH-related habits reflect an effect on the circuitry associated with goal-directed action control. Although the performance of animals in the METH context was consistent with an increase in habit, we found that these effects on decision-making were not mediated by changes in circuit commonly associated with the development of habitual actions and that is centered on the DLS (Killcross & Coutureau, 2003; Yin et al., 2004; Lingawi & Balleine, 2012). In contrast, these changes were localized to the circuit associated with goal-directed actions, centered on the DMS and which is strongly associated with the acquisition and expression of goal-directed actions (Yin et al., 2005a; Yin et al., 2005b; Balleine & O’Doherty, 2010; Shiflett et al., 2010).
These powerful effects of a METH-paired context on goal-directed action raise questions as to how context exposure affects the circuitry mediating goal-directed control. There are two relatively straightforward ways in which a METH context could rapidly alter striatal neuronal activity. One is direct: It has been repeatedly demonstrated that monoamine activity in the dorsal striatum is down regulated by methamphetamine exposure (Ricaurte et al., 1980). Furthermore, these changes appear to be associated with reduced immediate-early gene activity, particularly cytoplasmic ARC expression in D1 striatonigral projecting neurons (Daberkow et al., 2008). If such changes can become conditional on cues associated with methamphetamine exposure then context exposure could induce a direct change in D1 neuron excitability through this mechanism.
Alternatively, it is possible, perhaps via previously documented spiraling inhibitory relationship between striatum and midbrain dopamine neurons (Haber et al., 2000), that exposure to a methamphetamine-paired context causes a reduction in dopamine release to occur specifically in dorsomedial striatum. Such a reduction should be expected to replicate the effects observed here for several reasons: First, in other behavioral paradigms both context-mediated changes in behavior and in c-Fos expression are blocked by D1 antagonist infusion into the striatum suggesting that the ability of context to drive striatal-dependent behavior is D1 receptor dependent (Hamlin et al., 2007; Bossert et al., 2009). Similarly, in drug-induced sensitization, the psychomotor enhancement that results from repeated, intermittent exposure to low doses of psychostimulants is determined by the context where the drug is administered (Robinson et al., 1998) acting as an occasion-setter to gate the expression of the sensitized neural substrates (Anagnostaras et al., 2002). These neural substrates include the glutamatergic and dopaminergic systems of the cortex and striatum (Bell & Kalivas, 1996; Robinson & Badiani, 1998; Robinson et al., 1998; Corbit et al., 2014) and alterations in these could produce the deficits in the current study, given the similarities in the drug-pairing procedures. However, given that dopamine release enhances striatonigral and diminishes striatopallidal output from dorsal striatum (Gerfen & Surmeier, 2011), we favor an account based on a context-induced reduction in dopamine release. This should be anticipated to result in reduced D1 and D2 receptor activity and so reduced striatonigral and enhanced striatopallidal output.
Although speculative, these claims are consistent with our finding of reduced c-Fos–related activity in non-enkephalin expressing neurons in the DMS which, given the relative scarcity of enkephalin-negative neurons that are not D1 neurons (Steiner & Gerfen, 1998; Gerfen & Surmeier, 2011), implicated reduced activity in D1-expressing MSNs as the most likely source of the effect of exposure to the drug-paired context. Based on this we developed the further hypothesis that, if the loss of goal-directed control directly reflected the loss of D1-relative to D2-related activity, then reducing the latter might be expected to help recover function controlled by the former. To investigate this idea, we antagonized A2A receptors which are localized to D2-MSNs, a treatment known to reduce D2-MSN activity (Svenningsson et al., 1999). When the A2A antagonist ZM241385 was administered to the pDMS prior to the outcome devaluation and negative feedback tests in both the saline and METH-paired contexts we found that, although it had no effect on insensitivity to goal-directed action control in extinction, it fully restored sensitivity to negative feedback in these rats, allowing them to suppress the performance of an action associated with the delivery of a devalued outcome in the METH-paired context. We hypothesize, therefore, that treatment with ZM241385 acts to rebalance activity in the D1 and D2 pathways allowing restoration of behavioral control. This hypothesis is illustrated in Figure 4.
The fact that the A2A antagonist ZM241385 restored the rats’ ability to inhibit maladaptive habits in a drug-paired context suggests that such drugs may have therapeutic benefits and, indeed, A2A antagonists constitute a novel drug target in a range of psychiatric conditions associated with basal ganglia dysfunction, particularly Parkinson’s disease (Hickey & Stacy, 2012; Kulisevsky & Poyurovsky, 2012; Poewe et al., 2012) but also other disorders (Lara, 2010). As such, A2A antagonists may appear to be a promising treatment option for the effects of drug exposure (Soria et al., 2006; Castane et al., 2008; Yu et al., 2009). The caveat based on the current experiment is that, whereas antagonizing A2A receptors in the pDMS restored sensitivity to negative feedback, it failed to restore goal-directed control in the extinction component of the test. It is possible, however, that restoring the cognitive control may be more challenging in the absence of direct feedback; that, whereas treating the striatum is effective in altering sensitivity to negative feedback, changes in efferent structures, such as prefrontal cortex or the basolateral amygdala, may render performance in extinction less sensitive to treatment focused only on striatum and further research will be required to explore this possibility.
Supplementary Material
Acknowledgments
The research reported in the manuscript was supported by grants to BWB from the National Institutes of Health: NIAAA #AA018014, and the Australian Research Council, ARC #FL0992409, and to BWB and SK from the National Health and Medical Research Council of Australia, NHMRC #633268.
Footnotes
Author contributions.
BB and SK were responsible for the study concept and design. TF and ASAS contributed to the acquisition of the animal data. TF, LC, SK & BB assisted with data analysis and interpretation of findings. TF drafted the manuscript. LC, SK and BB provided critical revision of the manuscript and important intellectual content. All authors critically reviewed content and approved the final version for publication.
References
- Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26:703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacology (Berl) 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castane A, Wells L, Soria G, Hourani S, Ledent C, Kitchen I, Opacka-Juffry J, Maldonado R, Valverde O. Behavioural and biochemical responses to morphine associated with its motivational properties are altered in adenosine A(2A) receptor knockout mice. Br J Pharmacol. 2008;155:757–766. doi: 10.1038/bjp.2008.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-acetylcysteine. Neuropsychopharmacology. 2014;39:1893–1901. doi: 10.1038/npp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons. Neurotoxicity research. 2008;14:307–315. doi: 10.1007/BF03033855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hickey P, Stacy M. Adenosine A2A antagonists in Parkinson’s disease: what’s next? Curr Neurol Neurosci Rep. 2012;12:376–385. doi: 10.1007/s11910-012-0279-2. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kenneth Lloyd G, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nature reviews Drug discovery. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulisevsky J, Poyurovsky M. Adenosine A2A-receptor antagonism and pathophysiology of Parkinson’s disease and drug-induced movement disorders. European neurology. 2012;67:4–11. doi: 10.1159/000331768. [DOI] [PubMed] [Google Scholar]
- Lara DR. Caffeine, mental health, and psychiatric disorders. Journal of Alzheimer’s disease: JAD. 2010;20(Suppl 1):S239–248. doi: 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- Lingawi NW, Balleine BW. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J Neurosci. 2012;32:1073–1081. doi: 10.1523/JNEUROSCI.4806-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2014 doi: 10.1016/j.brainres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A, Adams C, Ruby CL, Choi DS. Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J Neurosci. 2013;33:4329–4338. doi: 10.1523/JNEUROSCI.3094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. On habits and addiction: An associative analysis of compulsive drug seeking. Drug discovery today. Disease models. 2008;5:235–245. doi: 10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT, Balleine BW. Alcohol-Paired Contextual Cues Produce an Immediate and Selective Loss of Goal-directed Action in Rats. Front Integr Neurosci. 2010:4. doi: 10.3389/fnint.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Academic Press; San Diego: 2007. [Google Scholar]
- Poewe W, Mahlknecht P, Jankovic J. Emerging therapies for Parkinson’s disease. Curr Opin Neurol. 2012;25:448–459. doi: 10.1097/WCO.0b013e3283542fde. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Badiani A. Drug-induced adaptations in catecholamine systems: on the inevitability of sensitization. Adv Pharmacol. 1998;42:987–990. doi: 10.1016/s1054-3589(08)60912-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Brown RA, Balleine BW. Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:2951–2959. doi: 10.1523/JNEUROSCI.1778-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2006;31:978–987. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr, Bryson EJ, Cadetti L, Thoreson WB. Endogenous adenosine reduces glutamatergic output from rods through activation of A2-like adenosine receptors. J Neurophysiol. 2003;90:165–174. doi: 10.1152/jn.00671.2002. [DOI] [PubMed] [Google Scholar]
- Surmeier D, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends in Neurosciences. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Tscherter A, Belcastro V, Tantucci M, Costa C, Picconi B, Centonze D, Calabresi P, Borsini F. Interaction of A2A adenosine and D2 dopamine receptors modulates corticostriatal glutamatergic transmission. Neuropharmacology. 2007;53:783–789. doi: 10.1016/j.neuropharm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur J Neurosci. 2005a;22:505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005b;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yu C, Gupta J, Chen JF, Yin HH. Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci. 2009;29:15100–15103. doi: 10.1523/JNEUROSCI.4215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.