Abstract
Purpose
Ultrasonic propulsion is a new technology using focused ultrasound energy applied transcutaneously to reposition kidney stones. We report the findings from the first human investigational trial of ultrasonic propulsion toward the applications of expelling small stones and dislodging large obstructing stones.
Materials and Methods
Subjects underwent ultrasonic propulsion either awake without sedation in clinic or during ureteroscopy while anesthetized. Ultrasound imaging and a pain questionnaire were completed before, during, and after propulsion.
The primary outcome was to reposition stones in the collecting system. Secondary outcomes included safety, controllable movement of stones, and movement of stones < 5 mm and ≥ 5 mm. Adverse events were assessed weekly for 3 weeks.
Results
Kidney stones were repositioned in 14 of 15 subjects. Of the 43 targets, 28 (65%) showed some level of movement while 13 (30%) were displaced > 3 mm to a new location. Discomfort during the procedure was rare, mild, brief, and self-limited. Stones were moved in a controlled direction with over 30 fragments being passed by 4 of 6 subjects who previously had a lithotripsy procedure. The largest stone moved was 10 mm. One patient experienced pain relief during treatment of a large stone at the UPJ. In 4 subjects a seemingly large stone was determined to be a cluster of small passable stones once moved.
Conclusions
Ultrasonic propulsion was able to successfully reposition stones and facilitate passage of fragments in humans with no adverse events associated with the investigational procedure.
Keywords: kidney calculi, lithotripsy, nephrolithiasis, residual fragment, ultrasound, ultrasonic propulsion
INTRODUCTION
The prevalence of kidney stones continues to increase and is estimated to affect nearly 9% of the US population.1 The unpredictability of stone movement and resultant pain cause anticipatory fear in many individuals with kidney stones, and it is not uncommon for a single stone episode to result in multiple emergency room visits and at least one surgical procedure.2 The annual medical expenditures of urinary stone disease has soared to $10 billion making it one of the most costly urologic conditions.3 While surgical treatment of kidney stones has evolved from large-incision surgery to noninvasive procedures, the current treatment options commonly leave behind residual stone fragments.4 Studies have shown that while most residual fragments will pass, others may grow, and in approximately 20%-40% of patients, lead to symptomatic events such as pain, emergency room visits, or additional procedures.5-9
Ultrasonic propulsion is a new technology developed to reposition kidney stones and facilitate passage using focused ultrasound energy applied transcutaneously.10-14 The proposed use is to expel stone fragments while they are small and passable. Other uses include moving larger stones back into the kidney to relieve acute renal obstruction and pain; and help small newly formed, or de novo, stones pass naturally under controlled conditions, rather than waiting for an unpredictable event or until stone growth requires surgery.
The ultrasonic propulsion technology has evolved over 5 years to a clinical prototype device.10-14 Safety and effectiveness have been demonstrated in a porcine model, and an Investigational Device Exemption (IDE) was obtained from the US Food and Drug Administration (FDA) to test the device in humans.15,16 Herein we report the findings of the first-in-human clinical trial of ultrasonic propulsion.
MATERIALS AND METHODS
A single-center, first in human, feasibility study was conducted at the University of Washington (UW) with approval from the UW Institutional Review Board and the US FDA through an IDE.
Study Objectives
The primary goal was to demonstrate the ability to reposition stones within the human collecting system. Secondary goals included to: (a) demonstrate the ability to move stones in a controlled direction, (b) demonstrate the ability to move both small (< 5mm) and larger (≥ 5mm) stones, and (c) determine any safety issues or discomfort associated with the investigational procedure. We further investigated the potential impact of stone size, stone location, patient position, treatment voltage, and stone type (de novo vs. fragment) on stone motion.
Investigational Device
The investigational system is essentially a diagnostic ultrasound platform capable of emitting longer-duration, slightly higher-amplitude, focused pulses (VDAS, Verasonics Inc, Redmond, WA). A graphical user interface and ultrasound image is displayed on a touchscreen monitor.
The custom-derived Push sequence was developed and optimized to work on the same diagnostic probe (HDI C5-2 curvilinear array, Philips Ultrasound, Andover, MA) as is used to image the kidney. The operator activates a single Push either by touching the stone on the touchscreen or clicking the mouse with the cursor on the stone. The Push sequence occurs between 2 B-mode imaging frames, giving real-time imaging feedback of stone motion. The Push can be applied to any location within the image. Two Push voltage settings are available: low (50V) and high (90V). Low power is used at shallow depths where there is less acoustic attenuation, and high power is employed at greater depths (≥ 7cm).
Study Population
Individuals presenting to a single provider's UW Urology clinic from November 2013 until October 2014 were screened. Inclusion and exclusion criteria are listed in Table 1.
Table 1.
Protocol inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • Patient of the UW urology clinic | • Under 18 years of age |
| • At least one kidney stone or fragment | • Nonechogenic stones (by ultrasound) |
| • Stone reported on clinic visit and observed on kidney-ureter-bladder x-ray, computed tomography, or ultrasound | • Coagulation abnormality or taking blood thinners at clinically significant levels |
| • Stone confirmed on screening visit with investigational ultrasound device | • Mobility issues that prevented the subject from lying on the bed for extended periods or rolling between their abdomen, side, and back |
| • Individuals belonging to a vulnerable group (e.g. currently pregnant, prisoner, disabled) |
UW, University of Washington
Because the ultimate application of this technology has a broad scope of use, restrictions on enrollment criteria were minimized. Group 1 (postlithotripsy) included subjects that had undergone a lithotripsy procedure within the last year with small (< 5mm) residual fragments. Group 2 included subjects with small (< 5mm) de novo stones. Group 3 included subjects with large (≥ 5mm) stones who would undergo a planned ureteroscopy the same day following ultrasonic propulsion. Group 4 included subjects with large (≥ 5mm) stones who would undergo simultaneous ultrasonic propulsion with ureteroscopy.
Study Procedure
Subjects underwent a screening ultrasound with the investigational device. Subjects then underwent the stone-pushing procedure by a sonographer and urologist. Raw ultrasound data and video was recorded. Subject position, stone location and motion were recorded. Stone motion was classified into 3 types for each Push: grade 1 reflected no motion, grade 2 indicated that the stone moved within a confined space, such as a calyx, or rolled back to the same position, and grade 3 meant that the stone translated to a new location (> 3mm). A maximum of 40 Pushes was delivered. Subjects were asked to move to different positions and to briefly hold their breath to help with targeting.
Safety Assessment
Subjects completed a 10-point pain questionnaire before and after the study. Direct feedback on any sensations was obtained after the first 3 Pushes and as noted thereafter. Research staff contacted the subjects weekly for 3 weeks and reviewed their medical charts over 90 days to assess for renal colic events, stone passage, or the need for additional intervention. An ultrasound was done after 4 weeks to rule out hydronephrosis or renal abnormalities.
Exceptions to Study Procedure
Subjects undergoing the investigational study during their ureteroscopic procedure were anesthetized and could not complete the pain questionnaire or provide feedback on any sensation felt during the procedure.
RESULTS
Subject Characteristics
15 subjects underwent ultrasonic propulsion, either awake without sedation (n=13) or anesthetized for simultaneous ureteroscopy (n=2) (Figure 1). Subject characteristics and baseline information are in Table 2. In total, 43 stone targets were visualized with ultrasound and targeted for stone pushing including: 24 lower pole, 10 interpolar, 5 upper pole, and 4 renal pelvis targets.
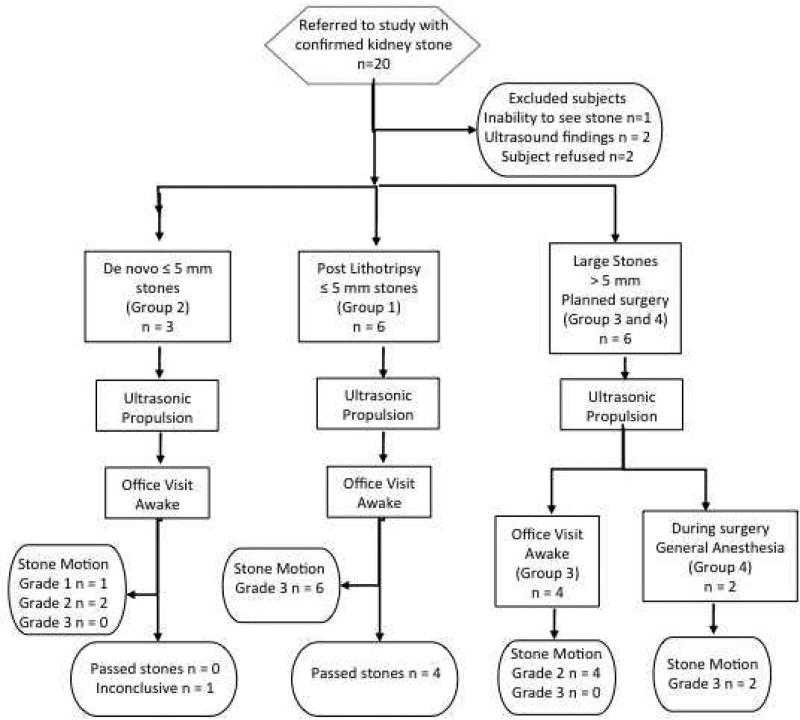
Figure 1.
Study design for ultrasonic propulsion clinical trial. Group 1 = postlithotripsy fragments; Group 2 = de novo < 5 mm stones, Group 3 = de novo > 5 mm treated before surgery, and Group 4 = de novo stones treated during ureteroscopy. Grade 1 = no movement; Grade 2 = movement < 3 mm; Grade 3 = movement > 3 mm or to new location.
Table 2.
Subject characteristics and baseline assessments of kidney stones (N = 15 subjects; n=16 renal units)
| VARIABLE | RESULT |
|---|---|
| Age, mean (SD), years | 56 (11) |
| BMI, mean (SD), kg/m2 | 29 (3) |
| Male, No. (%) | 11 (73) |
| Skin-to-stone distance, mean (SD) | |
| By CT, cm | 10.2 (2.0) |
| By ultrasound, cm | 7.1 (1.6) |
| Stone Size, range, mm | 1-14 |
| Stone location (side of kidney) | |
| Right | 10 |
| Left | 6 |
BMI, body mass index; CT, computed tomography; SD, standard deviation.
Stone Motion
Figure 2 shows Push motion results for postlithotripsy subjects differentiated by target location and size, where fragments that moved are depicted in green. Figure 3 shows Push motion results for the de novo subjects. Table 3 highlights the results for each subject, grouped by those with postlithotripsy fragments and those with de novo stones, and Table 4 summarizes results by management category.
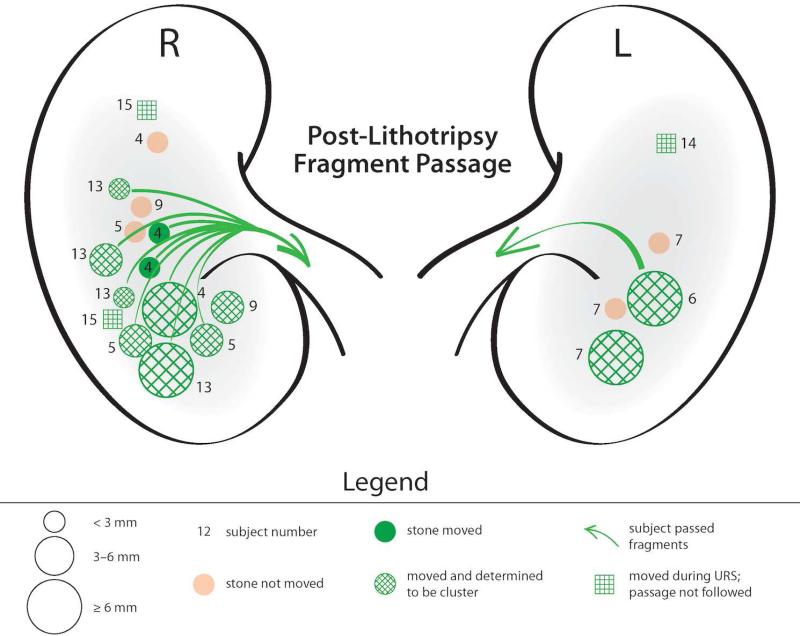
Figure 2.
Push motion results for subjects with postlithotripsy fragments. The number next to each target indicates the subject number. Different sized circles represent stone target sizes. Green represents stone movement and the green arrow highlights subjects that reported passing stones. The hash mark corresponds to a target that was identified as a single large stone on imaging but determined to be a cluster of small stones with ultrasonic propulsion.
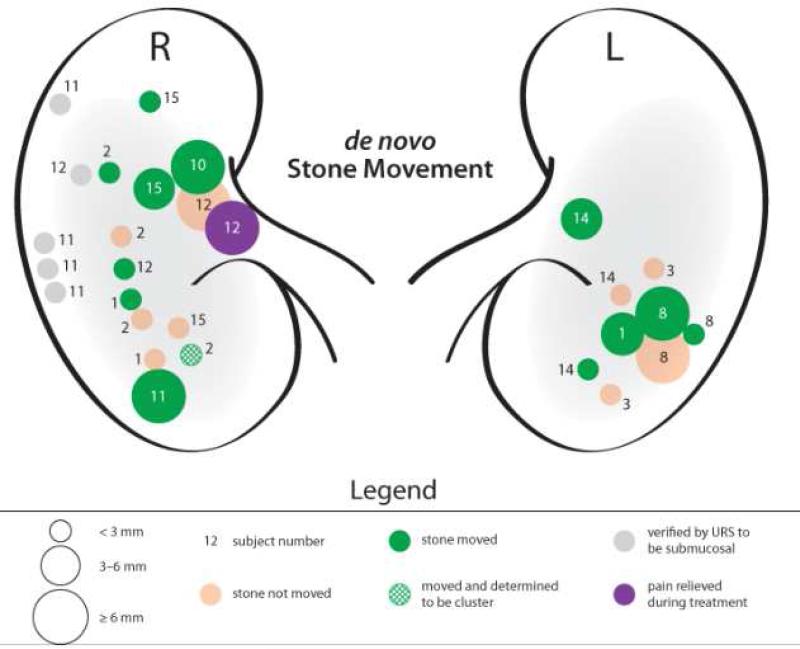
Figure 3.
Push motion results for subjects with small and large de novo stones. The number next to each target indicates the subject number. Different sized circles represent stone target sizes. Green represents stone movement. The purple stone highlights a subject that experienced a decrease in pain with movement of a renal pelvis stone. Only stones < 5 mm were monitored for passage (subjects #1-3), of which there were none reported. Gray indicates stones treated with the investigational device that were reported as submucosal based on ureteroscopy.
Table 3.
Stone motion results for each subject, organized into a postlithotripsy subject group and de novo subject group
| Subject No. | GroupA No. | Side | No. of TargetsB | Size(s), mm | Grade of Stone MovementC | Mean SSD, cm | Stone(s) Passed | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | US | CT | ||||||
| Postlithotripsy | ||||||||||
| 4 | 1 | R | 4 | ≤ 3 | 5 | 26 | 5 | 6.8 | 9.1 | Y |
| 5 | 1 | R | 3 | ≤ 2 | 17 | 14 | 8 | 4.9 | 7.3 | Y |
| 6 | 1 | L | 1 | ≤ 2 | 21 | 16 | 3 | 5.0 | 8.3 | Y |
| 7 | 1 | L | 3 | ≤ 2 | 12 | 15 | 13 | 5.4 | 8.3 | N |
| 9 | 1 | R | 2 | ≤ 2 | 15 | 20 | 5 | 5.5 | 10.1 | N |
| 13 | 1 | R | 3 | ≤ 3 | 10 | 19 | 11 | 8.5 | 13.3 | Y |
| Total | 16 | 77 (33%) | 113 (48%) | 45 (19%) | 4/6 | |||||
| De novo | ||||||||||
| 1 | 2 | L, R | 3 | 2-5 | 19 | 6 | 0 | 8.7 | N/A | N |
| 2 | 2 | R | 5 | 1-3 | 18 | 20 | 0 | 5.7 | 10.3 | N |
| 3 | 2 | L | 2 | 3 | 22 | 0 | 0 | 7.1 | 10.3 | N |
| 8 | 3 | L | 2 | 7, 8 | 22 | 5 | 0 | 6.1 | N/A | N/A |
| 10 | 3 | R | 1 | 7 | 10 | 6 | 0 | 7.6 | 8.7 | N/A |
| 11 | 3 | R | 3 | 1-3,10 | 24 | 6 | 0 | 7.0 | 9.4 | N/A |
| 12 | 3 | R | 3 | 2,10,14 | 24 | 3 | 0 | 8.8 | 12.8 | N/A |
| 14 | 4 | L | 4 | 1-2, 12 | 15 | 13 | 4 | 9.0 | 14.0 | N/A |
| 15 | 4 | R | 4 | 1-2, 8 | 11 | 7 | 4 | 9.9 | 10.9 | N/A |
| Total | 27 | 165 (69%) | 66 (28%) | 8 (3%) | 0/3 | |||||
| Summary | ||||||||||
| 15D |
10 R
6 L |
--- | 43 | --- | 242 (51%) | 179 (38%) | 53 (11%) | 7.1 | 10.2 | |
Group 1 = postlithotripsy fragments; Group 2 = de novo < 5-mm stones, Group 3 = de novo > 5 mm treated before surgery, and Group 4 = de novo stones treated during ureteroscopy.
Target for postlithotripsy subjects were clusters of stones; target for de novo were individual stones.
Grade 1 = no movement; Grade 2 = movement < 3 mm; Grade 3 = movement > 3 mm or to new location.
Total number of subjects on study. CT, computed tomography; L, left; NA, not applicable; R, right; SSD, skin-to-stone difference.
Table 4.
Summary of stone motion based on subject management groupA
| Postlithotripsy (Group 1) | De Novo (Group 2) | Pre-URS (Group 3) | Peri-URS (Group 4) | |
|---|---|---|---|---|
| Subjects, n | 6 | 3 | 4 | 2 |
| Stones, range, n | 5 to many | 2–5 | 1–3 | 3 |
| Stone size, mm | ≤ 3 | 1–5 | 1–2, 7–14 | 1–2, 8–12 |
| Pushes, mean (range) | 39 (37–40) | 30 (27–40) | 23 (17–32) | 28 (22–34) |
| % of Push w/ motionB | ||||
| Grade 1 | 35 | 75 | 81 | 58 |
| Grade 2 | 47 | 25 | 19 | 30 |
| Grade 3 | 18 | 0 | 0 | 12 |
| Stones Passed, n | 4 of 6 | 0 of 3 | N/A | N/A |
Group 1 = postlithotripsy fragments; Group 2 = de novo < 5 mm stones, Group 3 = de novo > 5 mm treated before surgery, and Group 4 = de novo stones treated during ureteroscopy.
Grade 1 = no movement; grade 2 = movement < 3 mm; grade 3 = movement > 3 mm or new location.
N/A, not applicable; URS, ureteroscopy.
Of the 43 targets, some level of motion was seen in 28 (65%), while 13 (30%) were displaced to a new location > 3mm (grade 3). Push motion results were consistent across stone locations and subject position, the most common being the lateral decubitus. Table 5 displays the number of Pushes and the resultant stone-motion grade based on target location (a), patient position (b), and output voltage (c).
Repositioning Small Stones
Grade 3 stone motion was achieved with postlithotripsy fragments (Group 1) and small de novo stones known to be loose (Group 4). Grade 2 motion was achieved in 2 of the 3 small de novo observational subjects (Group 2); no grade 3 motion occurred in these subjects.
Repositioning Large Stones
Stones > 5mm could be moved slightly (grade 2), but not displaced to a new location. The largest stone observed to move was 10mm. In the 2 subjects who underwent ultrasonic propulsion during ureteroscopy (Group 4), large stones in the renal pelvis (8mm and 12mm) were targeted but initially not moved. These stones were partially dusted with a laser until movement was achieved, which was estimated at 6mm. Skin-to-transducer distances were the greatest in these subjects (Table 3).
Clearance of Small Stones
Stone clearance was monitored in postlithotripsy subjects (Group 1) and in subjects with small de novo stones under observation (Group 2). Four of six postlithotripsy subjects passed more than 30 stone fragments within days following the procedure (Figure 2). One subject passed 2 small stones immediately afterwards (Figure 4). For the remaining two subjects, one was 10 months postlithotripsy and had new stone formation. The other was noted to have a long infundibulum in which a stone was pushed part way through. This subject felt discomfort consistent with passing a small stone but did not see a stone pass, and follow-up imaging proved inconclusive.
Figure 4.
Stones passed by subject #4 (Group 1). (a) Shows the 2 stones that the subject passed in clinic immediately after the investigational procedure. (b) Shows an additional 14 stone fragments passed over the next couple days after the investigational procedure.
No subjects in the small de novo Group 2 (n=3) reported passing a stone, although one subject was lost to follow-up and post procedure imaging in a second subject reported no stones.
For presurgical (Group 3) and perisurgical (Group 4) subjects, stone clearance was not applicable. However, subject #8 had a 3 mm stone in the proximal ureter at the time of ureteroscopy that may have been displaced from the lower pole during ultrasonic propulsion. Subject #11 had a 1 mm stone in the bladder at the time of surgery that also may have been displaced during ultrasonic propulsion. Although these two findings cannot be proven to be the result of ultrasonic propulsion, neither subject had a ureteral stone on preoperative imaging, or development of pain in the interim prior to their procedure, and neither had hydronephrosis or evidence of a ureteral stone at the time of ultrasonic propulsion.
Diagnostic Information
In at least 4 subjects in Group 1 (#6, 7, 9, 13), the clinical diagnostic report noted a single stone between 4-17mm, but ultrasonic propulsion revealed the single target was in fact a cluster of fragments small enough to pass. Figure 5 shows an example of the dispersion of a cluster of small stones.
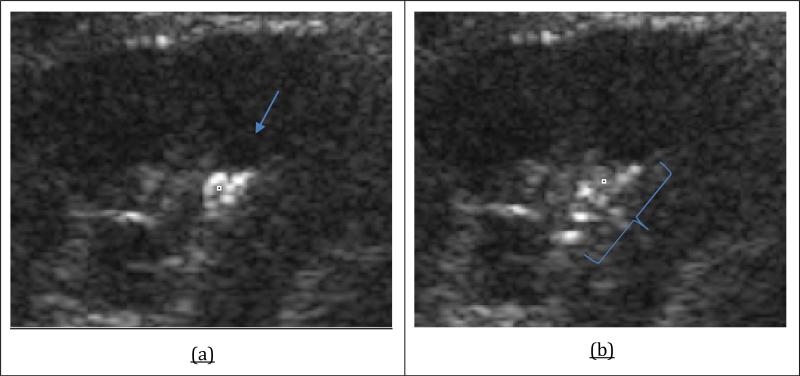
Figure 5.
Ultrasound images of the potential diagnostic role of ultrasonic propulsion. (a) A single large stone or possibly 2 medium-sized stones. Arrow indicates the direction of the Push. (b) Pushing of the stone revealed 6 or more small stone fragments.
Safety
No sensations were reported with the 50V Push. All subjects reported a warming of the skin from the transducer face with the 90V Push. Two subjects reported an internal sensation with 1-3 of the 90V Pushes. The sensation was brief and not considered painful. Changing the probe angle eliminated the sensation. Two subjects reported an increase in baseline pain from the beginning to the end of the study, which they attributed to lying on the research table.
No unanticipated or serious adverse events were reported. No device-related adverse events were reported. No subject required medical intervention associated with ultrasonic propulsion.
DISCUSSION
This study reports several findings of the first-in-human clinical trial for ultrasonic propulsion of kidney stones; first, ultrasonic propulsion facilitated the passage of stone fragments. Residual fragments often result in unplanned stone events such as pain, emergency room visits, or the need for an additional procedure.5-9 Unplanned visits were recently reported in 1 of 7 patients with average costs of $23,000-$32,000 after lithotripsy.4 By facilitating the movement of these stone fragments, ultrasonic propulsion might decrease the need for future and costly unplanned care.
Second, ultrasonic propulsion can be performed in a clinic setting without sedation and without adverse events related to the procedure. Stone motion was achieved at both low power (50V) and high power (90V). Although the percentage of grade 2 and grade 3 stone motion was higher with 50V Pushes, larger stones, which proved more difficult to move, often lay at a greater distance from the transducer in the renal pelvis, and therefore received predominantly 90V Push attempts.
Third, some degree of movement was seen in both small and large stones. The ability to confirm both the presence of a stone within the collecting space and its attachment status with ureteroscopy enhanced the accuracy of this study. For the first 3 de novo subjects (Group 2), no direct visualization of the kidney was performed as opposed to the presurgical (Group 3) and perisurgical groups (Group 4), and thus we were unable to confirm if targets that did not move were indeed true targets, submucosal, or stones attached to the urothelium. In subjects that underwent a ureteroscopic procedure following the investigational study, we were able to confirm that stones unmoved were submucosal or attached.
Whether ultrasonic propulsion can dislodge a stone that is attached (e.g., to a Randall's plaque) remains unclear. Because many stones develop in this manner17, they spontaneously come loose at some point. Theoretically, loosely attached stones would be more likely to dislodge than firmly attached stones. One subject from Group 3, had two 7-8 mm stones that were targeted—one that moved with ultrasonic propulsion and one that did not. On ureteroscopy, a firmly attached stone and a separate stone in a calyx, that appeared to have been detached from a Randall's plaque, were detected. Currently, we have no way of knowing a priori which stones might or might not be attached and to what strength they are adherent.
Although we do not know the limit for stone movement based on size, with the current system we did achieve grade 2 motion in stones 7-10mm. The ability to move larger stones will depend on the location and depth. In this study, our goal of moving stones that were too large to pass was to investigate the potential application of moving an obstructing stone in the UPJ back into the kidney to relieve pain and obstruction. This could avoid an urgent temporizing procedure such as a ureteral stent or nephrostomy tube. In one subject, we were able to rotate an approximately 10mm stone within the renal pelvis. Although the movement was slight, this subject reported a decrease in pain. Even a small movement of a stone at the UPJ could provide relief for an individual or enable a stone to roll to a new location with change in body position.
Lastly, an unexpected finding of ultrasonic propulsion was its diagnostic capability. In at least 4 subjects, what appeared as a larger stone on imaging was shown to be a cluster of small passable stones (Figure 5). This is particularly important, as stone size is a major factor in management of urolithiasis.18,19 Most stones < 5 mm will pass spontaneously, while stones ≥ 5 mm often require intervention.20,21 This result could have utility in determining indications for a primary or secondary lithotripsy procedure and may also help determine the endpoint of shockwave lithotripsy.
We acknowledge several limitations to this trial and barriers of this technology. It is unclear why certain stones do not move. Stone composition is not believed to be a significant factor based on in vitro studies and in this trial movement of calcium oxalate (monohydrate and dihydrate) as well as calcium phosphate (apatite and brushite) stones was achieved. One of the most important aspects of ultrasonic propulsion centers on finding the best alignment of the Push with, for example, the outflow (infundibulum) of a calyx. Gravity is also important, as is depth, and if there is not enough force to push a stone out of a calyx, it may fall back to the original location. The position of the patient can facilitate displacement of stones via gravity depending on the location of the stone and the renal anatomy. Other anatomical barriers include long, narrow, and steep infundibulopelvic angles, as these have also been described to decrease the passage of fragments after lithotripsy alone.22 A dilated collecting system is speculated to be beneficial since there is naturally more space for movement. Despite the barriers, it is believed that further and enhanced applications are possible with ongoing optimization of this technology.
CONCLUSION
This first-in-human feasibility study of ultrasonic propulsion was a success in accomplishing its primary and secondary goals. Further, in its current form, ultrasonic propulsion has a role in facilitating the passage of small kidney stones and can distinguish a cluster of small fragments from a larger stone.
Table 5a.
Motion grade results based on target location
| Push Results by GradeA, n (%) | |||
|---|---|---|---|
| Target location | 1 | 2 | 3 |
| Lower pole | 162 (46) | 147 (42) | 42 (12) |
| Mid pole | 40 (71) | 9 (16) | 7 (13) |
| Upper pole | 15 (52) | 10 (34) | 4 (14) |
| Renal pelvis | 41 (76) | 13 (24) | 0 (0) |
Grade 1 = no movement; grade 2 = movement < 3 mm; grade 3 = movement ≥ 3 mm or new location.
Table 5b.
Motion grade results based on subject position
| Push Results by GradeA, n (%) | |||
|---|---|---|---|
| Position | 1 | 2 | 3 |
| Prone | 44 (60) | 24 (32) | 6 (8) |
| Side | 137 (56) | 87 (35) | 23 (9) |
| Supine | 42 (42) | 46 (46) | 12 (12) |
Grade 1 = no movement; Grade 2 = movement < 3 mm; Grade 3 = movement ≥ 3 mm or new location.
Table 5c.
Motion grade results based system output voltage
| Push Results by GradeA, n (%) | |||
|---|---|---|---|
| Voltage, V | 1 | 2 | 3 |
| 50 | 135 (47) | 115 (40) | 35 (12) |
| 90 | 117 (61) | 56 (29) | 18 (9) |
Grade 1 = no movement; Grade 2 = movement < 3 mm; Grade 3 = movement ≥ 3 mm or new location.
ACKNOWLEDGMENTS
Taking a medical therapy device from concept to a successful clinical trial involves too many individuals and groups to thank them all. We appreciate the help of our colleagues at the UW Center for Industrial and Medical Ultrasound, UW Urology Department, the Consortium for Shock Waves in Medicine, and all those who helped us develop and test the system. This trial was supported by the NSBRI through NASA NCC 9-58. Research and development were supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK043881 and DK092197) and from the NSBRI. Additional funding was provided by the UW Applied Physics Laboratory, UW Department of Urology, CoMotion at the UW, The Wallace H. Coulter Foundation, and the UW Institute of Translational Health Sciences. We also would like to thank sonographer Marla Paun R.D.M.S., R.V.T.; study coordinators Alana Clark, Susan Ross R.N., and Jane Edelson; and finally, Michael Coburn, MD, FACS (Baylor College of Medicine) for overseeing the trial.
Glossary
- IDE
Investigational Device Exemption
- FDA
US Food and Drug Administration
- UW
University of Washington
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scales CD, Jr, Lin L, Saigal CS, et al. Emergency department revisits for patients with kidney stones in california. Acad Emerg Med. 2015;22(4):468. doi: 10.1111/acem.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litwin MS, Saigal CS. Urologic Diseases in America. National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, US Dept of Health and Human Services; Washington, DC: 2012. Table 14-47: economic impact of urologic disease. p. 486. NIH publication 12-7865. [Google Scholar]
- 4.Scales CD, Jr, Lai JC, Dick AW, et al. Comparative effectiveness of shock wave lithotripsy and ureteroscopy for treating patients with kidney stones. JAMA Surg. 2014;149(7):648. doi: 10.1001/jamasurg.2014.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raman JD, Bagrodia A, Gupta A, et al. Natural history of residual fragments following percutaneous nephrostolithotomy. J Urol. 2009;181(3):1163. doi: 10.1016/j.juro.2008.10.162. [DOI] [PubMed] [Google Scholar]
- 6.Rebuck DA, Macejko A, Bhalani V, et al. The natural history of renal stone fragments following ureteroscopy. Urology. 2011;77(3):564. doi: 10.1016/j.urology.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 7.Osman MM, Alfano Y, Kamp S, et al. 5-year-follow-up of patients with clinically insignificant residual fragments after extracorporeal shockwave lithotripsy. Eur Urol. 2005;47(6):860. doi: 10.1016/j.eururo.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Glowacki LS, Beecroft ML, Cook RJ, et al. The natural history of asymptomatic urolithiasis. J Urol. 1992;147(2):319. doi: 10.1016/s0022-5347(17)37225-7. [DOI] [PubMed] [Google Scholar]
- 9.Streem SB, Yost A, Mascha E. Clinical implications of clinically insignificant store fragments after extracorporeal shock wave lithotripsy. J Urol. 1996;155(4):1186. [PubMed] [Google Scholar]
- 10.Harper JD, Dunmire B, Wang YN, et al. Preclinical safety and effectiveness studies of ultrasonic propulsion of kidney stones. Urology. 2014;84(2):484. doi: 10.1016/j.urology.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorensen MD, Bailey MR, Hsi RS, et al. Focused ultrasonic propulsion of kidney stones: review and update of preclinical technology. J Endourol. 2013;27(10):1183. doi: 10.1089/end.2013.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harper JD, Sorensen MD, Cunitz BW, et al. Focused Ultrasound to Expel Calculi from the Kidney: Safety and Efficacy of a Clinical Prototype Device. J Urol. 2013;190(3):1090. doi: 10.1016/j.juro.2013.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah A, Harper JD, Cunitz BW, et al. Focused ultrasound to expel calculi from the kidney. J Urol. 2012;187(2):739. doi: 10.1016/j.juro.2011.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah A, Owen NR, Lu W, et al. Novel ultrasound method to reposition kidney stones. Urol Res. 2010;38(6):491. doi: 10.1007/s00240-010-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YN, Simon JC, Cunitz BW, et al. Focused ultrasound to displace renal calculi: threshold for tissue injury. J Ther Ultrasound. Mar 31. 2014;2:5. doi: 10.1186/2050-5736-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connors BA, Evan AP, Blomgren PM, et al. Comparison of Tissue Injury from Focused Ultrasonic Propulsion of Kidney Stones Versus Extracorporeal Shock Wave Lithotripsy. J Urol. 2013;191(1):235. doi: 10.1016/j.juro.2013.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evan AP, Lingeman JE, Coe FL, et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111(5):607. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coe FL, Keck J, Norton ER. The natural history of calcium urolithiasis. JAMA. 1977;238(14):1519. [PubMed] [Google Scholar]
- 19.Sterrett SP, Nakada SY. Medical expulsive therapy. Curr Opin Urol. 2008;18(2):210. doi: 10.1097/MOU.0b013e3282f51935. [DOI] [PubMed] [Google Scholar]
- 20.Ueno A, Kawamura T, Ogawa A. Relation of spontaneous passage of ureteral calculi to size. Urology. 1977;10(6):544. doi: 10.1016/0090-4295(77)90097-8. [DOI] [PubMed] [Google Scholar]
- 21.Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162(3 Pt 1):688. doi: 10.1097/00005392-199909010-00014. discussion 690. [DOI] [PubMed] [Google Scholar]
- 22.Elbahnasy AM, Shalhav AL, Hoenig DM, et al. Lower caliceal stone clearance after shock wave lithotripsy or ureteroscopy: the impact of lower pole radiographic anatomy. J Urol. 1998;159(3):676. [PubMed] [Google Scholar]