Abstract
Introduction
The aims of this study were to evaluate 1) grasping forces with the application of a tactile feedback system in vivo 2) the incidence of tissue damage incurred during robotic tissue manipulation. Robotic-assisted minimally invasive surgery has been shown to be beneficial in a variety of surgical specialties, particularly radical prostatectomy. This innovative surgical tool offers advantages over traditional laparoscopic techniques, such as improved wrist-like maneuverability, stereoscopic video displays, and scaling of surgical gestures to increase precision. A widely cited disadvantage associated with robotic systems is the absence of tactile feedback.
Methods and Procedure
Nineteen subjects were categorized into two groups: 5 experts (six or more robotic cases) and 14 novices (five cases or less). The subjects used the da Vinci with integrated tactile feedback to run porcine bowel in the following conditions: (T1: deactivated tactile feedback; T2: activated tactile feedback; and T3: deactivated tactile feedback). The grasping force, incidence of tissue damage, and the correlation of grasping force and tissue damage were analyzed. Tissue damage was evaluated both grossly and histologically by a pathologist blinded to the sample.
Results
Tactile feedback resulted in significantly decreased grasping forces for both experts and novices (P < 0.001 in both conditions). The overall incidence of tissue damage was significantly decreased in all subjects (P < 0.001). A statistically significant correlation was found between grasping forces and incidence of tissue damage (P = 0.008). The decreased forces and tissue damage were retained through the third trial when the system was deactivated (P > 0.05 in all subjects).
Conclusions
The in vivo application of integrated tactile feedback in the robotic system demonstrates significantly reduced grasping forces, resulting in significantly less tissue damage. This tactile feedback system may improve surgical outcomes and broaden the use of robotic-assisted minimally invasive surgery.
Keywords: haptic feedback, tactile feedback, robotic surgical system
Introduction
Robotic surgical systems were developed in the late 1990's to overcome inherent limitations of laparoscopic surgery (1-6). While still offering video-based, minimally-invasive approaches to surgical intervention, the evolving robotic systems provide surgeons with increased degrees of freedom to better mimic natural hand and wrist gestures, stereoscopic video displays to mimic more natural visual interpretation of the surgical field, and scaling of surgical gestures to enable precise movements (7-8).
Robotic surgery has found most acceptance in radical prostatectomy where estimates of cases performed robotically range from 67% to 85% (9). Urological procedures benefit from the improved dexterity and precision afforded by the robotic instruments due to anatomical restrictions limiting exposures.
Robotic surgery has also been used for hysterectomy, gastric bypass, cholecystectomy, adrenalectomy, mitral valve repair, and coronary artery bypass, among others (10-17). Despite the advantages of robotic surgery, traditional open or laparoscopic surgical techniques continue to be preferred for these types of procedures. While this is partly due to high equipment costs, the need for large equipment in a constrained operating room, and the need for additional training, the absence of haptic feedback has been widely accepted as a significant technical disadvantage (6, 18-19). The physical connection between the Surgeon's hands and the robotic instruments are removed, and haptic sensation such as the tension of a suture, texture of tissue, and even collisions between robotic arms are physically imperceptible. The lack of haptic feedback may prolong operative times, learning curves, and ultimately increase the risk of surgical errors (20). In addition, the robot system is capable of creating varying forces that far exceed tissue tolerances, which potentially create a dangerous environment without haptic feedback (21).
Robotic minimally invasive surgery (RMIS) with the da Vinci robot has become a progressively more popular option for many types of surgery since its FDA approval in 2000. Compared to open procedures, minimally invasive surgery is generally believed to reduce tissue damage, patient discomfort, and hospital stay duration, enabling a faster recovery, reduced morbidity, reduced pain, and improved cosmesis (22, 23). However there is also data that suggests RMIS can be slower and more expensive than non-robotic surgery, leading some to believe it offers no clear, significant advantage over standard laparoscopic techniques (24). With counter studies showing haptic feedback may reduce operative times, tissue damage, and excessive force it is apparent this technology is worth studying to understand its future for routine use broadly across surgical specialties (25, 26).
A tactile feedback system was previously designed and integrated with the da Vinci Surgical System (27-29). It measures forces normal to the tips of a robotic grasper and provides proportional forces to the fingertips of the operating surgeon. Preliminary investigation showed that adding supplemental tactile feedback significantly reduced grip force during robotic surgery training and with tissue phantoms (30). When tactile feedback was integrated with a non-robotic laparoscopic instrument, it was found that tactile feedback significantly decreased the grip force of novice subjects during laparoscopic training (31).
The following study further explored the restoration of tactile perception to the operating surgeon during robotic surgical tasks on live tissue, and quantified the impact of the feedback on grip force and tissue damage.
Methods and Procedures
Tactile Feedback System for In-Vivo Environments
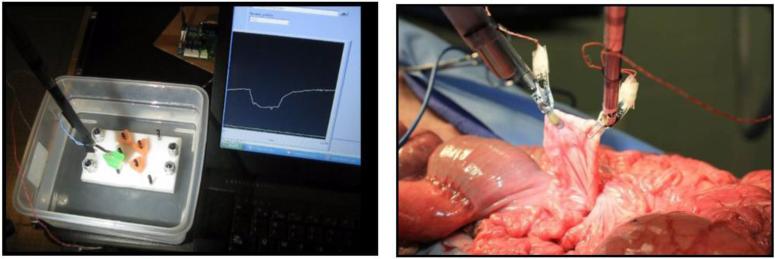
A previously designed tactile feedback system was modified for use in live tissue through the development of a moisture-resistant and biocompatible coating (Figure 1). The waterproof coating was validated through submersion tests, and grasping in both cadaveric and live tissue models (Figure 2). The effect of the seal on sensor response was characterized by applying known loads with an Instron Mechanical Loading system and evaluating the force output of the coated sensor using a LabVIEW data acquisition system (National Instruments®, Austin TX). From this experiment, the force conversion factor was determined to be 0.0056 N/ADC count (R2 > 0.98) for the coated sensor, and 0.0012 N/ADC count for the uncoated sensor (R2 > 0.99), indicating a 4.7x linear damping effect. This effect was countered by adjusting the gain of the hardware amplifier.
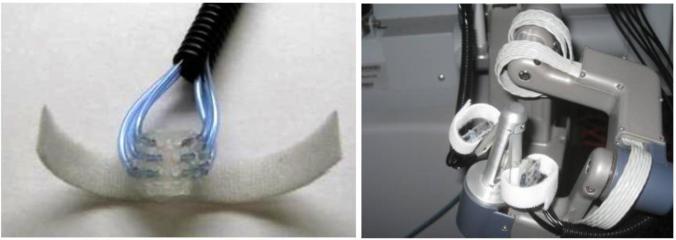
Figure 1.
Waterproof sensor mounted on da Vinci robotic instrument
Figure 2.
(Left) Sensors tested in a water tank and (Right) in a live tissue model
A control system was designed to convert forces detected at the grasper tips to pressures at the surgeon's fingertips. Pneumatic actuators provided pressure stimuli to fingertips using hemispherical silicone balloons, targeting the slow-adapting mechanoreceptors through constant deformation of the finger pad (Figure 3).
Figure 3.
(Left) Balloon actuator (Right) Actuator mounted on da Vinci controls
Sensors were calibrated by having an expert surgeon grasp a foam block. Pneumatic regulators were adjusted to provide the pressure outputs shown on table 1 as determined by surgeon feedback. These pressure outputs that created the largest impulse were from level 0 to level 1, indicating a force sufficient to hold the object.
Table 1.
Pressure Outputs for Live Tissue Experiments
| Level 0 | 0 PSI |
| Level 1 | 25 PSI |
| Level 2 | 30 PSI |
| Level 3 | 35 PSI |
| Level 4 | 40 PSI |
Software thresholds were adjusted so that the minimal force required to hold the tissue would cause a transition to the first inflation level. Integration of the tactile feedback system into the da Vinci robot was validated by grasping foam blocks using the system and observing actuator inflation in response to grasping events.
Study Design
Nineteen subjects (five robotic surgery experts, and fourteen novices) used the da Vinci surgical system with integrated tactile feedback to pass porcine bowel from one grasper to the other (“run the bowel”), until they had grasped the bowel approximately ten times with each hand. An expert robotic surgeon explained the task, and then verbally guided subjects during the procedure.
Subjects who had performed six or more robotic surgery cases were considered experts, and this included four attending surgeons and one senior urology resident. The fourteen novice subjects consisted of eleven surgical residents and three attending surgeons who had performed five or fewer robotic surgery cases.
For each subject, the bowel was run in the following three sequential conditions: (T1) tactile feedback off, (T2) tactile feedback on, and (T3) tactile feedback off. This staggered structure was designed to help establish short term learning effects using the surgical system. After each subject completed the task, an observing surgeon harvested the segment of grasped bowel. The bowel segment was photographed for gross analysis. Subjects were then asked to fill out a survey that contained questions concerning the subject's level of experience in the medical profession, experience with regard to robotic surgery, and questions concerning tactile feedback technology and the experiment.
A porcine model was used because it is a well-established and proven model for abdominal surgery. A total of four pigs were used for all studies, with 5-10 subjects per pig. The study was approved by the Animal Research Committee (ARC) under protocol number 2008-172-12A. Work with human subjects was approved by the Institutional Review Board (IRB) under protocol #11-000077.
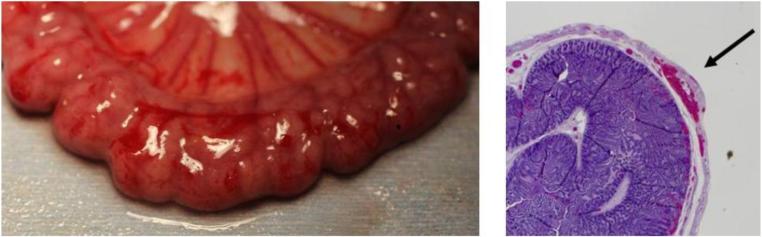
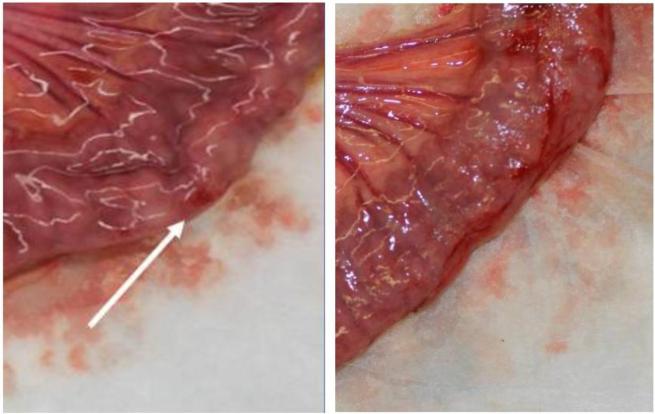
Prior to the experiments, a dry run was performed by research collaborators to provide bowel tissue for analysis. In a double blind fashion two pathologist scored grasped segments of tissue through gross pathological inspection, and again by inspecting the histology. These evaluations found that tissue damage rankings from the histological examination matched closely with those from the gross exam (Figure 4). From this, it was concluded that clinically significant damage could be evaluated from gross inspection alone. The pathologists counted the number of sites of observable damage and scored each damage site as either level 1 (light), level 2 (medium), or level 3 (heavy) (Figure 5). An example of light (L1) damage was a faint or superficial hemorrhage. An example of medium (L2) damage was a 2 – 3 mm raised hematoma or an intermediate (grade 2) lesion. An example of heavy (L3) damage was disrupted serosa or a hemorrhagic area with a 2 mm abrasion and 6 mm area of discoloration.
Figure 4.
Example of damaged bowel. (Left) Gross exam indicated hemorrhagic tissue (Right) Corresponding histology image shows focal hemorrhage in muscularis propria
Figure 5.
(Left) An example of light damage. (Right) An example of heavy damage where pathologist noted a possible disrupted serosion
Statistical Analysis
At the end of the experiment, data consisted of tissue damage scores, measured grasping forces, and answers to survey questions. Both the grasping forces data and tissue damage results showed non-Gaussian distributions. This distribution was due to a saturation of high grasping forces (4.5 N) and due to a majority of segments ranked with zero or one site of damage. For these reasons all statistical analyses were performed using non-parametric methods. Because the data for grasping force and tissue damage was non-Gaussian it is expressed as median [quartiles], rather than mean and standard deviation.
Results
Because of the multiple different analyses performed during data acquisition and interpretation, the corresponding statistical methodology is described with each set of results for the sake of clarity.
Population Analysis of Grasping Force
Friedman's Test was performed with seven repetitions for each subject, and with each subject serving as their own control. Friedman's test was run four times: once across all three measures, and then three pair-wise comparisons. Because four comparisons were made, a Bonferonni correction was used and P < 0.0125 considered for significance.
For the dominant hand of novice subjects, median force decreased from 3.5 [3.0 – 4.0] N to 2.3 [1.4 – 3.3] N when tactile feedback was activated (P = 1.56e-012). When tactile feedback was subsequently deactivated in the third condition, median force was 2.5 [1.7 – 3.6] N, which was statistically similar to when tactile feedback was active (P =0.339), and lower than the initial case (P = 3.31e-010).
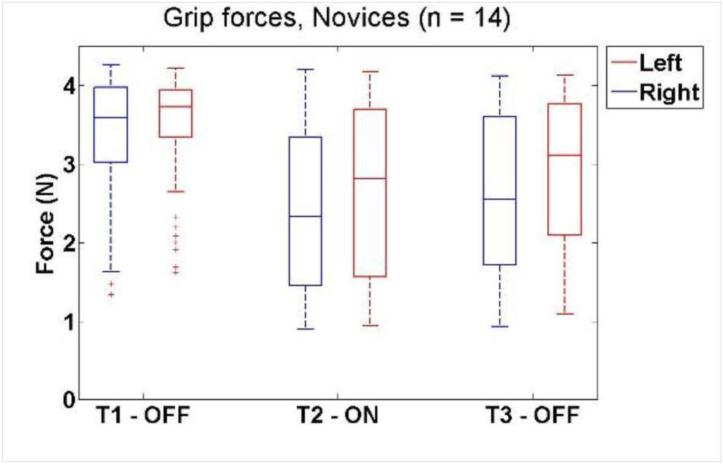
Similar results were seen for the non-dominant hand. Median force in the first condition decreased from 3.7 [3.4 – 3.9] N to 2.7 [1.6 – 3.8] N when tactile feedback was activated (P = 1.75e-008), and remained low in the third condition (3.1 [2.1 – 3.8] N) (P = 0.0877). The force in the third condition was lower than the first (P = 1.74e-007), despite tactile feedback being off in both cases. These results are shown in Figure 6. Because the saturation point of the sensors artificially decreased the upper limit, some novice forces were likely higher.
Figure 6.
Box and whisker plots of grip force versus tactile feedback condition for novice subjects. The data showed a substantial decrease between T1 and T2, but no difference between T2 and T3.
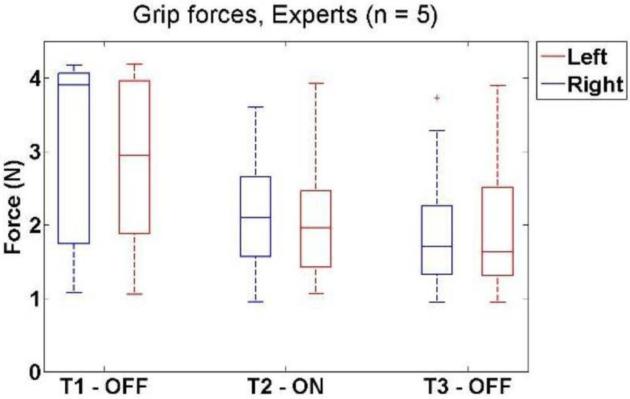
Expert subjects had a similar result, but showed a wider variability in the first condition due to less frequent saturation. For the dominant hand, median forces decreased from 3.8 [1.8 – 4.1] N to 2.2 [1.5 – 2.7] N when tactile feedback was activated (P = 4.22e-6). The force in the third condition (1.7 [1.3 – 2.2] N) was statistically similar to the second (P = 0.0814) and lower than the first (P = 0.000228).
The same was true for the non-dominant hand of experts. The median force decreased from 2.8 [1.8 – 4.0] N to 2.0 [1.5 – 2.3] N when tactile feedback was activated (P = 0.000228), and remained low in the third condition (1.7 [1.2 – 2.5] N) (P = 0.71). The third condition was statistically lower than the first (P = 0.000115). The results for experts are shown in Figure 7. Hypothesis testing for both groups is summarized in Table 2.
Figure 7.
Box and whisker plots of grip force versus tactile feedback condition for Expert subjects. The data showed a substantial decrease between T1 and T2, and no differences between T2 and T3.
Table 2.
P-Values for force data hypothesis testing using Friedman's test
| Group | Hand | P (All) | P (T1 / T2) | P (T2 / T3) | P (T1 / T3) |
|---|---|---|---|---|---|
| Experts (n = 5) | Dominant | 5.68e-006*** | 4.22e-006*** | 0.0814 | 0.000228*** |
| Non-Dominant | 6.03e-005*** | 0.000228*** | 0.71 | 0.000115*** | |
| Novices (n = 14) | Dominant | 4.18e-014*** | 1.56e-012*** | 0.339 | 3.31e-010*** |
| Non-Dominant | 8.11e-010*** | 1.75e-008*** | 0.0877 | 1.74e-007*** |
Population Analysis of Tissue Damage
Damage scores were compared using Friedman's test across all three measures with one repetition per subject. Pair-wise comparisons between conditions were made using a Wilcoxon Signed Rank Test. There was not enough statistical power to analyze the different levels of damage independently, so significance testing was performed on the total number of damage sites. Because four comparisons were made, Bonferonni's correction was used and P < 0.0125 considered for significance.
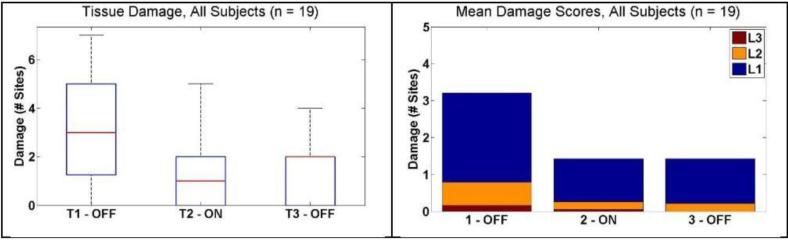
For the subject pool as a whole, there was a significant decrease in median number of sites of damage from 3 [1.3 – 5] to 1 [0 – 2] (P = 0.0018) when the tactile feedback system was activated.. When tactile feedback was deactivated in the third condition, the number of damage sites remained low (2 [0 – 2]) (P = 0.96). These results are shown in Figure 8. For comparison, mean scores for each of the damage intensity levels are displayed alongside.
Figure 8.
Tissue damage results for all subjects. (Left) Box and whisker plots show decrease in median number of damage sites from T1 to T2. (Right) Mean number of damage sites ranked by intensity, L1 – Light, L2 – Medium, and L3 – Heavy. A Majority of the sites were ranked as level 1.
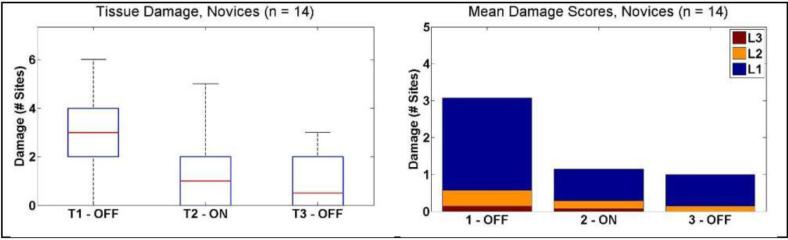
Novices showed a similar result, with a decrease in median number damage sites from 3 [2 – 4] to 1 [0 – 2] when the tactile feedback was activated (P = 0.0046) . This median decreased slightly in the third condition (0.5 [0 – 2]) although this was not significant (P = 0.69). These results are shown in Figure 9.
Figure 9.
Tissue damage results for novice subjects. (Left) Box and whisker plots showing median number of damage sites decrease from T1 to T2. (Right) Mean number of damage sites ranked by intensity L1 – Light, L2 – Medium, and L3 – Heavy.
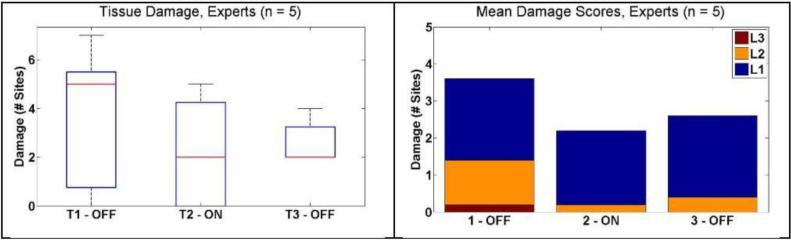
For expert subjects, the median number of damage sites decreased from 5 [0.75 – 5.5] to 2 [0 – 4.25] when the system was activated, although this was not significant (P = 0.25). In the third condition, the median number of damage sites was 2 [2 – 3.25]. The low number of expert subjects resulted in a wider distribution of damage sites due to the increased impact of each particular subject. These results are shown in Figure 10.
Figure 10.
Tissue damage results for expert subjects. (Left) Box and whisker plots showing median number of damage sites decrease from T1 to T2. (Right) Mean number of damage sites ranked by intensity L1 – Light, L2 – Medium, and L3 – Heavy.
Results from the hypothesis testing is summarized in Table 3. Each subject group showed a decrease in mean number of damage sites between T1 and T2, but statistical significance was only obtained for all subjects (n=19) and novice subjects (n=14). It is possible that the statistical power of the study limits our ability to show significance in the expert group, however it is also possible that these users, with already low forces, do not benefit from this system to the degree of novice users.
Table 3.
Tissue damage hypothesis testing.
| Group | P (All)a | P (T1 / T2)b | P (T2 / T3)b | P (T1 / T3)b |
|---|---|---|---|---|
| All Subjects (n = 19) | 0.00082** | 0.0018** | 0.96 | 0.017 |
| Experts (n = 5) | 0.47 | 0.25 | 1.0 | 0.69 |
| Novices (n = 14) | 0.0011** | 0.0046* | 0.69 | 0.0098* |
Friedman's Test, one rep per subject
Wilcoxon Signed Rank Test
Force / Damage Correlation Analysis
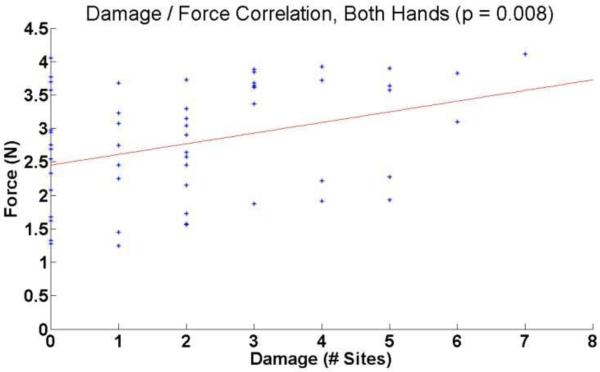
The results showed a significant correlation (P= 0.008) between the mean force applied to a section of bowel and the number of sites of damage (Figure 11).
Figure 11.
Correlation analysis of damage and mean grasping force.
For bowel segments with fewer than two damage sites, there were 21 occasions where mean force applied to that bowel was above 2.5 N, and 17 occasions were the mean force was below 2.5 N. For bowel segments with three or more damage sites, there were fourteen occasions of mean force greater than 2.5 N, and only five occasions of force lower than 2.5 N (Table 4). This suggests that that using consistently higher levels of force may lead to higher incidence of damage.
Table 4.
Incidence of Damage at High and Low Force.
| Incidence | Two or Fewer Damage Sites | Three or More Damage Sites |
|---|---|---|
| Force Below 2.5 N | 21 | 5 |
| Force Above 2.5 N | 17 | 14 |
Classification of Subjects by Force Data
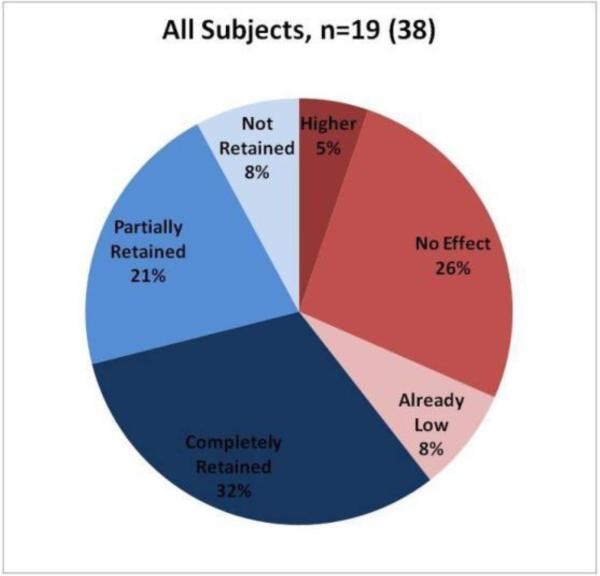
The purpose of the subject-by-subject analysis was to classify subjects into categories based on statistical differences in grasping force in response to tactile feedback. Time-averaged grip force values were compared using a Wilcoxon Rank Sum test. Pair-wise comparisons were made. In this analysis, subjects were classified into one of six categories based on their individual p-values (Table 5). Each subject's hand was analyzed separately so for 19 subjects, there were 38 subject-hands. The results of the classification is shown in Figure 12.
Table 5.
Categories for Subject-by-Subject Analysis
| Category | Classification |
|---|---|
| No Effect | Subject showed no significant differences between T1 and T2, and the mean forces in all conditions were greater than 2.5 N. |
| Already Low | Subject showed no significant differences between T1 and T2, and the mean forces in all conditions were less than 2.5 N. |
| Higher | Subject used more force with tactile feedback. (T2 > T1) |
| Lower and Completely Retained | Subject used less force with tactile feedback. When system was turned off in T3, this low force was completely retained. T2 and T3 were indistinguishable. |
| Lower & Not Retained | Subject used less force with tactile feedback. When the system was turned off in T3, the force increased to the level of T1. T1 and T3 were indistinguishable. |
| Lower and Partially Retained | Subject used less force with tactile feedback. When the system was turned off in T3, the force increased, but not as high as T1. T1, T2, and T3 were different, and T1 > T3 > T2. |
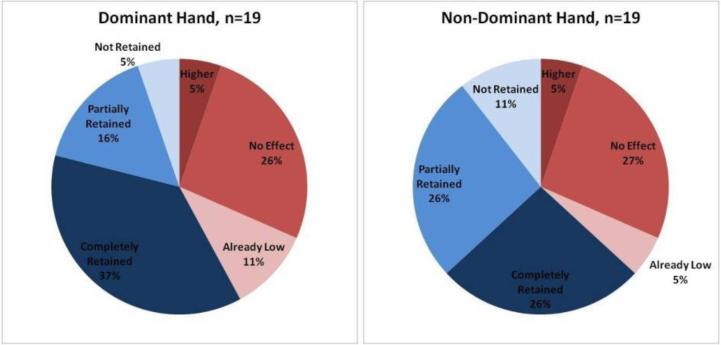
Figure 12.
Classification of all subjects, both hands.
Approximately 39% of subjects saw no change or slightly higher forces when tactile feedback was activated (T1 vs. T2). A few of these subjects (8% of total) subjects were experts who used forces lower than 2.5 N, and the remaining group (31% of total) used forces higher than 2.5 N.
Approximately 61% of subjects showed decreased forces when tactile feedback was activated (T1 vs. T2). When tactile feedback was deactivated (T2 vs. T3) approximately half of these subjects retained the lower forces (32% of total), whereas the other half did not retain (8% of total) or only partially retained this information (21% of total).
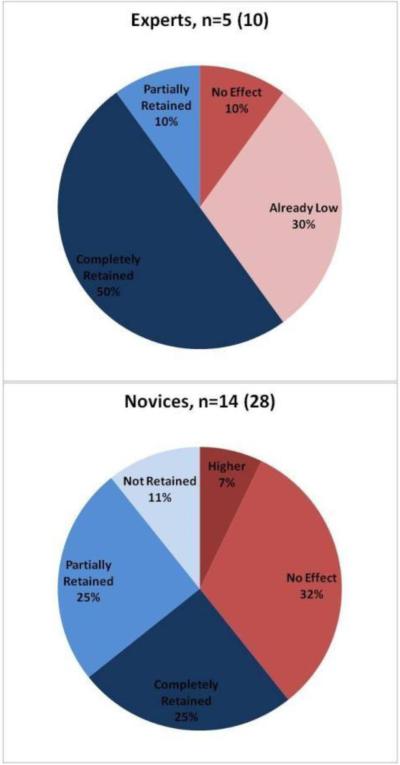
Experts and novices are observed separately in Figure 13. A majority of expert subjects (80%) had low forces in the third condition (T3), due to either already low forces (30%), or complete retention (50%). One expert subject showed a force of approximately 2.8 N for his non-dominant hand and was classified as “No Effect.”
Figure 13.
(Left) Classification of expert subjects (Right) Classification of novice subjects
For novice subjects, 61% showed significant decreases in grip force when tactile feedback was activated. Most of these novices at most partially retained the low forces (25% partial, 11% no retention), indicating that the while there may have been some learning, the impact due to tactile feedback was more appreciable. Thirty-nine percent (39%) of novice subjects saw no improvements across the experiment and all of these subjects used forces higher than 2.5 N.
A comparison of dominant and non-dominant hand (Figure 14) showed that the dominant hand had a higher incidence of initially low forces, and when improvement was observed, a higher incidence of complete retention.
Figure 14.
Subject-by-Subject Analysis: Classification of all subjects, (Left) only dominant hand (Right) Non-dominant hand.
Survey Analysis
At the end of the experiment, subjects filled out a survey (Table 6). For each statement, subjects indicated their level of agreement with one of five answers, which were scored on the following scale: Strongly Disagree: 1, Disagree: 2, Neither Agree nor Disagree: 3, Agree: 4, Strongly Agree: 5.
Table 6.
Survey Questions
| Number | Question |
|---|---|
| 1. | Tactile feedback helped me perform the task |
| 2. | Tactile feedback had no effect on my performance |
| 3. | With tactile feedback, I grasped tissue with less force |
| 4. | The balloon inflations were easy to feel |
| 5. | I found myself ignoring the balloon inflations |
| 6. | The balloon inflations were intuitive |
| 7. | The inflations became more difficult to feel over time |
| 8. | Avoiding drops was my highest priority |
| 9. | Delicate grasping was my highest priority |
Table 7 shows the results from the survey analysis.
Table 7.
Mean scores in survey responses, grouped by experience level
| Question | Mean Score (± Standard Deviation) | ||
|---|---|---|---|
| All Subjects (n = 19) | Experts (n = 5) | Novices (n = 14) | |
| Tactile Feedback helped me perform the task | 3.84 (±0.69) | 3.60 (±0.89) | 3.93 (±0.62) |
| Tactile feedback had no effect on my performance | 2.37 (±1.01) | 2.80 (±1.10) | 2.21 (±0.97) |
| With tactile feedback, I grasped tissue with less force | 4.11 (±0.81) | 3.80 (±0.84) | 4.21 (±0.80) |
| The balloon inflations were easy to feel | 4.37 (±0.76) | 4.40 (±0.55) | 4.36 (±0.84) |
| I found myself ignoring the balloon inflations | 1.84 (±0.76) | 2.00 (±0.00) | 1.79 (±0.89) |
| The balloon inflations were intuitive | 3.79 (±0.79) | 3.80 (±0.84) | 3.79 (±0.80) |
| The inflations became more difficult to feel over time | 2.00 (±0.75) | 2.00 (±0.71) | 2.00 (±0.78) |
| Avoiding drops was my highest priority | 2.84 (±1.01) | 2.80 (±0.84) | 2.86 (±1.10) |
| Delicate grasping was my highest priority | 3.53 (±0.84) | 3.60 (±0.55) | 3.50 (±0.94) |
Three new groups were also analyzed (No Effect, One Hand, Both Hands). The mean scores for these groups are shown in Table 8.
Table 8.
Mean scores in survey responses, grouped by grasping force results
| Question | Mean Score (± Standard Deviation) | ||
|---|---|---|---|
| No Effect (n = 5) | One Hand (n = 5) | Both Hands (n = 9) | |
| Tactile Feedback helped me perform the task | 3.60 (±0.55) | 3.80 (±0.84) | 4.00 (±0.71) |
| Tactile feedback had no effect on my performance | 2.80 (±0.84) | 2.40 (±1.52) | 2.11 (±0.78) |
| With tactile feedback, I grasped tissue with less force | 3.60 (±0.55) | 4.20 (±0.84) | 4.33 (±0.87) |
| The balloon inflations were easy to feel | 4.00 (±1.22) | 4.60 (±0.55) | 4.44 (±0.53) |
| I found myself ignoring the balloon inflations | 2.40 (±1.14) | 1.60 (±0.55) | 1.67 (±0.50) |
| The balloon inflations were intuitive | 3.20 (±0.84) | 4.40 (±0.89) | 3.78 (±0.44) |
| The inflations became more difficult to feel over time | 2.60 (±0.89) | 2.00 (±0.71) | 1.67 (±0.50) |
| Avoiding drops was my highest priority | 2.80 (±1.10) | 3.40 (±1.14) | 2.56 (±0.88) |
| Delicate grasping was my highest priority | 3.20 (±0.84) | 3.40 (±0.89) | 3.78 (±0.83) |
In general, the subject groups tended to give similar answers to the survey questions. Subjects noted that tactile feedback helped performance and decreased grasping force. Responses indicated that the balloon inflations were perceptible and not ignored, and that efforts were directed more towards delicate grasping.
There were also several notable differences. Expert subjects tended towards neutral when asked about the impacts of feedback on performance. Additionally, the group that did not benefit from tactile feedback (No Effect) agreed more with the statements: Tactile feedback did not effect performance, I found myself ignoring balloon inflations, and the inflations became more difficult to feel over time. This group disagreed more with the statements: With tactile feedback, I grasped tissue with less force, the balloon inflations were easily to feel, the balloon inflations were intuitive, and delicate grasping was my highest priority.
Discussion
Surgeons often cite the lack of tactile feedback as a limitation of current commercially available robotic systems, especially within General Surgery (32). Procedures where it may be of particular value in General Surgery include Heller myotomy, paraesophageal hernia repair, gastric bypass, gastric resection for neoplasm, biliary reconstructive surgery, transhiatal esophagectomy, transthoracic esophageal surgery, distal pancreatectomy with splenic preservation, and selected colorectal procedures. It may hold promise for pancreatic head resection and hepatectomy, but experience to date is limited. In resections for neoplasm, robotic surgery may help to enhance the completeness of lymph node dissection. According to the SAGES consensus 2014, although there is a substantial cost disadvantage to using the robot for simple procedures such as cholecystectomy and fundoplication, these procedure may present an excellent opportunity for surgeons early in their robotic learning curve to acquire increasingly more advanced skills. The results of this study show that with the addition of tactile feedback, less forces were used which correlated with less tissue damage to the bowel. Significance was only represented within the novice group. It is possible with a higher level of power experts would demonstrate this same significance, however these effects may also be limited to level of training. Further studies will need to address these issues including a sub-analysis that looks at the degree of training each individual has with Robotic Systems. From our study it appears there would be value in equipping robotic systems and trainers with tactile feedback to advance novice users towards mastery.
The population analysis also showed both grasping forces and number of sites of damage, remained low when feedback was subsequently deactivated. This suggests that there is a learning effect that could be due to the presence of tactile feedback, increased familiarity with the task, or some combination of the two. Future studies are needed to distinguish between these two possibilities and to quantify the impact and actual duration of retained tactile memory.
A linear regression showed a significant correlation between high grasping forces and incidence of damage. While other factors, such as location of grasp (i.e., proximity to delicate vessels), the duration of the hold, and the effect of tugging on the bowel, may also contribute to tissue damage, this correlation suggests that using high grip forces increases the potential for damage, and that reducing grip force may have measurable benefit to clinical outcomes.
The wide distribution of grasping force and tissue damage in the second and third conditions indicated that improvements were subject-specific. The results from the subject classification analysis lent further evidence to this claim. This subject classification analysis showed that approximately 61% of subjects had a significant decrease in grip force when the feedback system was activated, and that 39% showed no change across the experiment. For experts, this was largely due to baseline forces within 2.5 N. For novices, the survey showed that one subject had difficulty perceiving the balloon inflations, and the remaining three were unsure how to interpret them. This suggests that the system should be improved for more intuitive use, or should be paired with an additional period of training.
For 36% of novice subjects, there was a decrease in force when the system was activated and limited to no retention of these low forces when system was later deactivated. These results match the original robotic surgery training study performed prior to this research [25], and further indicate that tactile feedback might be necessary as a permanent fixture in surgical robotic systems in order to decrease forces and damage while grasping.
Many subjects, especially experts, showed a high incidence of retention. This could be due to re-familiarization with the task after a warm-up period or due to re-attunement to feedback present in the visual display. It is also possible that tactile feedback served as a trigger for kinesthetic memory for fine control of grip when using the robotic surgery system. The latter case would once again suggest benefit from the use of tactile feedback during robotic surgery training, with novices possibly acquiring expert-level force levels after repeated use.
Tactile feedback appeared to be an intuitive mechanism for novice users to automatically use lower grasping forces as they learned how to use the robotic surgical systems and perform tasks. As expected, these individuals showed the largest gains due to their inexperience and lack of kinesthetic memory. Due to limitations in our system with force saturations met by many novice users the degree to which the tactile feedback system reduced these forces cannot be fully appreciated. The expert users, already with kinesthetic memory, and low grasping forces still had evidence of benefit from tactile feedback as it pertains to force. It is clear in both instances tactile feedback could act as a safeguard while manipulating tissues and restricting grasping forces. Perhaps the most interesting aspect of our findings is the lack of a demonstrated learning curve when employing tactile feedback; that is, the effect was immediate, and more pronounced in the novice group, indicating some innate mechanism.
We are at the forefront of advancing technology available for robotic surgical systems. It is clear that further research needs to be performed to further improve these systems to benefit patient care by allowing more surgical specialties the ability to use these tools safely. Our group believes that the evidence gathered thus far strongly suggests that accurate tactile feedback provides useful information, especially to novices, which does not induce cognitive distraction or cognitive overload. Due to the nature of this research studies have been limited to small cohorts. To better appreciate the role of tactile feedback systems larger studies are needed. This could be approached through a robotic surgical curriculum with randomization to include systems with or without tactile feedback.
Conclusion
The in vivo application of integrated tactile feedback in the robotic system demonstrates significantly reduced grasping forces, resulting in significantly less tissue damage. These results are suggestive of both innate and adaptive learning processes, which may be better defined with further study. The daVinci robotic system is capable of generating forces that go well beyond tissue tolerances, thus creating a potentially dangerous situation whenever tissue is being handled without some form of feedback. This tactile feedback system may improve surgical outcomes and broaden the use of robotic-assisted minimally invasive surgery in a wider spectrum of clinical care.
Acknowledgements
The authors most gratefully appreciate funding provided by the National Institutes of Health (NIH)/ National Institute of Biomedical Imaging and Bioengineering (NIBIB) under award 1-R21-EB-013832-01A1.
The authors would also like to thank Dr. Joanne Sohn, Dr. Charles Gates, Guillermo Moreno and the rest of staff at the Division of Laboratory Animal Medicine (DLAM), Dr. Clara Magyar and the staff of the Translational Pathology Core Laboratory (TPCL), Dr. Jim Sayre for his contributions to statistical methods, CASIT administrator Holly Chung for her assistance with organizing experiments, Dr. Shane White and Dalene Sederstrom for use of their Instron, and the surgeons and surgical residents who volunteered their time to participate in this study.
Footnotes
Disclosures
The authors have no conflicts of interest or financial ties to disclose.
Citations
- 1.Satava RM. Surgical robotics: the early chronicles: a personal historical perspective. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2002;12(1):6–16. doi: 10.1097/00129689-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Schurr MO, Buess G, Neisius B, Voges U. Robotics and telemanipulation technologies for endoscopic surgery. Surgical endoscopy. 2000;14(4):375–381. doi: 10.1007/s004640020067. [DOI] [PubMed] [Google Scholar]
- 3.Berkelman PJ, Whitcomb LL, Taylor RH, Jensen P. A miniature instrument tip force sensor for robot/human cooperative microsurgical manipulation with enhanced force feedback. Medical Image Computing and Computer-Assisted Intervention–MICCAI. 2000;1935:897–906. [Google Scholar]
- 4.Louw DF, Fielding T, McBeth PB, et al. Surgical robotics: a review and neurosurgical prototype development. Neurosurgery. 2004;54(3):525–537. doi: 10.1227/01.neu.0000108638.05274.e9. [DOI] [PubMed] [Google Scholar]
- 5.Jacob BP, Gagner M. Robotics and general surgery. Surgical Clinics of North America. 2003;83(6):1405–1419. doi: 10.1016/S0039-6109(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 6.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Annals of Surgery. 2004;239(1):14. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moorthy K, Munz Y, Dosis A, et al. Dexterity enhancement with robotic surgery. Surgical Endoscopy And Other Interventional Techniques. 2004;18(5):790–795. doi: 10.1007/s00464-003-8922-2. [DOI] [PubMed] [Google Scholar]
- 8.Munz Y, Moorthy K, Dosis A, et al. The benfits of stereoscopic vision in robotic-assisted performance on bench models. Surgical Endoscopy And Other Interventional Techniques. 2004;18(4):611–616. doi: 10.1007/s00464-003-9017-9. [DOI] [PubMed] [Google Scholar]
- 9.Lowrance WT, Eastham JA, Savage C, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. The Journal of Urology. 2012;87(6):2087–2093. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagotti A, Gagliardi ML, Fanfani F, et al. Perioperative outcomes of total laparoendoscopic single-site hysterectomy versus total robotic hysterectomy in endometrial cancer patients: A multicentre study. Gynecologic Oncology. 2012;125(3):552–555. doi: 10.1016/j.ygyno.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Markar SR, Karthikesalingam AP, Venkat Ramen V, et al. Robotic vs. laparoscopic Roux-en-Y gastric bypass in morbidly obese patients: systematic review and pooled analysis. The International Journal of Medical Robotics and Computer Assisted Surgery. 2011;7(4):393–400. doi: 10.1002/rcs.414. [DOI] [PubMed] [Google Scholar]
- 12.Tieu K, Allison N, Snyder B, et al. Robotic-assisted Roux-en-Y gastric bypass: update from 2 high-volume centers. Surgery for Obesity and Related Diseases. 2012;9(2):284–288. doi: 10.1016/j.soard.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Breitenstein S, Nocito A, Puhan M, et al. Robotic-assisted versus laparoscopic cholecystectomy: outcome and cost analyses of a case-matched control study. Annals of surgery. 2008;247(6):987–993. doi: 10.1097/SLA.0b013e318172501f. [DOI] [PubMed] [Google Scholar]
- 14.Karabulut K, Agcaoglu O, Aliyev S, et al. Comparison of intraoperative time use and perioperative outcomes for robotic versus laparoscopic adrenalectomy. Surgery. 2012;151(4):537–542. doi: 10.1016/j.surg.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Suri RM, Antiel RM, Burkhart HM, et al. Quality of life after early mitral valve repair using conventional and robotic approaches. The Annals of Thoracic Surgery. 2012;93(3):761–769. doi: 10.1016/j.athoracsur.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 16.Schmitto JD, Mokashi SA, Cohn LH. Past, present, and future of minimally invasive mitral valve surgery. Journal of Heart Valve Disease. 2011;20(5):493. [PubMed] [Google Scholar]
- 17.Turner WF, Jr, Sloan JH. Robotic-assisted coronary artery bypass on a beating heart: initial experience and implications for the future. The Annals of thoracic surgery. 2006;82(3):790–794. doi: 10.1016/j.athoracsur.2006.03.112. [DOI] [PubMed] [Google Scholar]
- 18.Oehler MK. Robotic assisted Surgery in Gynecology. Australian and New Zealand Journal of Obsterics and Gynaecology. 2009;49(2):124–129. doi: 10.1111/j.1479-828x.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- 19.Kontarinis DA, Son JS, Peine W, Howe RD. A tactile shape sensing and display system for teleoperated manipulation. IEEE. International Conference on Robotics and Automation. 1995;1:641–646. [Google Scholar]
- 20.Toledo L, Gossot D, Fritsch S, Revillon Y, Reboulet C. [Study of sustained forces and the working space of endoscopic surgery instruments]. Ann Chir. 1999;53(7):587–97. [PubMed] [Google Scholar]
- 21.Mucksavage P, Kerbl DC, Pick DL, Lee JY, Mcdougall EM, Louie MK. Differences in grip forces among various robotic instruments and da Vinci surgical platforms. J Endourol. 2011;25(3):523–8. doi: 10.1089/end.2010.0306. [DOI] [PubMed] [Google Scholar]
- 22.Van der meijden OA, Schijven MP. The value of haptic feedback in conventional and robot-assisted minimal invasive surgery and virtual reality training: a current review. Surg Endosc. 2009;23(6):1180–90. doi: 10.1007/s00464-008-0298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bethea BT, Okamura AM, Kitagawa M, et al. Application of haptic feedback to robotic surgery. J Laparoendosc Adv Surg Tech A. 2004;14(3):191–5. doi: 10.1089/1092642041255441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutt CN, Oniu T, Mehrabi A, Kashfi A, Schemmer P, Büchler MW. Robot-assisted abdominal surgery. Br J Surg. 2004;91(11):1390–7. doi: 10.1002/bjs.4700. [DOI] [PubMed] [Google Scholar]
- 25.Van der meijden OA, Schijven MP. The value of haptic feedback in conventional and robot-assisted minimal invasive surgery and virtual reality training: a current review. Surg Endosc. 2009;23(6):1180–90. doi: 10.1007/s00464-008-0298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdea G, Burdea GC. Force and Touch Feedback for Virtual Reality. Wiley; New York: 1996. pp. 3–4. [Google Scholar]
- 27.Okamura AM. Haptic feedback in robot-assisted minimally invasive surgery. Current opinion in urology. 2009;19(1):102. doi: 10.1097/MOU.0b013e32831a478c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Culjat MO, Bisley JW, King CH, et al. Tactile feedback in surgical robotics. In: Rosen J, Hannaford B, Satava RM, editors. Surgical Robotics: Systems, Applications, and Visions. Springer; New York: 2011. pp. 449–468. [Google Scholar]
- 29.Culjat MO, King CH, Franco ML, et al. A tactile feedback system for robotic surgery. Engineering in Medicine and Biology Society. 2008;2008:1930–1934. doi: 10.1109/IEMBS.2008.4649565. [DOI] [PubMed] [Google Scholar]
- 30.King CH, Culjat MO, Franco ML, et al. Tactile feedback induces reduced grasping force in robot-assisted surgery. IEEE Transactions on Haptics. 2009;2(2):103–110. doi: 10.1109/TOH.2009.4. [DOI] [PubMed] [Google Scholar]
- 31.Wottawa CR, Cohen JR, Fan RE, et al. The role of tactile feedback in grip force during laparoscopic training tasks. Surgical endoscopy. 2012;27(4):1111–8. doi: 10.1007/s00464-012-2612-x. [DOI] [PubMed] [Google Scholar]
- 32.Simorov A, Otte RS, Kopietz CM, Oleynikov D. Review of surgical robotics user interface: what is the best way to control robotic surgery?. Surg Endosc. 2012;26(8):2117–25. doi: 10.1007/s00464-012-2182-y. [DOI] [PubMed] [Google Scholar]