Abstract
Serum periostin is a potential biomarker of response to therapies that target type 2 inflammation in asthma. The objectives of this study were to describe: 1) the distribution of serum periostin levels in adults with symptomatic airflow obstruction; 2) its relationship with other variables, including type 2 biomarkers; and 3) the effect of inhaled corticosteroids on periostin levels.
Serum periostin levels were measured in a cross-sectional study exploring phenotypes and biomarkers in 386 patients aged 18–75 years who reported wheeze and breathlessness in the past 12 months. In 49 ICS-naïve patients, periostin levels were measured again after 12 weeks of budesonide (800 μg·day−1).
The distribution of serum periostin levels was right skewed (mean±sd 57.3±18.6 ng·mL−1, median (interquartile range) 54.0 (45.1–65.6) ng·mL−1, range 15.0–164.7 ng·mL−1). Periostin was positively associated with exhaled nitric oxide (Spearman's rho=0.22, p<0.001), blood eosinophil count (Spearman's rho=0.21, p<0.001), and total IgE (Spearman's rho=0.14, p=0.007). The Hodges–Lehmann estimator (95% CI) of change in periostin level after ICS therapy was −4.8 (−6.7– −3.2) ng·mL−1 (p<0.001).
These findings provide data on the distribution of serum periostin in adults with symptomatic airflow obstruction, the weak associations between periostin and other type 2 markers, and the reduction in periostin with inhaled corticosteroid therapy.
Short abstract
Serum periostin levels may be a distinct marker of type 2 inflammation in adults with symptomatic airways disease http://ow.ly/XAKyK
Introduction
In asthma, biomarkers have been used to explore underlying pathogenesis, define specific disease phenotypes, and identify subgroups of patients who preferentially respond to different treatments [1, 2]. Sputum eosinophilia and elevated exhaled nitric oxide fraction (FeNO) identify an asthma phenotype highly responsive to inhaled corticosteroid (ICS) therapy, and in which ICS treatment may be titrated by measurements of these biomarkers [3, 4]. Biomarkers may also identify a phenotype of severe asthma, refractory to ICS therapy, which may show greater response to novel treatments targeting eosinophilic type 2 inflammation. Clinical trials of mepolizumab and reslizumab, monoclonal antibodies directed against interleukin (IL)-5, have provided evidence that a responder group characterised by eosinophilic airways inflammation can be identified through measurement of sputum eosinophils, blood eosinophils or FeNO levels [5–8]. Peripheral blood eosinophil count, FeNO and serum periostin have also shown utility in identifying responder groups to omalizumab, an anti-IgE monoclonal antibody therapy [9], and lebrikizumab, an anti-IL-13 monoclonal antibody therapy [10, 11].
Periostin, a matricellular protein detectable in serum, has a strong association with airways eosinophilia in severe asthma [12, 13]. The gene coding for periostin is among the most highly up-regulated genes in asthma [14]. Periostin expression in airway epithelial cells can be regulated by the key T-helper (Th)2 cytokines IL-4 and IL-13 [15]. Periostin has a role in stimulating the transforming growth factor (TGF)-β signalling pathway involving matrix metalloproteinase-2 and -9, with epithelial cells overexpressing periostin causing TGF-β-dependent secretion of type 1 collagen by airway fibroblasts [16]. Periostin gene expression in airway epithelial cells is associated with an asthma phenotype driven by increased expression of IL-5 and IL-13, with high levels of serum IgE, systemic and lung eosinophilia, increased thickness of the reticular basement membrane and responsiveness to corticosteroids [14, 17].
The relationships between serum periostin and severity of airflow obstruction, symptoms, health status and potential confounding factors, such as comorbidity, smoking status and ethnicity, are unknown. This information would be useful for clinical interpretation of periostin levels in an individual.
Serum periostin was measured as part of the New Zealand Respiratory Health Survey (NZRHS), in which the phenotypes of obstructive airways disease were investigated by cluster analysis [18]. The aims of this secondary exploratory analysis of the NZRHS were to determine: 1) the distribution of periostin levels in a random sample of adult subjects with symptoms of obstructive airways disease; 2) its relationship with other clinical and pathophysiological features, including type 2 biomarkers; and 3) the effect of ICS treatment on periostin levels in ICS-naïve subjects.
Methods
The design of the NZRHS has been reported previously [18]. In summary, the NZRHS was a cross-sectional study designed to explore phenotypes and biomarkers in adults aged 18–75 years, with symptoms of obstructive airways disease. Participants with self-reported wheeze and breathlessness in the past year were drawn from a random population sample of the electoral roll in the Wellington region of New Zealand. Doctor-diagnosed asthma or chronic obstructive pulmonary disease were not a requirement for inclusion. Participants attended for detailed assessment, with complete lung function and phenotype data available in 389 individuals, of whom 386 had baseline serum periostin measurements and are included in this analysis. The 125 participants not receiving ICS at baseline completed a 12-week, open-label trial of budesonide (400 μg via turbuhaler twice daily) followed by repeat assessment. Following a protocol amendment during the study, blood samples were also drawn for a subset of 49 subjects after 12 weeks of ICS for repeat measurement of serum periostin to enable an exploratory analysis of the effects of ICS therapy on serum periostin levels.
Ethical approval was given by the Central Regional Ethics Committee of New Zealand (CEN/09/12/095) and written informed consent was obtained from all participants prior to testing. The NZRHS was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12610000666022).
Lung function and reversibility testing
Lung function testing was performed in accordance with American Thoracic Society (ATS) guidelines [19], with lung volumes measured by body plethysmography (Masterscreen Body; Erich–Jaeger, Friedberg, Germany). Post-bronchodilator measurements of forced expiratory volume in 1 s (FEV1) were performed 30 min after metered dose inhaler administration of 400 μg salbutamol through a spacer (Volumatic; GlaxoSmithKline, Brentford, UK). FeNO was determined according to ATS guidelines [20] with an online nitric oxide monitor (NiOX; Aerocrine AB, Solna, Sweden).
Blood tests
Serum periostin levels were determined from blood samples obtained on the day of lung function testing. Full blood count and differential (Sysmex platform, Mundelein, IL, USA) were performed immediately; blood samples were coagulated for 30 min, centrifuged and the serum stored at −80°C prior to analysis of: total IgE (Roche modular, Indianapolis, IN, USA); high-sensitivity C-reactive protein (Roche modular); and serum Phadiatop (Phadia, Uppsala, Sweden), a composite test for a panel of specific IgE with a positive test signifying atopy. Serum periostin levels were determined using the clinical trial version of the Elecsys Periostin assay (Roche Diagnostics, Penzberg, Germany). This is an automated electrochemiluminescence immunoassay, based on the sandwich principle, utilising the same antibodies as those previously reported by Jia et al. [12].
Questionnaires and medical history
Disease control and health status were assessed with the seven point Asthma Control Questionnaire (ACQ-7) [21] and the validated New Zealand version of the St George's Respiratory Questionnaire (SGRQ) [22]. Symptoms and medical history were obtained using questions drawn from a series of validated questionnaires, with comorbidities recorded using questions from the ATS Division of Lung Diseases questionnaire (ATS-DLD-78) [23].
Statistical methods
The relationships between serum periostin and other continuous variables were explored by scatter plots and the strengths of the associations were assessed by the rank-based Spearman coefficient because of the skew distribution of some of the continuous variables. A post hoc analysis of the relationship between serum periostin and each of IgE, FeNO and eosinophil count, in relation to whether participants had or did not have doctor-diagnosed asthma, was performed by ANCOVA. This method assumes that there is a relationship, measured by a slope term (corresponding to a correlation coefficient in the simpler analyses), between serum periostin and each of the continuous variables. An interaction term between the categorical variable of having or not having doctor-diagnosed asthma and each of IgE, FeNO and eosinophil count tests if the slope relationship is the same in those with and without doctor-diagnosed asthma. If the interaction term is statistically significant this means that the strength of the association is different in these two groups. To meet normality assumptions, IgE, FeNO and periostin were logarithm transformed for these analyses. The Mann–Whitney or Kruskal–Wallis tests examined the strength of association between categorical variables and serum periostin. For dichotomous variables, where appropriate, a location shift was estimated using the Hodges–Lehmann method and associated confidence interval. Although a nominal value of 0.05 is used for statistical significance, in this exploratory study we did not adjust for multiple statistical testing. SAS version 9.3 (SAS Institute, Carey, NC, USA) and R version 3.02 (www.r-project.org/about.html) were used for the statistical analysis.
Results
Sample description
The participants are described in table 1. There was a wide range of demographic characteristics, risk factors for lung disease, severity of airflow obstruction and symptoms.
TABLE 1.
Description of study sample#
| Mean±sd | Median (IQR) | Range | n (%) | |
| Continuous variables | ||||
| Age years | 48.9±13.9 | 49.5 (40–59) | 19–76 | |
| Periostin ng·mL−1 | 57.3±18.6 | 54.0 (45.1–65.6) | 15.0–164.7 | |
| Pre-bronchodilator FEV1 L | 2.97±0.97 | 2.93 (2.36–3.60) | 0.50–5.86 | |
| Salbutamol reversibility % | 10.0±11.8 | 6.8 (3.2–12.5) | −10.7–121.9 | |
| KCO % pred | 99.2±17.5 | 100.6 (90.6–109.2) | 34.5–143.4 | |
| FeNO ppb | 33.7±35.2 | 21.9 (13.5–39.5) | 2.7–262.8 | |
| IgE IU·L−1 | 342.7±1162 | 81.1 (20.7–247) | 0.1–18 083 | |
| Eosinophil ×109·L−1 | 0.2±0.19 | 0.2 (0.1–0.3) | 0.0–1.5 | |
| ACQ-7 | 0.89±0.77 | 0.71 (0.29–1.29) | 0–3.86 | |
| SGRQ | 23.7±16.7 | 19.5 (10.4–35.0) | 0–84.1 | |
| Categorical variables | ||||
| Males | 179 (46.4) | |||
| ICS in past 12 months | 140 (36.3) | |||
| Doctor-diagnosed asthma | 285 (73.8) | |||
| Doctor-diagnosed COPD | 17 (4.4) | |||
| Smoking status | ||||
| Current | 58 (15.0) | |||
| Ex | 132 (34.2) | |||
| Never | 196 (50.8) |
IQR: interquartile range; FEV1: forced expiratory volume in 1 s; KCO: transfer coefficient of the lung for carbon monoxide; FeNO: exhaled nitric oxide fraction; ACQ-7: seven point Asthma Control Questionnaire; SGRQ: St George's Respiratory Questionnaire; ICS: inhaled corticosteroids; COPD: chronic obstructive pulmonary disease. #: n=386.
Distribution of serum periostin, FeNO, blood eosinophil and serum IgE levels
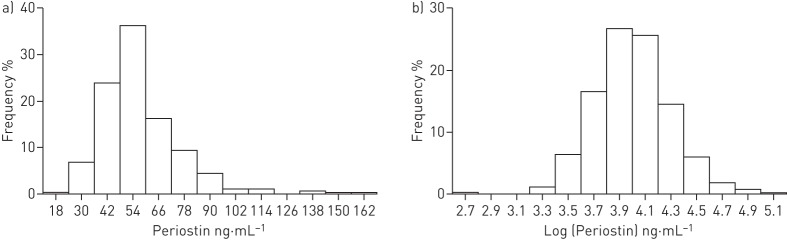
The distribution of serum periostin values was right skewed. The natural logarithm transformation leads to a more symmetric distribution (figure 1). The mean±sd serum periostin was 57.3±18.6 ng·mL−1 (median (interquartile range (IQR)) 54.0 (45.1–65.6) ng·mL−1, range 15.0–164.7 ng·mL−1) (table 1). Doctor-diagnosed asthma was observed in 285 (73.8%) out of 386 participants. Table 2 shows data summaries for the distribution of variables in participants with and without asthma. The median (IQR) of serum periostin for participants with and without a diagnosis of asthma was 53.7 (45.2–65.7) ng·mL−1 and 54.6 (44.5–63.9) ng·mL−1, respectively (table 2, figure S1).
FIGURE 1.
a) Distribution of baseline serum periostin and b) the same data plotted on a natural logarithm transformed scale.
TABLE 2.
Data description by asthma diagnosis
| Variable | Doctor-diagnosed asthma | |||
| Yes# | No¶ | |||
| Serum periostin ng·mL−1 | 57.7±19.4 | 53.7 (45.2–65.7) | 56.3±16.1 | 54.6 (44.5–63.9) |
| FeNO ppb | 35.6±36.8 | 22.6 (13.5–41.4) | 26.8±26.7 | 20.5 (12.9–25.5) |
| IgE IU·L−1 | 400.5±1320 | 117.8 (28–272.7) | 189.2±507.2 | 39.0 (12.0–139) |
| Eosinophils ×109·L−1 | 0.25±0.20 | 0.2 (0.1–0.3) | 0.21±0.15 | 0.2 (0.1–0.3) |
| FEV1 % pred | 81.3±17.4 | 83.1 (72.3–92.9) | 83.3±22.9 | 86.5 (77.4–97.2) |
| Salbutamol reversibility % | 10.9±12.3 | 8.0 (3.8–13.3) | 7.7±10.0 | 5.1 (2.2–8.9) |
| SGRQ | 23.0±15.5 | 19.2 (10.5–32.9) | 25.7±19.6 | 19.9 (10.4–35.2) |
Data are presented as mean±sd or median (interquartile range). FeNO: exhaled nitric oxide fraction; FEV1: forced expiratory volume in 1 s; SGRQ: St George's Respiratory Questionnaire. #: n=285; ¶: n=101.
The distribution of FeNO was also right skewed. The median (IQR) for FeNO in participants with and without asthma was 22.6 (13.5–41.4) ppb and 20.5 (12.9–25.5) ppb, respectively. The distribution of serum periostin in participants with high versus low FeNO levels is shown in figure S2. For serum IgE levels, the median (IQR) was 117.8 (28–272.7) IU·L−1 and 39 (12.0–139) IU·L−1 in participants with and without asthma, respectively. For blood eosinophils the median (IQR) was 0.2 (0.1–0.3) cells×109·L−1 for both groups.
Associations with clinical variables
Markers of type 2 patterns of disease
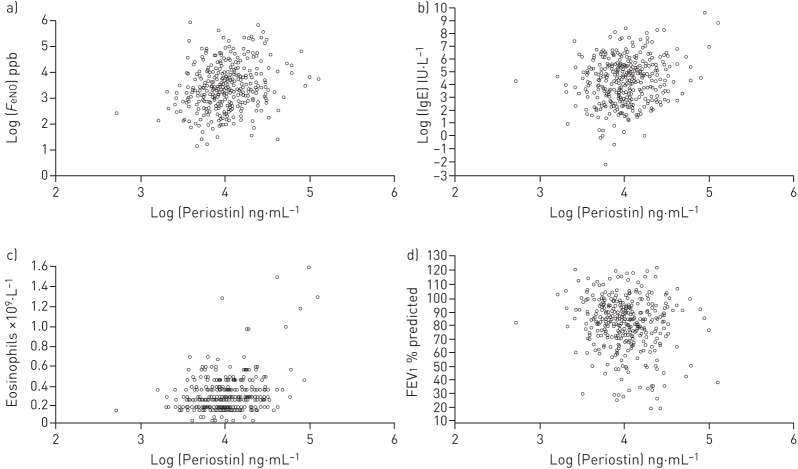
There were significant associations between logarithm serum periostin and logarithm FeNO (Spearman's rho=0.22, p<0.001), blood eosinophil count (Spearman's rho=0.21, p<0.001) and logarithm total IgE (Spearman's rho=0.14, p=0.007) (figure 2a–c). Atopy, defined by Phadiatop, was associated with a higher serum periostin: median (IQR) 55.9 (47.3–67.1) ng·mL−1 and 51.0 (42.2–59.9) ng·mL−1 in 241 adults with and 145 adults without atopy. Hodges–Lehmann estimator of location shift (95% CI) was 4.8 (1.8–7.9) ng·mL−1 (p=0.002). There were no significant associations between serum periostin level and a history of rhinitis, eczema or baseline ICS use.
FIGURE 2.
Scatter plots of log serum periostin and a) log exhaled nitric oxide fraction (FeNO), b) log IgE, c) blood eosinophil count and d) forced expiratory volume in 1 s (FEV1).
In the post hoc interaction analysis there was no evidence of a difference in the slope of the relationship between logarithm periostin and either logarithm FeNO, eosinophil count or logarithm IgE based on whether the participant had or did not have doctor-diagnosed asthma. The p-values for the interaction terms were 0.99, 0.15 and 0.40 for logarithm FeNO, eosinophil count and logarithm IgE respectively.
For those with doctor-diagnosed asthma the logarithm FeNO (95% CI) was 0.19 (0.01–0.38) higher (p=0.042), which is equivalent to a ratio of mean FeNO of 1.21, and the slope of the logarithm FeNO to logarithm periostin increase was 0.60 (0.33–0.87; p<0.001). This is consistent with a ratio of mean FeNO of 1.5 per doubled periostin level. For those with doctor-diagnosed asthma, the median (95%) eosinophil count was 0.03 (−0.0006–0.08) cells×109·L−1 higher (p=0.092) and the slope of the eosinophil count to logarithm periostin increase was 0.20 (0.14–0.26; p<0.001), which is consistent with an increased eosinophil count of 0.14 cells×109·L−1 per doubled periostin level. The difference (95% CI) in logarithm IgE between those with and without doctor-diagnosed asthma was 1.07 (0.50–1.65), which is consistent with a mean ratio of IgE of 2.1. The slope of logarithm IgE to logarithm periostin increase was 0.76 (0.37–1.15; p=0.02), which is consistent with a ratio of mean IgE of 1.7 per doubled periostin level.
Severity of airflow obstruction
There was a weak negative association between serum periostin and FEV1 % predicted (Spearman's rho −0.13, p=0.014) (figure 2d), and a weak positive association with functional residual capacity % predicted (Spearman's rho 0.15, p=0.004). There were no statistically significant associations between serum periostin and any of the following: FEV1/FVC ratio, transfer factor, reversibility to salbutamol or peak flow variability (Spearman's rho between −0.08 and 0.08, p≥0.10).
Medical history and risk factors
There was a weak negative association between serum periostin and body mass index (Spearman's rho −0.15, p=0.003). A diagnosis of gastro-oesophageal reflux disease (GORD) was associated with a lower serum periostin: median (IQR) 50.9 (42.3–62.4) ng·mL−1 and 55.2 ng·mL−1 (47.2–68.5) in 117 participants with and 268 without GORD, respectively. Hodges-Lehmann estimator of location shift (95% CI) was −4.9 (−8.1– −1.7) ng·mL−1 (p=0.003).
There were no statistically significant associations between serum periostin and a wide range of other variables. For the continuous variables age, tobacco exposure as pack-years, age at onset of respiratory symptoms and high-sensitivity C-reactive protein Spearman's rho was between −0.05 and 0.03 (p>0.20). For categorical variables sex, smoking status, history of cardiovascular disease, history of diabetes and a history of hypertension the p-value using the Mann-Whitney test or Kruskal-Wallis test was p≥0.10.
Ethnicity
The data distribution of serum periostin by ethnicity is shown in table 3. There was no overall difference in serum periostin by ethnicity (Kruskal–Wallis test p=0.31), although participants self-reporting Asian ethnicity had a higher mean periostin than those reporting European ethnicity (69.9 ng·mL−1 versus 56.9 ng·mL−1, respectively).
TABLE 3.
Periostin and ethnicity
| Patients n | Mean±sd | Median (IQR) | Range | |
| Asian | 9 | 69.6±24.4 | 59.5 (55.7–78.8) | 46.1–119.6 |
| European | 334 | 56.9±18.6 | 53.7 (45.1–63.9) | 15.0–164.7 |
| Maori | 23 | 57.3±15.6 | 58.0 (45.1–69.0) | 34.6–87.8 |
| Pacific | 9 | 62.0±17.5 | 65.1 (44.6–75.5) | 38.9–88.3 |
| Other | 10 | 57.3±18.7 | 55.1 (43.5–60.2) | 34.8–90.3 |
IQR: interquartile range.
Cohort study of ICS, serum periostin and FeNO
The open-label cohort study of ICS had 125 ICS-naïve participants. The baseline mean±sd FeNO was 37.6±39.4 ppb (median (IQR) 23.6 (14.6–43.1) ppb, range 5.5–240.2 ppb). The baseline mean±sd serum periostin was 56.6±14.1 ng·mL−1 (median (IQR) 53.9 (46.6–65.7) ng·mL−1, range 30.6–90.3 ng·mL−1). The change in asthma-related variables in response to ICS therapy is shown in table 4. There was no significant association between baseline serum periostin and change in ACQ, FeNO, FEV1, peak expiratory flow rate variability or SGRQ (Spearman's rho between −0.14 and 0.05, p≥0.1 for all). There was also no association between baseline FeNO and change in ACQ, FEV1, peak expiratory flow rate variability or SGRQ (Spearman's rho between −0.13 and 0.07, p>0.1).
TABLE 4.
Change in asthma related variables in the full cohort of 125 inhaled corticosteroid (ICS) study participants
| Variable | Mean±sd | Median (IQR) | Range |
| ACQ-7 | −0.17±0.55 | −0.14 (−0.42–0.0) | −2.0–3.0 |
| FEV1 % pred | 0.7±3.9 | 0.9 (−1.8–3.2) | −11.1–11.5 |
| Pre-bronchodilator FEV1 L | 0.02±0.15 | 0.03 (−0.07–0.12) | −0.5–0.5 |
| FeNO ppb | −13.7±28.7 | −2.6 (−15.4–1.1) | −16.5–16.7 |
| PEF variability# | −5.0±10.5 | −4.2(−9.0–1.0) | −48.5–24.1 |
| SGRQ total | −5.6±9.9 | −4.7 (−9.3– −0.3) | −42.1–40.9 |
Change in variable is calculated as post-ICS value minus pre-ICS value. IQR: interquartile range; ACQ-7: seven point Asthma Control Questionnaire; FEV1: forced expiratory volume in 1 s; FeNO: exhaled nitric oxide fraction; PEF: peak expiratory flow; SGRQ: St George's Respiratory Questionnaire. #: n=123.
Serum periostin values were obtained at both the beginning and end of the open-label cohort study for 49 out of 125 ICS participants. Table 5 shows baseline periostin, post-ICS periostin and change in baseline periostin for this sub-group. The Hodges–Lehmann estimator (95% CI) of change in serum periostin was −4.8 (−6.7– −3.2) ng·mL−1 (p<0.001). There were no statistically significant associations between the change in serum periostin and change in the asthma-related variables, (Spearman's rho between 0.05 and 0.21, p>0.15). In a post hoc analysis there were no differences in response to ICS, based on high versus low FeNO, serum periostin or blood eosinophil count (tables S1 and S2).
TABLE 5.
Change in asthma related variables in the 49 inhaled corticosteroid (ICS) study participants with end of study periostin measurements
| Variable | Mean±sd | Median (IQR) | Range |
| Baseline periostin ng·mL−1 | 52.9±14.9 | 49.8 (42.7–58.5) | 30.6–88.3 |
| Post-ICS periostin ng·mL−1 | 47.6±14.4 | 47.3 (38.3–50.9) | 27.2–95.5 |
| Change in serum periostin ng·mL−1 | −5.3±6.3 | −4.3 (−8.3– −1.4) | −21.9–8.2 |
IQR: interquartile range.
Discussion
This study describes the distribution of serum periostin values in a random adult population with symptoms of obstructive airways disease. Periostin showed a weak association with markers of type 2 inflammation and a weak inverse association with the severity of airflow obstruction. ICS therapy was associated with a reduction in serum periostin level.
The notable features of the distribution of serum periostin levels in this sample were the wide, 11-fold range from 15–165 ng·mL−1 and the marked right skew distribution. The median serum periostin of 54 ng·mL−1 was similar to the median of 50.2 ng·mL−1 previously reported in adults with moderate and severe asthma inadequately controlled despite ICS therapy [10]. Likewise, the mean serum periostin of 57 ng·mL−1 was similar to the 53 ng·mL−1 reported in severe persistent allergic uncontrolled asthma despite treatment with high-dose ICS plus long-acting β-agonist therapy [9]. This suggests that periostin is not a measure that can usefully discriminate patients with severe asthma from a population with symptomatic but nonspecific airflow obstruction.
In order for serum periostin to be useful in guiding personalised asthma therapy, physicians need to be able to interpret periostin values in the context of an individual patient's characteristics, risk factors and medical history. The findings of this study suggest that periostin values do not need to be adjusted to take account of a patient's age, sex or smoking history. Likewise, although periostin expression in cardiac tissues is upregulated after cardiac injury [24, 25], there was no association between serum periostin and an individual's history of cardiovascular disease, hypertension or diabetes. The major potential confounding variable that physicians might need to be aware of when interpreting periostin levels in airways disease is GORD, which, in this study, was associated with lower levels of serum periostin. It is not clear whether the presence of GORD downregulates periostin expression or if participants with significant GORD had respiratory symptoms secondary to non-type 2-related inflammatory pathways. There was a weak inverse correlation between body mass index and serum periostin, which may be due to a decreased incidence of eosinophilic inflammation in some obese people with asthma [26, 27].
The associations between serum periostin and FeNO, IgE, blood eosinophil count and atopic status were statistically significant, albeit weak. The associations were similar in those with and without doctor-diagnosed asthma. Together with the observations that FeNO and blood eosinophil counts are not clearly related [28, 29] and that FeNO, IgE and blood eosinophil counts, both separately and combined, have variable accuracy in predicting sputum eosinophil percentages [30–32], this suggests that these biomarkers, including periostin, might identify different aspects of type 2 inflammation. This complexity of type 2 inflammation does not reduce the observed utility of periostin and other biomarkers as predictors of response to monoclonal antibody therapy directed against components such as IL-5, IL-13 or IgE [5–11].
Serum periostin was only weakly associated with the degree of airflow obstruction as measured by FEV1 % predicted, and was not correlated with FEV1/FVC ratio. Together with our observation that periostin levels in this cohort with predominately mild-to-moderate airflow obstruction is similar to that previously reported in adults with severe poorly controlled asthma [9, 10], this suggests that periostin levels might not be a clinically relevant marker of asthma severity. However, relevant to this interpretation we observed in our ICS responsiveness study in ICS-naïve subjects that 12 weeks of treatment with ICS was associated with a reduction in serum periostin of 4.8 ng·mL−1 (∼10% of the baseline value). This suggests that one of the reasons why serum periostin levels were similar in this population of adults with mild-to-moderate airflow obstruction to those with severe asthma [9, 10] might be because the high-dose ICS therapy of severe asthmatics might have reduced the periostin levels.
We did not identify a relationship between baseline periostin and response to ICS apart from a weak association with the ACQ-7 score. This contrasts with the previous demonstration that asthma patients with a “Th2-high” phenotype, characterised by high periostin gene expression in the airway, preferentially respond to ICS therapy with improvements in lung function, compared to a “Th2-low” phenotype [17]. It is probable that the participants in our study did not have a sufficiently strong type 2 pattern of disease or asthma severity to show a relationship between these biomarkers and ICS responsiveness. This interpretation would be suggested by our inability to show an effect of ICS on lung function, or an association between baseline FeNO and response to ICS, which has been previously demonstrated [1, 2].
The NZRHS used a random population sampling approach and our findings are, therefore, likely to be applicable to the general population of individuals with symptoms of obstructive airways disease. We did not employ purposive sampling of different ethnic groups, and the finding of higher mean serum periostin in participants self-identifying as Asian requires validation. The reference range of serum periostin in different populations, including disease-free populations, is not known and should be a focus for future research. The associations we have found should be considered and interpreted cautiously, as we did not adjust for performing multiple statistical tests and so some of our associations may be due to type I error rate inflation and require validation in other samples.
In summary, we provide data on the distribution of serum periostin levels in adults with symptoms of obstructive airways disease. The findings support the interpretation that serum periostin might be a distinct marker of a type 2 pattern of airway disease and, therefore, potentially a predictor of response to biological agents targeting this pathway [9, 12].
Acknowledgements
We are grateful to the study participants for their involvement in the study. J. Fingleton would like to acknowledge the respiratory medicine specialty training programme (South West Peninsula Deanery, Plymouth, UK) for research placement approval.
The members of the NZRHS Study Group are as follows. Steering Committee: J. Fingleton (clinical coordinating investigator), J. Travers, I. Braithwaite, M. Weatherall (study biostatistician), R. Beasley (principal investigator), M. Williams, P. Shirtcliffe (designated safety reviewer), T. Charles, D. Bowles, R. Strik, N. Dooney, T. Baker, M. Patel, M. Holliday, M. Stretch, A. Pritchard, D. Fabian, C. Munro, A. Hosking, A. Brinded (all Medical Research Institute of New Zealand, Wellington, New Zealand) and G. Purdie (Otago University, Wellington, New Zealand).
Footnotes
This article has supplementary material available from erj.ersjournals.com
Clinical trial: The NZRHS was registered with the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au ACTRN12610000666022).
Support statement: This study was funded by the Ministry of Health, New Zealand (Health Research Council of New Zealand; 10/174) and Genentech Inc., USA. Funding information for this article has been deposited with FundRef.
Conflict of interest: Disclosures can be found alongside the online version of this article at erj.ersjournals.com
References
- 1.Taylor DR. Using biomarkers in the assessment of airways disease. J Allergy Clin Immunol 2011; 128: 927–934. [DOI] [PubMed] [Google Scholar]
- 2.Szefler SJ, Wenzel S, Brown R, et al. . Asthma outcomes: biomarkers. J Allergy Clin Immunol 2012; 129: Suppl. 3, S9–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petsky HL, Cates CJ, Lasserson TJ, et al. . A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax 2012; 67: 199–208. [DOI] [PubMed] [Google Scholar]
- 4.Malerba M, Ragnoli B, Radaeli A, et al. . Usefulness of exhaled nitric oxide and sputum eosinophils in the long-term control of eosinophilic asthma. Chest 2008; 134: 733–739. [DOI] [PubMed] [Google Scholar]
- 5.Castro M, Mathur S, Hargreave F, et al. . Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2011; 184: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 6.Haldar P, Brightling CE, Hargadon B, et al. . Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009; 360: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair P, Pizzichini MM, Kjarsgaard M, et al. . Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 2009; 360: 985–993. [DOI] [PubMed] [Google Scholar]
- 8.Pavord ID, Korn S, Howarth P, et al. . Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. [DOI] [PubMed] [Google Scholar]
- 9.Hanania NA, Wenzel S, Rosén K, et al. . Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187: 804–811. [DOI] [PubMed] [Google Scholar]
- 10.Corren J, Lemanske RF, Hanania NA, et al. . Lebrikizumab treatment in adults with asthma. N Engl J Med 2011; 365: 1088–1098. [DOI] [PubMed] [Google Scholar]
- 11.Hanania NA, Noonan M, Corren J, et al. . Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax 2015; 70: 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia G, Erickson RW, Choy DF, et al. . Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 2012; 130: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair P, Kraft M. Serum periostin as a marker of TH2-dependent eosinophilic airway inflammation. J Allergy Clin Immunol 2012; 130: 655–656. [DOI] [PubMed] [Google Scholar]
- 14.Woodruff PG, Boushey HA, Dolganov GM, et al. . Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 2007; 104: 15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takayama G, Arima K, Kanaji T, et al. . Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 2006; 118: 98–104. [DOI] [PubMed] [Google Scholar]
- 16.Sidhu SS, Yuan S, Innes AL, et al. . Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 2010; 107: 14170–14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodruff PG, Modrek B, Choy DF, et al. . T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingleton J, Travers J, Williams M, et al. . Treatment responsiveness of phenotypes of symptomatic airways obstruction in adults. J Allergy Clin Immunol 2015, 136: 601–609. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. . Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide 2005. Am J Respir Crit Care Med 2005; 171: 912–930. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Svensson K, Mork AC, et al. . Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005; 99: 553–538. [DOI] [PubMed] [Google Scholar]
- 22.Jones PW, Quirk FH, Baveystock CM, et al. . A self-complete measure of health status for chronic airflow limitation. The St George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. [DOI] [PubMed] [Google Scholar]
- 23.Ferris BG. Recommended respiratory disease questionnaires for use with adults and children in epidemiological research: epidemiology standardization project. Am Rev Respir Dis 1978; 118: 7–53. [PubMed] [Google Scholar]
- 24.Dorn GW., II Periostin and myocardial repair, regeneration, and recovery. N Engl Med J 2007; 357: 1552–1554. [DOI] [PubMed] [Google Scholar]
- 25.Kϋhn B, del Monte F, Hajjar RJ, et al. . Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 2007; 13: 962–969. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland TJ, Cowan JO, Young S, et al. . The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med 2008; 178: 469–475. [DOI] [PubMed] [Google Scholar]
- 27.Telenga ED, Tideman SW, Kerstjens HA, et al. . Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy 2012; 67: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 28.Pavord ID, Bafadhel M. Exhaled nitriic oxide and blood eosinophilia: independent markers of preventable risk. J Allergy Clin Immunol 2013; 132: 828–829. [DOI] [PubMed] [Google Scholar]
- 29.Malinovschi A, Fonseca JA, Jacinto T, et al. . Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol 2013; 132: 821–827. [DOI] [PubMed] [Google Scholar]
- 30.Hastie AT, Moore WC, Li H, et al. . Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol 2013; 132: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagener AH, de Nijs SB, Lutter R, et al. . External validation of blood eosinophils, FeNo and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 2015; 70: 115–120. [DOI] [PubMed] [Google Scholar]
- 32.Arron J, Izuhara K. Asthma biomarkers: what constitutes a ‘gold standard’? Thorax 2015; 70: 105–107. [DOI] [PubMed] [Google Scholar]