Abstract
Objective
The brain is one of the main targets of hypertension. However, little is known about the relation between hypertension and motor performances. We studied the association between hypertension and walking speed in a cohort of elderly people.
Methods
Analyses are based on participants (65–85 years) from the Dijon (France) center of the Three-City study (n=3604), followed every two years. Persistent hypertension was defined by the use of antihypertensive drugs at baseline or at first follow-up, or by high blood pressure (≥140/90 mmHg) at baseline and first follow-up. Walking speed was measured over 6 meters, at baseline and fourth follow-up (n=1774) after a mean (SD) duration of 7.0 (0.5) years. Brain MRI was performed in 1590 participants. Generalized linear models were used to assess the relation between hypertension and baseline walking speed or walking speed change.
Results
At baseline, mean (SD) walking speed (m/s) was lower in hypertensive subjects (1.51 [0.31]) than in non-hypertensive subjects (1.59 [0.30], P<0.001). During follow-up, hypertensive subjects had a higher mean annual decline in walking speed (cm/s per year; 2.30 [3.4]) than non hypertensive subjects (1.87 [3.3], P=0.004). The number of antihypertensive drugs was associated with lower walking speed at baseline and higher walking speed decline. Adjustment for MRI white matter abnormalities attenuated these relations.
Conclusion
Persistent hypertension was associated with both lower walking speed and higher decline in walking speed in the elderly. These results may be partly explained by white matter abnormalities, and support the hypothesis of a contribution of vascular risk factors to motor dysfunction.
Keywords: Aged, Brain, Female, Humans, Hypertension, Magnetic Resonance Imaging, Male, Risk Factors, Walking
Introduction
The etiology of gait disorders and decreased motor performances in the elderly is likely to be multifactorial [1], and recent evidence points to the role of vascular risk factors such as diabetes mellitus [2], increased homocysteine [3], or low HDL cholesterol [4]. Hypertension is highly prevalent among the elderly [5], and is a major risk factor for cerebrovascular disease, including clinical stroke and subclinical cerebral white matter lesions [6]. While some studies have shown that hypertension is a risk factor for cognitive decline and dementia [7, 8], little is known about its relation with motor function in the elderly.
Walking speed is a simple and reliable measure that is considered as a surrogate for overall quality of gait and motor function [9], and provides similar information as does a more comprehensive summary measure of physical performance [10]. Decreased walking speed has been shown to be strongly associated with adverse health-related events such as falls [11], disability [12], hospitalization [13], and death [10].
The aim of this study was therefore to investigate the cross-sectional and longitudinal association between hypertension and walking speed in a sample of community-dwelling nondisabled elderly subjects, as part of the Three-City (3C) study.
Methods
Study Population
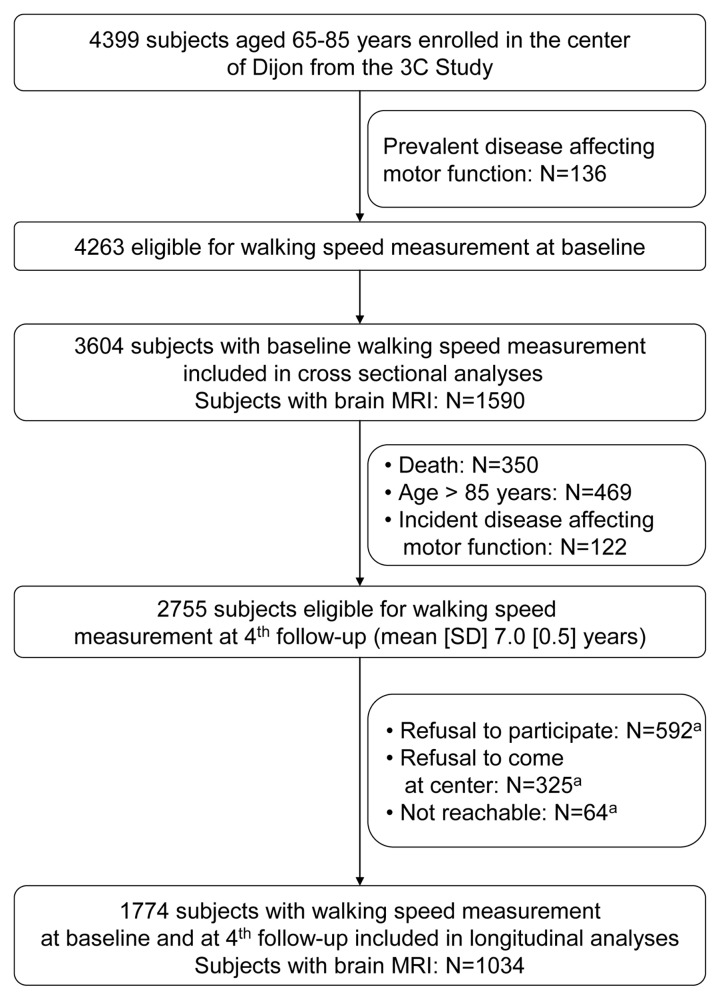
The 3C study is a prospective cohort of community-dwelling persons aged 65 years and over living in three French cities (Bordeaux, Dijon, and Montpellier). The study design has been described elsewhere [14]. A specific sub-study on motor function was performed in Dijon (N=4399) where walking speed was measured at baseline (1999–2000) in subjects aged 85 years old or younger. At baseline, 3604 participants were included for cross-sectional analyses, after exclusion of those with conditions that strongly affected motor function (dementia, disabling stroke, hip fracture, Parkinson’s disease). Following the baseline examination, participants were seen at 4 follow-up surveys. The first, second, and fourth follow-up examinations took place over periods of 2 years. They consisted in at home interviews followed by an examination at the study center. The third follow-up consisted in self-administered questionnaire mailed at home, and data collection took place during one year. After the baseline measure of walking speed, it was measured again at the fourth follow-up, after a mean (SD) duration of 7.0 (0.5) years. A total of 1774 subjects were included for longitudinal analyses, after exclusion of subjects with incident diseases affecting motor function (dementia, disabling stroke, hip fracture, Parkinson’s disease) and those with missing values for a walking speed measure at the fourth follow-up. The flow-chart of participants enrolled in the analyses is presented in Figure 1.
Figure 1. Selection of the participants for cross-sectional and longitudinal analyses.
aSubjects with a missing value for walking speed at the fourth follow-up, who were included in sensitivity analyses based on multiple imputation.
The study protocol was approved by the Ethical Committee of the University-Hospital of Bicêtre (France) and written informed consent was obtained from each participant.
Data Collection
Baseline demographic and medical data were collected at home during face-to-face interviews using a standardized questionnaire administered by trained psychologists. Education level was defined as no school or primary without diploma, primary with diploma, secondary without a baccalaureate degree, baccalaureate or university degree. Weight and height were measured and used to calculate body mass index (BMI). Physical activity level was defined based on the daily duration of walking and athletic activities; low physical activity level was considered for subjects who answered “very little or none” for the daily duration of walking and had no athletic activity. The interview also included an inventory of all drugs prescribed during the preceding month. The name of the medication was recorded, and all drugs were subsequently coded according to the French translation of the WHO ATC classification [15]. Chronic consumption of non-steroidal anti-inflammatory drugs (NSAIDs) for joint pain was assessed and used as surrogate for rheumatic disease. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) [16]. Global cognitive function was assessed by the Mini-Mental State Examination (MMSE) [17]. Subjects were considered as diabetic if they used antidiabetic agents or if their fasting glucose level was equal to or greater than 7.0 mmol/L. Hypercholesterolemia was defined as total cholesterol ≥ 7.25 mmol/L or by use of lipid-lowering drugs. Smoking status was classified as current, past, or never. History of coronary heart disease and peripheral artery disease was assessed by questionnaire. Exertional dyspnea was self-declared using the New York Heart Association classification [18].
Definition of Hypertension
At each examination, blood pressure (BP) was measured twice at home by the interviewers after at least 5 minutes of rest in a seated position, with an appropriately sized cuff placed on the right arm and using a validated digital electronic tensiometer (OMRON M4). The mean of both measures was used in the analyses. Use of antihypertensive drugs was determined according to the number and the type of antihypertensive drugs taken to lower blood pressure: diuretics, beta-blockers, angiotensin converting enzyme inhibitors, calcium channel blockers, and others (ATC code C02).
Participants were considered as having persistent hypertension if they were treated by antihypertensive drugs for hypertension at baseline or at first follow-up, or if they had high blood pressure (systolic BP ≥140 mmHg or diastolic BP ≥ 90 mmHg) at baseline and at first follow-up. This definition of persistent hypertension was therefore based on two measures taken at two different time-points (baseline and first follow-up examinations; mean [SD] delay between the two measures = 1.8 [0.17] years) in order to reduce the risk of misclassifying hypertensive status [19, 20].
Walking Speed Assessment
At baseline, following the home interview, participants aged 85 years old or younger were invited to the study center where walking speed was measured. Two photoelectric cells connected to a chronometer were placed in a corridor 6 meters apart. Participants were asked to walk in the corridor at their maximum speed. The time needed to cover 6 meters was measured by the photoelectric cells, and walking speed was computed as 6 meters divided by time in seconds.
To assess the reproducibility of this measure, we performed a test-retest study in 51 subjects (mean [SD] age = 71.1[3.4] years). Two measures of maximum walking speed were taken five minutes apart. The intraclass correlation coefficient (SE) was 0.92 (0.02), thus showing high reproducibility, as in previous studies.[21]
Walking speed was measured again using the same protocol at the fourth follow-up, in subjects aged 85 years old or younger at that time who agreed to come to the study center. Annual walking speed change (in centimeters per second per year) was defined as the baseline walking speed measurement minus the follow-up walking speed measurement, divided by the duration between the two measurements.
Brain MRI
Brain MRI was proposed to all participants aged ≤ 80 years enrolled between June 1999 and September 2000 (N=2673); 2285 subjects (82%) accepted but only 1924 scans were performed because of financial limitations. Exclusion criteria for MRI were cardiac pace-maker, valvular prosthesis, or other internal electrical/magnetic devices; history of neurosurgery/aneurysm; claustrophobia; and presence of metal fragments. The detailed MRI protocol has been previously described [22, 23]. MRI acquisition was performed on a 1.5T Magnetom (Siemens, Erlangen, Germany). Fully automated image processing software was developed to measure white matter lesions (WML) volume [23]. The presence of lacunar infarcts was assessed visually by a neurologist using a standardized assessment grid.
Statistical Analysis
Baseline characteristics of the study population are presented overall and according to tertiles of walking speed and to hypertensive status, and were compared using the chi-square statistic for categorical variables or the Student t-test for continuous variables.
Generalized linear models were used to study the relation between walking speed (considered as the dependent variable) and hypertensive status. In a first model, in addition to hypertension, three covariates strongly associated with walking speed and hypertension (age, sex, BMI) were included. In a second model, we included additional covariates associated both with walking speed and hypertension in univariate analyses (education level, MMSE, physical activity, hypercholesterolemia, smoking, NSAIDs use). In a third model, we included variables associated with either hypertension or walking speed. In a fourth and final model, we adjusted for potential mediators: history of coronary heart disease or peripheral artery disease, and exertional dyspnea.
For longitudinal analyses, the characteristics of the study sample are presented overall and according to tertiles of annual walking speed change, and were compared using the chi-square statistic for categorical variables or the Student t-test for continuous variables. Generalized linear models were used to study the relation between annual walking speed decline (considered as the dependent variable) and hypertension. In a first model, in addition to hypertension, age, sex, baseline walking speed, and BMI were included. In a second model, we included additional covariates associated both with walking speed decline and hypertension (education level, hypercholesterolemia, smoking, physical activity level, diabetes mellitus, psychotropic drug use). In a final model, we included history of coronary heart disease and peripheral artery disease, as well as exertional dyspnea as covariates.
We studied the relation between the number of different antihypertensive drugs used at the same time and baseline walking speed or annual walking speed decline; we considered that a higher number of antihypertensive drugs represents a surrogate marker for the severity of hypertension [24, 25].
Because of a large difference in walking speed in men and women, all analyses were systematically stratified by sex.
To investigate differences in walking speed across the main categories of antihypertensive drugs, we restricted the analyses to subjects treated for hypertension with a monotherapy and we defined the following 4-level categorical variable: users of diuretics, beta-blockers, angiotensin converting enzyme inhibitors, calcium channel blockers; mean walking speed was compared across the levels of this variable using the Tukey-Kramer method to adjust for multiple comparisons.
We performed sensitivity analyses. As beta-blockers can induce fatigue or dyspnea during physical exercise [26], we excluded participants using beta-blockers, to investigate whether the relations observed were explained by this category of drugs. We also used multiple imputation procedures to impute missing values of walking speed at the fourth follow-up using a general linear regression model (10 imputations) including baseline predictors (age, gender, BMI, walking speed, hypertensive status) and the delay between baseline and follow-up examinations (SAS Proc MI). Imputed data sets were analyzed as complete data and the 10 sets of results were pooled using SAS Proc MIANALYZE.
Finally, to investigate whether MRI abnormalities related to hypertension (WML volume, lacunar infarcts) explained the association between hypertension and walking speed, we repeated our cross-sectional and longitudinal analyses among subjects who performed a brain MRI at baseline. In a first model we adjusted for age, sex, and BMI. In a second model, we added WML volume and presence of lacunar infarcts.
All P values were two-tailed; P ≤ 0.05 was considered to be statistically significant. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of the study population (N=3604) are presented in Table 1. The mean (SD) age of the participants was 73.4 (4.6) years, 61.9% of them were women, and 71.4% had persistent hypertension. Subjects who walked slower were older, heavier, more often women, and were more likely to have depressive symptoms and exertional dyspnea than those who walked faster. They also had a lower educational, MMSE, and physical activity level, were more often treated for hypercholesterolemia, and used more frequently NSAIDs and psychotropic drugs. These associations remained significant after adjustment for age and sex. Subjects who walked faster were more often ever-smokers and current alcohol drinkers than those who walked slower; however, this relation was explained by a strong confounding effect of sex and age, and it was no longer significant after adjustment for these two variables (smoking, p=0.88; alcohol, p=0.14). Hypertension was associated with older age, male sex, higher BMI, lower education, MMSE, and physical activity level, and other vascular risk factors (diabetes, hypercholesterolemia, smoking), exertional dyspnea, and history of coronary and peripheral artery disease (Table 1); these associations remained significant after adjustment for age and sex, except for peripheral artery disease (p=0.11) and smoking (p=0.46).
Table 1.
Baseline characteristics of the study population, overall and by tertiles of walking speed and by hypertensive status.
| Characteristics | All | Tertiles of walking speed
|
Hypertensiona
|
|||||
|---|---|---|---|---|---|---|---|---|
| < 1.43 m/s | 1.43–1.62 m/s | > 1.62 m/s | pb | No | Yes | pb | ||
| N | 3604 | 1304 | 1013 | 1287 | 1029 | 2575 | ||
| Age, years, mean (SD) | 73.4 (4.6) | 74.9 (4.6) | 73.4 (4.6) | 71.9 (4.1) | <0.001 | 72.2 (4.3) | 73.9 (4.7) | <0.001 |
| Women, n (%) | 2231 (61.9) | 1059 (81.2) | 663 (65.5) | 509 (39.6) | <0.001 | 709 (68.9) | 1522 (59.1) | <0.001 |
| BMI, kg.m−2, mean (SD) | 25.7 (4.0) | 26.4 (4.5) | 25.5 (3.8) | 25.1 (3.4) | <0.001 | 24.3 (3.4) | 26.2 (4.1) | <0.001 |
| Low education level, n (%)c | 1240 (34.3) | 618 (47.4) | 306 (30.2) | 316 (24.6) | <0.001 | 299 (29.1) | 941 (36.5) | 0.001 |
| MMSE score, mean (SD) | 27.5 (1.9) | 27.0 (2.0) | 27.6 (1.8) | 27.8 (1.7) | <0.001 | 27.6 (1.9) | 27.4 (1.9) | 0.001 |
| Depressive symptoms, n (%)d | 460 (12.9) | 225 (17.5) | 112 (11.1) | 123 (9.6) | <0.001 | 129 (12.7) | 331 (13.0) | 0.80 |
| Low physical activity, n (%)e | 599 (17.3) | 291 (23.5) | 149 (15.3) | 159 (12.6) | <0.001 | 147 (14.8) | 452 (18.3) | 0.01 |
| Diabetes mellitus, n (%) | 324 (9.2) | 123 (9.7) | 93 (9.4) | 108 (8.7) | 0.65 | 34 (3.4) | 290 (11.6) | <0.001 |
| Hypercholesterolemia, n (%)f | 1422 (40.2) | 550 (42.9) | 418 (42.0) | 454 (36.0) | 0.002 | 343 (33.8) | 1079 (42.8) | <0.001 |
| Ex- or current smokers, n (%) | 1386 (38.5) | 360 (27.6) | 376 (37.1) | 650 (50.5) | <0.001 | 352 (34.2) | 1034 (40.2) | 0.001 |
| Current drinker, n (%) | 2844 (79.1) | 961 (73.8) | 796 (78.8) | 1087 (84.7) | <0.001 | 815 (79.3) | 2029 (79.0) | 0.92 |
| Psychotropic drugs use, n (%) | 911 (25.3) | 455 (34.6) | 254 (25.1) | 206 (16.0) | <0.001 | 255 (24.8) | 656 (25.5) | 0.66 |
| NSAIDs for chronic joint pain, n (%) | 535 (15.0) | 275 (21.4) | 123 (12.3) | 137 (10.8) | <0.001 | 138 (13.6) | 397 (15.6) | 0.12 |
| History of coronary disease, n (%) | 369 (10.2) | 145 (11.1) | 105 (10.4) | 119 (9.3) | 0.29 | 34 (3.3) | 335 (13.0) | <0.001 |
| Peripheral artery disease, n (%) | 112 (3.2) | 49 (3.8) | 27 (2.7) | 36 (2.8) | 0.22 | 20 (2.0) | 92 (3.6) | 0.01 |
| Exertional dyspnea, n (%) | 494 (13.7) | 273 (21.0) | 119 (11.8) | 102 (7.9) | <0.001 | 106 (10.3) | 388 (15.1) | 0.01 |
Antihypertensive treatment or blood pressure ≥ 140/90 mmHg at baseline and first follow-up.
Chi-square statistic for categorical variables or the Student t-test for continuous variables.
Primary without diploma.
Assessed by the Center of Epidemiological Studies–Depression Scale.
Walking less than one hour a day and no sport activity.
Lipid-lowering treatment or cholesterol level ≥ 7.25 mmol/L.
The cross-sectional relation between hypertension and walking speed at baseline is presented in Table 2. Hypertensive subjects had a lower mean walking speed (1.51 [0.31] m/s) than non-hypertensive subjects (1.59 [0.30]). This difference was significant after adjustment for age, sex, and BMI (model 1, P value <0.001). There was a progressive decrease in mean walking speed with an increasing number of antihypertensive drugs used (p for trend <0.001). Further adjustment for potential confounders (models 2 and 3) or mediators (model 4) yielded similar findings. This relation was present and of the same magnitude in men and women (Table 2). Among subjects treated for hypertension with a monotherapy, there were no significant differences in baseline walking speed across the main types of antihypertensive drugs (p-values ranging from 0.11 to 0.99 after adjustment for age, sex, and BMI).
Table 2.
Cross sectional association between hypertension and baseline walking speed
| Characteristics | N (%) | Walking Speed mean (SD), m/s | Model 1a
|
Model 2b
|
Model 3c
|
Model 4d
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |||
| Overall (N=3604) | ||||||||||
| No hypertension | 1029 (28.6) | 1.59 (0.30) | Ref. | Ref. | Ref. | Ref. | ||||
| Hypertensione | 2575 (71.4) | 1.51 (0.31) | −0.045 (0.010) | <.001 | −0.041 (0.010) | <.001 | −0.037 (0.010) | <.001 | −0.033 (0.010) | <.001 |
| 0 AHDf | 1780 (49.4) | 1.58 (0.30) | Ref. | Ref. | Ref. | Ref. | ||||
| 1 AHD | 1112 (30.9) | 1.50 (0.30) | −0.041 (0.010) | <.001 | −0.034 (0.010) | .001 | −0.028 (0.010) | .005 | −0.024 (0.010) | .02 |
| 2 AHD | 561 (15.5) | 1.46 (0.30) | −0.064 (0.013) | <.001 | −0.057 (0.013) | <.001 | −0.051 (0.013) | <.001 | −0.043 (0.013) | .001 |
| ≥3 AHD | 151 (4.2) | 1.43 (0.33) | −0.101 (0.022) | <.001 | −0.089 (0.022) | <.001 | −0.086 (0.023) | <.001 | −0.071 (0.023) | .002 |
| P for linear trend | <.001 | <.001 | <.001 | <.001 | ||||||
| Men (N=1373) | ||||||||||
| No hypertension | 320 (23.3) | 1.76 (0.29) | Ref. | Ref. | Ref. | Ref. | ||||
| Hypertensione | 1053 (76.7) | 1.66 (0.30) | −0.049 (0.018) | .007 | −0.051 (0.018) | .006 | −0.045 (0.018) | .01 | −0.043 (0.018) | .02 |
| Women (N=2231) | ||||||||||
| No hypertension | 709 (31.8) | 1.51 (0.27) | Ref. | Ref. | Ref. | Ref. | ||||
| Hypertensione | 1522 (68.2) | 1.40 (0.27) | −0.043 (0.012) | <.001 | −0.036 (0.012) | .002 | −0.033 (0.012) | .004 | −0.030 (0.012) | .01 |
Adjusted for age, sex, BMI. We present regression coefficients (β) and their standard error (SE).
Additional adjustment for education level, MMSE, physical activity, hypercholesterolemia, smoking, and NSAIDs use for chronic joint pain.
Additional adjustment for depressive symptoms, diabetes mellitus, alcohol and psychotropic drugs use.
Additional adjustment for coronary heart disease, peripheral artery disease, and exertional dyspnea.
Antihypertensive treatment or blood pressure ≥ 140/90 mmHg at baseline and first follow-up.
AHD = antihypertensive drugs.
Among the 2755 subjects eligible for a walking speed assessment at the fourth follow-up, a second measure was not available for 981 subjects (Figure 1). They walked slower and were older, heavier, more often women, and more likely to have hypertension, depressive symptoms, diabetes mellitus, and a low physical activity level at baseline than subjects with a second walking speed measure. Table 3 presents the baseline characteristics of the participants included in longitudinal analyses (N=1774). A higher annual decline in walking speed was associated with older age, male sex, higher BMI, higher baseline walking speed, lower education and physical activity level, smoking, use of psychotropic drugs, diabetes mellitus, coronary disease, and exertional dyspnea.
Table 3.
Baseline characteristics of the population included in longitudinal analyses, overall and according to tertiles of walking speed decline
| Characteristics | All | Tertiles of annual walking speed decline, cm/s per year
|
Pa | ||
|---|---|---|---|---|---|
| < 0.87 | 0.87–3.06 | >3.06 | |||
| N | 1774 | 586 | 584 | 604 | |
| Age, years, mean (SD) | 71.5 (3.6) | 71.2 (3.7) | 71.5 (3.6) | 71.9 (3.6) | <0.001 |
| Women, n (%) | 1092 (61.6) | 379 (64.7) | 368 (63.0) | 345 (57.1) | <0.001 |
| BMI, kg.m−2, mean (SD) | 25.4 (3.7) | 25.0 (3.6) | 25.6 (3.6) | 25.7 (3.8) | <0.001 |
| Low education level, n (%)b | 572 (32.2) | 197 (33.6) | 212 (36.3) | 163 (27.0) | <0.001 |
| MMSE score, mean (SD) | 27.8 (1.7) | 27.8 (1.7) | 27.7 (1.8) | 27.8 (1.7) | 0.48 |
| Depressive symptoms, n (%)c | 193 (10.9) | 68 (11.7) | 57 (9.8) | 68 (11.3) | 0.29 |
| Low physical activity, n (%)d | 242 (14.0) | 70 (12.2) | 83 (14.6) | 89 (15.3) | <0.001 |
| Diabetes mellitus, n (%) | 138 (7.9) | 41 (7.1) | 46 (8.0) | 51 (8.6) | 0.03 |
| Hypercholesterolemia, n (%)e | 688 (39.3) | 239 (41.1) | 237 (41.2) | 212 (35.7) | 0.24 |
| Ex- or current smokers, n (%) | 666 (37.5) | 196 (33.4) | 225 (38.5) | 245 (40.6) | 0.31 |
| Current drinker, n (%) | 1427 (80.7) | 475 (81.2) | 466 (79.9) | 486 (80.9) | 0.18 |
| Psychotropic drugs use, n (%) | 234 (13.3) | 87 (15.0) | 80 (13.8) | 67 (11.2) | 0.004 |
| NSAIDs for chronic joint pain, n (%) | 340 (19.2) | 116 (19.8) | 108 (18.5) | 116 (19.2) | 0.27 |
| History of coronary disease, n (%) | 137 (7.7) | 37 (6.3) | 50 (8.6) | 50 (8.3) | 0.08 |
| Peripheral artery disease, n (%) | 31 (1.8) | 8 (1.4) | 11 (1.9) | 12 (2.0) | 0.43 |
| Exertional dyspnea, n (%) | 173 (9.8) | 55 (9.4) | 58 (9.9) | 60 (9.9) | 0.006 |
| Baseline walking speed, m/s, mean (SD) | 1.61 (0.29) | 1.50 (0.25) | 1.58 (0.26) | 1.74 (0.30) | <0.001 |
Adjusted for baseline walking speed.
Primary without diploma.
Assessed by the Center of Epidemiological Studies–Depression Scale.
Walking less than one hour a day and no sport activity.
Lipid-lowering treatment or cholesterol level ≥ 7.25 mmol/L.
Analyses of the relation between hypertension and annual walking speed decline are presented in Table 4. After adjustment for baseline walking speed, age, sex, and BMI (model 1), subjects with hypertension had a higher mean annual decline in walking speed (2.30 [3.4] cm/s per year) than non-hypertensive subjects (1.87 [3.3], p=0.004). An increasing number of antihypertensive drugs was also associated with a higher annual rate of walking speed decline (model 1, p for linear trend <0.001). Further adjustment for potential confounders (model 2) or mediators (model 3) yielded similar results. This association was not modified by gender (interaction p-values ranging from 0.87 to 0.93). Among subjects treated for hypertension with a monotherapy, there were no significant differences in annual decline in walking speed across the main types of antihypertensive drugs (p-values ranging from 0.40 to 0.99 after adjustment for age, sex, BMI, and baseline walking speed).
Table 4.
Longitudinal association between hypertension and annual decline in walking speed
| Characteristics | N, % | Mean (SD) annual walking speed decline, cm/s/yr | Model 1a
|
Model 2b
|
Model 3c
|
|||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | |||
| Overall (N=1774) | ||||||||
| No hypertension | 603 (34.0) | 1.87 (3.3) | Ref. | Ref. | Ref. | |||
| Hypertensiond | 1171 (66.0) | 2.30 (3.4) | 0.44 (0.15) | 0.004 | 0.43 (0.16) | 0.006 | 0.37 (0.16) | 0.02 |
| 0 AHDe | 1026 (57.8) | 1.99 (3.31) | Ref. | Ref. | Ref. | |||
| 1 AHD | 497 (28.0) | 2.38 (3.34) | 0.47 (0.16) | 0.004 | 0.40 (0.17) | 0.02 | 0.33 (0.17) | 0.06 |
| ≥2 AHD | 251 (14.2) | 2.38 (3.58) | 0.60 (0.21) | 0.004 | 0.59 (0.22) | 0.006 | 0.52 (0.22) | 0.02 |
| P for linear trend | <0.001 | 0.002 | 0.01 | |||||
| Men (N=682) | ||||||||
| No hypertension | 183 (26.8) | 1.95 (3.7) | Ref. | Ref. | Ref. | |||
| Hypertensiond | 499 (73.2) | 2.51 (3.6) | 0.49 (0.27) | 0.07 | 0.55 (0.28) | 0.05 | 0.44 (0.28) | 0.12 |
| Women (N=1092) | ||||||||
| No hypertension | 420 (38.5) | 1.83 (3.18) | Ref. | Ref. | Ref. | |||
| Hypertensiond | 672 (61.5) | 2.15 (3.18) | 0.39 (0.18) | 0.03 | 0.38 (0.19) | 0.05 | 0.34 (0.19) | 0.08 |
Adjusted for baseline walking speed, age, sex, BMI. We present regression coefficients (β) and their standard error (SE).
Additional adjustment for education level, MMSE, hypercholesterolemia, smoking, baseline physical activity level, diabetes mellitus, and psychotropic drug use.
Additional adjustment for coronary heart disease, peripheral artery disease, and exertional dyspnea.
Antihypertensive treatment or blood pressure ≥ 140/90 mmHg at baseline and first follow-up.
AHD = antihypertensive drugs.
In sensitivity analyses, we excluded beta-blocker users from the analyses (cross-sectional analysis, N=717; longitudinal analysis, N=300). At baseline, after adjustment for age, sex and BMI, hypertension was still associated with lower walking speed (model 1, β [SE] = −0.033 [0.011], p=0.002). After adjustment for baseline walking speed, age, sex and BMI, hypertension was still associated with a higher annual decline in walking speed (model 1, β [SE] = 0.42 [0.16], p=0.009).
In longitudinal analyses including multiple imputation of missing values of walking speed at the fourth follow-up in 981 participants, we found that, after adjustment for baseline walking speed, age, sex, and BMI, hypertension was still associated with higher annual decline in walking speed (model 1, β [SE] = 0.31 [0.14], p=0.03). Additional adjustment for potential confounders or mediators did not modify these relations (model 3, β [SE] = 0.28 (0.14), p=0.04).
At baseline, a brain MRI was available for 1590 subjects (figure 1). The mean (SD) age of subjects with a MRI was 73.2 (4.1) years, 60.5% of them were women, and 68.2% were hypertensive. The mean (SD) WML volume was 5.5 (5.0) cm3, and 7.0% of subjects had at least one lacunar infarct. Table 5 presents the relation between hypertension and walking speed among subjects who performed a brain MRI at baseline. Adjustment for WML volume and the presence of lacunar infarcts attenuated the relation between hypertension and baseline walking speed (decrease in regression coefficient = 16.3%), and walking speed decline (decrease in regression coefficient = 18.4%).
Table 5.
Relationship between hypertension and baseline walking speed or walking speed decline among subjects who performed a brain MRI at baseline
| N (%) | Model 1a
|
Model 2b
|
|||
|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | ||
| Cross-sectional analysesc | |||||
| No hypertension | 505 (31.8) | Ref. | Ref. | ||
| Hypertensiond | 1085 (68.2) | −0.043 (0.017) | 0.01 | −0.036 (0.017) | 0.03 |
| Longitudinal analysese | |||||
| No hypertension | 375 (36.3) | Ref. | Ref. | ||
| Hypertensiond | 659 (63.7) | 0.38 (0.19) | 0.04 | 0.31 (0.19) | 0.10 |
Adjusted for age, sex, and BMI.
Additional adjustment for volume of white matter lesions (WML) (log transformed) and presence of lacunar infarcts
Relation between hypertensive status and baseline walking speed.
Antihypertensive treatment or blood pressure ≥ 140/90 mmHg at baseline and first follow-up.
Relation between hypertensive status and walking speed change over follow-up. Models are also adjusted for baseline walking speed.
Discussion
In this large prospective cohort of non-institutionalized elderly individuals, hypertension was associated with lower walking speed at baseline and with a higher annual rate of decline in walking speed over follow-up. Using the number of antihypertensive drugs used as a surrogate for the severity of hypertension, we found that these associations were characterized by a dose-effect pattern. These relations were present both in men and women, and remained after adjustment for several potentials confounders and mediators. Adjustment for WML volume and presence of lacunar infarcts at MRI attenuated these relations.
Few studies have investigated the relation between hypertension and motor function in the elderly. In the Women’s Health Initiative, hypertensive women aged 50 to 79 years old had a lower walking speed as part of their baseline assessment [27]. In other studies, hypertension was associated with slower long-distance corridor walk [28], and increasing systolic blood pressure was associated with the decline in a composite measure of lower limb function [29]. Hypertension was also found to be associated with direct or indirect markers of physical fitness like activities of daily living [30], frailty [31], or increased time to complete the Up and Go test [32].
To study the relation between motor performances and hypertension, we used a simple and reliable test, i.e. walking speed, that can be assessed with high reliability in many settings. In addition, using the number of drugs used as a surrogate for hypertension severity [24, 25], our findings are suggestive of dose-effect pattern. Finally, we adjusted our analyses for several potential confounders or mediators and found consistent results in cross-sectional and longitudinal analyses.
We defined persistent hypertension based on two measures of blood pressure taken at two different time points in order to reduce the risk of misclassifying hypertensive status. Indeed, the prevalence of high blood pressure (≥140/90 mm Hg) was considerably higher at baseline (67.6%) than at the first (47.6%) or second follow-up (46.4%) exams. An excess of participants with high blood pressure at baseline in cohort studies has already been described [20], and may be partly explained by a white-coat effect. Participants who had high blood pressure at two subsequent time points were more likely to be truly hypertensive; our approach is in agreement with international recommendations requiring two separate measures of elevated blood pressure for the diagnosis of hypertension [33].
Various mechanisms may account for the association between hypertension and lower walking speed. Hypertension is a major risk factor for cerebrovascular disease, including stroke and WML seen on cerebral MRI [6]. Several studies have shown that WML are associated with worse motor performances [34, 35], suggesting perturbations of neural networks due to small vessel pathology [36]. In our analyses, adjustment for white matter abnormalities attenuated the relations between hypertension and baseline walking speed, and walking speed decline. This result suggests that white matter abnormalities may be an intermediate factor in the relation between hypertension and lower walking speed in the elderly. We therefore hypothesize that the relation between hypertension and walking speed may be, at least partly, explained by the central neurological consequences of hypertension.
Alternative mechanisms may be involved. Hypertension is a major risk factor for atherosclerosis and leads to peripheral artery and coronary disease, which may be responsible for poorer physical performances. However, adjustment for these conditions did not modify the observed relations. A class-effect of antihypertensive drugs is unlikely as no difference was found for the different antihypertensive drugs used; further, exclusion of subjects treated by beta-blockers did not modify our results. Similarly, a small trial performed in healthy subjects found no effect of beta-blockers on walking speed [37].
This study has several strengths, including its large size, the measure of walking speed using of an automated method, the inclusion of numerous potential confounders in the analyses, and the confirmation of the association in both sexes and in both cross-sectional and longitudinal analyses.
The main limitation of this study is a non-negligible proportion of missing values for walking speed at the fourth follow-up; participants were able to choose if they wanted to be interviewed at home or at the study center and a walking speed could not be measured in those who were interviewed at home or refused to participate. In complete-case analyses (i.e., analyses based on available data), observations with missing information for any of the predictors are deleted from the analyses [38]. This approach reduces the precision of parameter estimates, may introduce bias, and yields different sample sizes depending on the predictors included in the model. Simple approaches (e.g., missing indicators method) do not in general correct this bias, and require that missingness be independent of the outcome. Multiple imputation is more generally appropriate and allows asymptotically unbiased estimation of exposure effects under the weaker assumption of missing at random conditional on measured variables, including the outcome [38]. This approach is considered to decrease bias without strongly affecting standard errors of regression parameters [39]. In order to assess whether missingness may have biased the association measures, we conducted a sensitivity analysis, by imputing missing values of walking speed based on its main determinants. We found that the associations remained in analyses based on multiple imputation; this finding is reassuring and suggests that missing values were not responsible for an important bias. Another limitation of the study is that, given its observational nature, we were unable to study the consequence of blood pressure control on walking speed decline among treated hypertensive subjects.
In conclusion, hypertension was associated with lower walking speed at baseline and with a higher annual decline in walking speed over follow-up in a large cohort of non-institutionalized elderly individuals. These findings suggest that hypertension plays a role in motor decline in the elderly, partly explained by its consequences on brain vessels. These data are in line with the involvement of hypertension and other vascular risk factors in cognitive decline and dementia. Motor decline in the elderly represents a great challenge for our aging societies, and the optimization of hypertension care may contribute to prevent motor decline and its severe consequences in the elderly.
Acknowledgments
The 3C study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (INSERM), the Victor Segalen – Bordeaux II University, and the Sanofi-Synthélabo Company. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The 3C Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Haute Autorité de la Santé, Institut National de Prévention et d’Education pour la Santé (INPES), Conseils Régionaux of Bourgogne, Fondation de France, Ministry of Research-INSERM Program, “Cohortes et collections de données biologiques,” Mutuelle Générale de l’Education Nationale, Institut de la Longévité, Conseil Général de la Côte d’or. Julien Dumurgier was supported by a PhD grant from the Fondation pour la Recherche Médicale.
Abbreviations
- 3C
Three-City Study
- BMI
body mass index
- BP
blood pressure
- MMSE
Mini-Mental State Examination
- NSAID
non-steroidal anti-inflammatory drug
- WMH
white matter hypersignal
Footnotes
There are no conflicts of interest.
References
- 1.Alexander NB, Goldberg A. Gait disorders: search for multiple causes. Cleve Clin J Med. 2005;72:586, 589–90, 592–4. doi: 10.3949/ccjm.72.7.586. passim. [DOI] [PubMed] [Google Scholar]
- 2.Brach JS, Talkowski JB, Strotmeyer ES, Newman AB. Diabetes mellitus and gait dysfunction: possible explanatory factors. Phys Ther. 2008;88:1365–74. doi: 10.2522/ptj.20080016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soumare A, Elbaz A, Ducros V, Tavernier B, Alperovitch A, Tzourio C. Cross-sectional association between homocysteine and motor function in the elderly. Neurology. 2006;67:985–90. doi: 10.1212/01.wnl.0000237325.16502.08. [DOI] [PubMed] [Google Scholar]
- 4.Volpato S, Ble A, Metter EJ, Lauretani F, Bandinelli S, Zuliani G, et al. High-density lipoprotein cholesterol and objective measures of lower extremity performance in older nondisabled persons: the InChianti study. J Am Geriatr Soc. 2008;56:621–9. doi: 10.1111/j.1532-5415.2007.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 6.van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension. 2004;44:625–30. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- 7.Tzourio C, Dufouil C, Ducimetiere P, Alperovitch A. Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. EVA Study Group. Epidemiology of Vascular Aging. Neurology. 1999;53:1948–52. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- 8.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–22. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 9.Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: a review. Arch Phys Med Rehabil. 2008;89:865–72. doi: 10.1016/j.apmr.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostir GV, Kuo YF, Berges IM, Markides KS, Ottenbacher KJ. Measures of lower body function and risk of mortality over 7 years of follow-up. Am J Epidemiol. 2007;166:599–605. doi: 10.1093/aje/kwm121. [DOI] [PubMed] [Google Scholar]
- 11.Morita M, Takamura N, Kusano Y, Abe Y, Moji K, Takemoto T, et al. Relationship between falls and physical performance measures among community-dwelling elderly women in Japan. Aging Clin Exp Res. 2005;17:211–6. doi: 10.1007/BF03324599. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–7. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 14.The 3C Study Group. Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316–25. doi: 10.1159/000072920. [DOI] [PubMed] [Google Scholar]
- 15.Centre National Hospitalier d’Information sur le Médicament. 2002. http://www.theriaque.org.
- 16.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9. Boston, Mass: Little, Brown & Co; 1994. pp. 253–256. [Google Scholar]
- 19.Turner MJ, van Schalkwyk JM. Blood pressure variability causes spurious identification of hypertension in clinical studies: a computer simulation study. Am J Hypertens. 2008;21:85–91. doi: 10.1038/ajh.2007.25. [DOI] [PubMed] [Google Scholar]
- 20.Bovet P, Gervasoni JP, Ross AG, Mkamba M, Mtasiwa DM, Lengeler C, et al. Assessing the prevalence of hypertension in populations: are we doing it right? J Hypertens. 2003;21:509–17. doi: 10.1097/00004872-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci L, Guralnik JM, Salive ME, Fried LP, Bandeen-Roche K, Brock DB, et al. Effect of age and severity of disability on short-term variation in walking speed: the Women’s Health and Aging Study. J Clin Epidemiol. 1996;49:1089–96. doi: 10.1016/0895-4356(96)00231-4. [DOI] [PubMed] [Google Scholar]
- 22.Soumare A, Elbaz A, Zhu Y, Maillard P, Crivello F, Tavernier B, et al. White matter lesions volume and motor performances in the elderly. Ann Neurol. 2009;65:706–15. doi: 10.1002/ana.21674. [DOI] [PubMed] [Google Scholar]
- 23.Maillard P, Delcroix N, Crivello F, Dufouil C, Gicquel S, Joliot M, et al. An automated procedure for the assessment of white matter hyperintensities by multispectral (T1, T2, PD) MRI and an evaluation of its between-centre reproducibility based on two large community databases. Neuroradiology. 2008;50:31–42. doi: 10.1007/s00234-007-0312-3. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava PM, Calafiore P, Macisaac RJ, Patel SK, Thomas MC, Jerums G, et al. Prevalence and predictors of cardiac hypertrophy and dysfunction in patients with Type 2 diabetes. Clin Sci (Lond) 2008;114:313–20. doi: 10.1042/CS20070261. [DOI] [PubMed] [Google Scholar]
- 25.Wallenius S, Kumpusalo E, Parnanen H, Takala J. Drug treatment for hypertension in Finnish primary health care. Eur J Clin Pharmacol. 1998;54:793–9. doi: 10.1007/s002280050553. [DOI] [PubMed] [Google Scholar]
- 26.Butler J, Khadim G, Belue R, Chomsky D, Dittus RS, Griffin M, et al. Tolerability to beta-blocker therapy among heart failure patients in clinical practice. J Card Fail. 2003;9:203–9. doi: 10.1054/jcaf.2003.34. [DOI] [PubMed] [Google Scholar]
- 27.McGinn AP, Kaplan RC, Verghese J, Rosenbaum DM, Psaty BM, Baird AE, et al. Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke. 2008;39:1233–9. doi: 10.1161/STROKEAHA.107.500850. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. Jama. 2006;295:2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 29.Shah RC, Wilson RS, Bienias JL, Arvanitakis Z, Evans DA, Bennett DA. Blood pressure and lower limb function in older persons. J Gerontol A Biol Sci Med Sci. 2006;61:839–43. doi: 10.1093/gerona/61.8.839. [DOI] [PubMed] [Google Scholar]
- 30.Newman AB, Arnold AM, Sachs MC, Ives DG, Cushman M, Strotmeyer ES, et al. Long-Term Function in an Older Cohort-The Cardiovascular Health Study All Stars Study. J Am Geriatr Soc. 2009 doi: 10.1111/j.1532-5415.2008.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samper-Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ. Relationship Between Frailty and Cognitive Decline in Older Mexican Americans. J Am Geriatr Soc. 2008 doi: 10.1111/j.1532-5415.2008.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hausdorff JM, Herman T, Baltadjieva R, Gurevich T, Giladi N. Balance and gait in older adults with systemic hypertension. Am J Cardiol. 2003;91:643–5. doi: 10.1016/s0002-9149(02)03332-5. [DOI] [PubMed] [Google Scholar]
- 33.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 34.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 35.Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, Chabriat H, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–42. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 36.Guttmann CR, Benson R, Warfield SK, Wei X, Anderson MC, Hall CB, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54:1277–83. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 37.Rechnitzer PA, Cunningham DA, Howard JH. The self-selected walking pace test and beta blockade. Can J Sport Sci. 1989;14:178–81. [PubMed] [Google Scholar]
- 38.Klebanoff MA, Cole SR. Use of multiple imputation in the epidemiologic literature. Am J Epidemiol. 2008;168:355–7. doi: 10.1093/aje/kwn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allison PD. Imputation of categorical variables with PROC MI. SUGI 30 Proceedings. 2005:1–14. [Google Scholar]