Abstract
Previous evidence suggests that exposure to outdoor air pollution during pregnancy could alter fetal metabolic function, which could increase the risk of obesity in childhood. However, to our knowledge, no epidemiologic study has investigated the association between prenatal exposure to air pollution and indicators of fetal metabolic function. We investigated the association between maternal exposure to nitrogen dioxide and fine particulate matter (aerodynamic diameter ≤2.5 µm) and umbilical cord blood leptin and adiponectin levels with mixed-effects linear regression models among 1,257 mother-infant pairs from the Maternal-Infant Research on Environmental Chemicals (MIREC) Study, conducted in Canada (2008–2011). We observed that an interquartile-range increase in average exposure to fine particulate matter (3.2 µg/m3) during pregnancy was associated with an 11% (95% confidence interval: 4, 17) increase in adiponectin levels. We also observed 13% (95% confidence interval: 6, 20) higher adiponectin levels per interquartile-range increase in average exposure to nitrogen dioxide (13.6 parts per billion) during pregnancy. Significant associations were seen between air pollution markers and cord blood leptin levels in models that adjusted for birth weight z score but not in models that did not adjust for birth weight z score. The roles of prenatal exposure to air pollution and fetal metabolic function in the potential development of childhood obesity should be further explored.
Keywords: adiponectin, air pollution, birth cohort, umbilical cord blood, leptin, maternal exposure, pregnancy
The prevalence of childhood obesity has increased dramatically in the past 30 years, having more than doubled in children and quadrupled in adolescents in the United States (1). This trend has emerged as a major public health concern because obesity is associated with lifelong health consequences, such as the development of type 2 diabetes mellitus and cardiovascular disease (2). One mechanism proposed for the increase in childhood obesity is that exposure to environmental factors such as outdoor air pollution might alter metabolic function, predisposing affected children to the development of obesity (2, 3). Metabolic function can be evaluated by measuring blood levels of leptin and adiponectin, 2 adipocyte-secreted hormones commonly termed adipokines that are important in energy, glucose, and lipids homeostasis (4, 5). Elevated adipokine levels in cord blood are considered potential predictors of the early development of obesity (6–9) and have been associated with higher birth weight (10) and being born large for gestational age (11).
Investigators have examined the relationship of cord blood leptin and adiponectin levels with childhood obesity-related outcomes. Among 80 Mexican-American children in the CHAMACOS birth cohort, leptin levels were positively related to body mass index (BMI) throughout the childhood period (9). Although the relationship between cord blood adiponectin and childhood BMI is less clear, higher adiponectin levels at birth seem to be related to increases in central adiposity and elevated lipid levels during infancy (7, 9). Recently, higher levels of arsenic, lead, cadmium, and phthalate metabolites measured in blood or urine were associated with elevated leptin levels in umbilical cord blood (12–14). However, to our knowledge, the association between prenatal exposure to air pollution and cord blood adipokine levels has not yet been investigated.
Associations between prenatal exposure to air pollution and adverse birth outcomes have been previously reported (15–18). In addition, previous evidence suggests that exposure to ambient air pollution during pregnancy could increase the risk of obesity in childhood (19), although evidence remains sparse. For example, in previous laboratory studies, investigators have shown that prenatal or early-life exposure to air pollution is associated with weight gain, increases in fat mass, and increases in measures of adiposity in offspring mice (20, 21). This might occur when in utero exposure to air pollution inappropriately activates receptors of genes important to adipocyte differentiation and regulation of metabolic efficiency (2, 20). A better understanding of the association between prenatal environmental exposures and metabolic function at birth could provide further insight into the mechanism linking such exposures to childhood obesity.
The aim of the present study was to investigate the associations between maternal exposure to ambient air pollution and umbilical cord blood levels of leptin and adiponectin among the cohort of mother-infant pairs enrolled in the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. We used measurements of nitrogen dioxide and particulate matter with an aerodynamic diameter less than or equal to 2.5 µm (PM2.5).
METHODS
Study population
The MIREC Study has been described in detail previously (22). Briefly, a total of 2,001 women were recruited from 10 sites across Canada (Vancouver, Edmonton, Winnipeg, Toronto, Hamilton, Sudbury, Kingston, Ottawa, Montreal, and Halifax). Women were eligible for inclusion in the study if they were in the first trimester of pregnancy (<14 weeks of gestation), 18 years of age or older, able to communicate in French or English, and planning to deliver at a local participating hospital. Women were excluded from the study if they had any serious medical complications or if there were known fetal or chromosomal anomalies in the current pregnancy (22). Ethics approval for the present study was received from Health Canada and each participating study site.
Women consented to participate and provided biospecimens during scheduled visits to the study clinics during each trimester of pregnancy. Eighteen women withdrew and asked that all their data be destroyed. Of the 1,983 subjects remaining in the study, 1,363 had infants with a cord blood sample and consented to having biospecimens stored in the MIREC Biobank. An additional 106 subjects were excluded, for the following reasons: multiple birth, preterm delivery, cord blood sample unsuitable for analysis, missing data for any of the exposures, or unknown infant sex. The final sample size for the present analysis was 1,257.
Exposure assessment
Information about each participant's residential location (the first 3 characters of the 6-digit postal code) was collected using questionnaires completed in the first and third trimesters. We obtained the population-weighted latitude and longitude coordinates for each participant's 3-digit postal code during the first- and third-trimester visits using Postal Code Conversion File Plus software, version 5K (Statistics Canada, Ottawa, Ontario, Canada) (23). These locations were used to estimate the geographic location of each residence during pregnancy. There were 458 different geographic units among participants' 3-digit postal codes, with an average size of 10.8 km2 (range, 0.6–32.4 km2). Prenatal exposure to PM2.5 was assigned to women's residential histories based on monthly surfaces of a North American land use regression (LUR) model that incorporated observations from fixed-site monitoring stations and satellite-derived estimates of PM2.5 (24, 25). A Bayesian maximum entropy interpolation method (26) was used to create a spatiotemporal prediction model of the space-time residuals from the LUR model that were added to the LUR prediction estimates. The normalized cross-validated R2 value for the PM2.5 LUR model was 0.53 for Canada (25).
In addition, we used data from an updated national LUR model, including satellite nitrogen dioxide estimates and geographic predictors (27) to obtain information on maternal residential exposure to nitrogen dioxide. To capture fine-scale variation in vehicle emissions, kernel density functions describing densities of roadways were incorporated into the LUR model predictions. This model explained 73% of the variation in nitrogen dioxide measurements for 2006, with a root mean square error of 2.9 parts per billion. We used fixed-site monitoring stations to temporally scale nitrogen dioxide estimates to capture trimester-specific estimates (27–29). Exposure was assigned based on the monthly surfaces in which a participant spent most of her pregnancy. These models allowed us to estimate exposure for each study subject across each trimester of pregnancy while accounting for residential mobility during pregnancy. Whole-pregnancy exposure estimates for nitrogen dioxide and PM2.5 were obtained by averaging trimester-specific estimates.
Fetal markers of metabolic function
As part of the MIREC Study, stored umbilical cord blood samples were analyzed to measure levels of leptin and adiponectin using Meso Scale Discovery immunoassay kits (Meso Scale Diagnostics, Rockville, Maryland) at Mount Sinai Laboratory (Toronto, Ontario, Canada). Repeated analysis was performed on all samples with a coefficient of variation greater than 15% (12). The inter- and intraassay coefficients of variation, respectively, were 11.8% and 9.3% for leptin and 8% and 9% for adiponectin. All samples were above the assay's limits of detection.
Covariates
Covariates were identified as potential confounders based on a priori knowledge of their relationships with the exposures and outcomes (12, 14–16). Covariates were assessed during study clinic visits throughout pregnancy or at delivery, and included maternal age at delivery (≤24, 25–29, 30–34, or ≥35 years), prepregnancy BMI (weight (kg)/height (m)2), using categories set by the World Health Organization (30), gestational weight gain according to the Institute of Medicine's recommendations (inadequate, adequate, or excessive) (31), parity (nulliparous or parous), maternal educational level (high school diploma or less, trade school or some college, undergraduate university degree, or graduate university degree), annual household income (in Canadian dollars; ≤30,000, 30,001–50,000, 50,001–100,000, or >100,000), ethnicity (white or nonwhite), maternal smoking (never smoked or quit before pregnancy, quit smoking when pregnancy was confirmed, or current smoker), infant sex, and birth weight z score (which was used as a surrogate of fetal fat mass) (12–14). Previous literature has shown associations between outdoor air pollution and low birth weight (15), and size at birth has been shown to be positively associated with cord blood leptin levels (8). Thus, birth weight z score could be in the causal pathway between outdoor air pollution and cord blood leptin levels. For this reason, the main analyses were conducted with and without adjustment for birth weight z score.
Statistical analyses
We first evaluated whether there were differences in leptin and adiponectin levels by infant sex using a t test of significance with a P value of 0.05. Mixed-effects linear regression models were used to evaluate the associations between average prenatal exposure to ambient air pollutants and natural log-transformed leptin and adiponectin concentrations. The natural log transformation was used to normalize the distributions of adipokine levels (11, 14, 32). Mixed-effects models with random intercepts across the 10 communities and across 3-digit postal codes were used to account for potential clustering of the outcome on a small-scale spatial level. Air pollution exposures (PM2.5 and nitrogen dioxide) were evaluated in separate models as well as in a joint model. Results are presented as the percentage change (accompanied by the 95% confidence interval) in plasma leptin and adiponectin concentrations per interquartile-range (IQR) increase in average exposure to PM2.5 and nitrogen dioxide. We also modeled PM2.5 and nitrogen dioxide as categorical variables (quartiles) to assess potential nonlinearity of exposure-outcome relationships with adipokine levels (32). Natural cubic splines with 3 degrees of freedom were also used to further characterize the functional form of the relationship between ambient air pollutants and adipokine concentrations.
Evaluation of confounding in the multivariable models was done using a backward deletion approach (33); this was accomplished by adjusting for all potential confounders and then removing the least significant confounding variables one by one as long as the total proportional change in the air pollutant estimate compared with the fully adjusted model was less than 10%. Covariates that were not confounders but increased the precision of the estimates were kept in the final model (33). Sensitivity analyses were conducted by excluding women with gestational diabetes (n = 32) and women with impaired glucose tolerance (n = 44) and by conducting distinct analyses for each trimester-specific exposure variable. Gestational diabetes and impaired glucose tolerance were defined in accordance with guidelines from the Canadian Diabetes Association and the Society of Obstetricians and Gynaecologists of Canada as described in a previous publication (34).
We also evaluated effect modification of infant sex by including a term for interaction between infant sex and each exposure. In addition, analyses were stratified by infant sex because in utero exposure to environmental contaminants might be associated with cord blood adipokine levels in a sex-dependent manner (12). All models for leptin and adiponectin analyses adjusted for maternal age, parity, prepregnancy BMI, and infant sex. We performed the analyses with and without adjustment for birth weight z score. Analyses were performed using Stata, version 12.1 (StataCorp LP, College Station, Texas), and R, version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The study participants were predominantly white and ranged in age from 19 years to 48 years at recruitment. The majority had a university degree and had a household income greater than $50,000 (Table 1). Leptin concentrations ranged from 0.086 ng/mL to 243 ng/mL, and adiponectin concentrations ranged from 0.19 µg/mL to 239 µg/mL. Leptin concentrations were significantly higher among female infants (median, 16.0 ng/mL; IQR, 26.3 ng/mL) than among male infants (median, 8.7 ng/mL; IQR, 3.6 ng/mL). There were no differences in adiponectin concentrations by infant sex. Mean levels of nitrogen dioxide and PM2.5 were 14.4 (standard deviation, 7.6) parts per billion and 7.7 (standard deviation, 1.9) µg/m3, respectively. Nitrogen dioxide and PM2.5 were moderately correlated (Pearson correlation coefficient = 0.49).
Table 1.
Characteristics of a Sample of Participants (n = 1,257) in the Maternal-Infant Research on Environmental Chemicals Study, Canada, 2008–2011
| Characteristic | No. | % | Mean (SD) |
|---|---|---|---|
| Maternal age, years | 33.1 (5.0) | ||
| Prepregnancy body mass indexa | 24.9 (5.4) | ||
| Gestational weight gain | |||
| Inadequate | 212 | 18.3 | |
| Adequate | 342 | 29.5 | |
| Excessive | 605 | 52.2 | |
| Missing | 98 | ||
| Nulliparous | 726 | 57.8 | |
| Educational level | |||
| High school diploma or less | 104 | 8.3 | |
| Trade school or some college | 361 | 28.7 | |
| Undergraduate university degree | 478 | 38.0 | |
| Graduate university degree | 312 | 24.8 | |
| Missing | 2 | ||
| Household income, Canadian dollars | |||
| ≤30,000 | 91 | 7.2 | |
| 30,001–50,000 | 117 | 9.3 | |
| 50,001–100,000 | 514 | 40.9 | |
| >100,000 | 487 | 38.7 | |
| Missing | 48 | ||
| White | 1,087 | 86.5 | |
| Maternal smoking | |||
| Never smoked or quit before pregnancy | 1,113 | 88.5 | |
| Quit smoking when pregnancy confirmed | 79 | 6.3 | |
| Current smoker | 65 | 5.2 | |
| Male infant sex | 672 | 53.5 | |
| Birth weight, g | 3,533.7 (453.0) | ||
| Cord blood leptin, ng/mL | 19.9 (25.5) | ||
| Cord blood adiponectin, µg/mL | 18.2 (12.1) |
a Body mass index was calculated as weight (kg)/height (m)2.
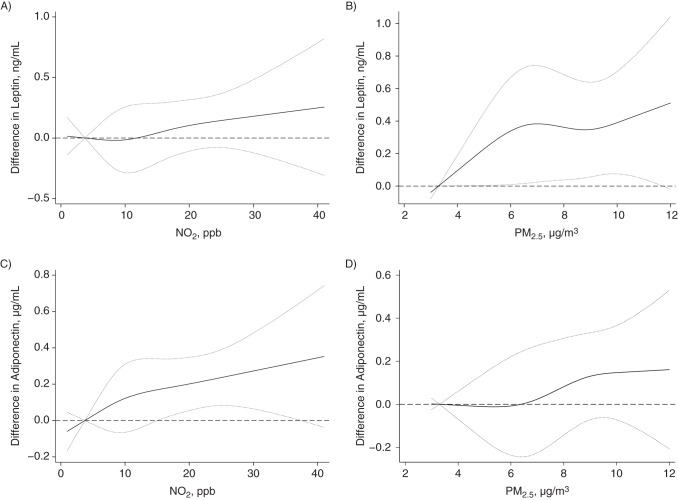
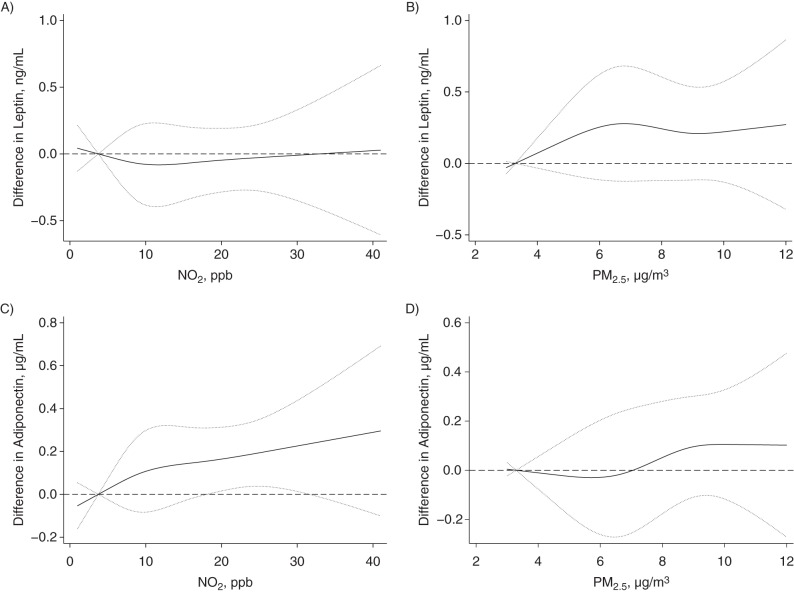
PM2.5 appeared to be positively associated with umbilical cord blood leptin levels in models adjusting for birth weight z score (Table 2). For example, we found that an IQR increase (3.2 µg/m3) in PM2.5 was associated with 11% (95% confidence interval (CI): 1, 21) higher leptin levels. We also found 12% (95% CI: 2, 23) higher leptin levels associated with an IQR (13.6 parts per billion) increase in nitrogen dioxide. However, most associations between PM2.5 (per IQR increase, 3%, 95% CI: −8, 14) or nitrogen dioxide (per IQR increase, 3%, 95% CI: −9, 15) and umbilical cord blood leptin levels decreased and were not statistically significant in models that did not adjust for birth weight z score (Table 3). Figures 1 and 2 show the natural cubic spline representations of nitrogen dioxide and PM2.5, suggesting that the adjusted associations (with and without adjustment for birth weight z score) with leptin and adiponectin levels were approximately linear, except for the association between PM2.5 and leptin levels when no adjustment was made for birth weight z score.
Table 2.
Associations Between Percentage Change in Umbilical Cord Blood Leptin and Adiponectin Levelsa and Exposure to PM2.5 and NO2 During Pregnancy by Quartile and Interquartile-Range Increase, With Adjustment for Birth Weight z Score, Maternal-Infant Research on Environmental Chemicals Study, Canada, 2008–2011
| Pollutant and Quartile of Concentration | Leptin |
Adiponectin |
||
|---|---|---|---|---|
| % Change | 95% CI | % Change | 95% CI | |
| PM2.5, µg/m3 | ||||
| Quartile 1 (3.3–6.0) | 0 | Referent | 0 | Referent |
| Quartile 2 (6.0–8.3) | 26 | 10, 42 | 19 | 8, 30 |
| Quartile 3 (8.3–9.2) | 14 | −2, 31 | 13 | 2, 24 |
| Quartile 4 (9.2–11.6) | 22 | 6, 38 | 19 | 9, 30 |
| IQR (3.2) | 11 | 1, 21 | 11 | 4, 17 |
| NO2, ppb | ||||
| Quartile 1 (3.7–7.1) | 0 | Referent | 0 | Referent |
| Quartile 2 (7.1–13.7) | −5 | −22, 11 | 12 | 1, 23 |
| Quartile 3 (13.7–20.7) | 5 | −12, 23 | 14 | 2, 26 |
| Quartile 4 (20.7–41.4) | 11 | −6, 28 | 22 | 11, 34 |
| IQR (13.6) | 12 | 2, 23 | 13 | 6, 20 |
| Multipollutant model | ||||
| IQR (3.2) increase in PM2.5 | 0 | −13, 13 | 8 | 0, 17 |
| IQR (13.6) increase in NO2 | 12 | 1, 25 | 9 | 1, 17 |
Abbreviations: CI, confidence interval; IQR, interquartile range; NO2, nitrogen dioxide; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 µm; ppb, parts per billion.
a Adjusted for maternal age, parity, prepregnancy body mass index, birth weight z score, and infant sex.
Table 3.
Associations Between Percentage Change in Umbilical Cord Blood Leptin and Adiponectin Levelsa and Exposure to PM2.5 and NO2 During Pregnancy by Quartile and Interquartile-Range Increase, Without Adjustment for Birth Weight z Score, Maternal-Infant Research on Environmental Chemicals Study, Canada, 2008–2011
| Pollutant and Quartile of Concentration | Leptin |
Adiponectin |
||
|---|---|---|---|---|
| % Change | 95% CI | % Change | 95% CI | |
| PM2.5, µg/m3 | ||||
| Quartile 1 (3.3–6.0) | 0 | Referent | 0 | Referent |
| Quartile 2 (6.0–8.3) | 18 | 1, 36 | 17 | 6, 28 |
| Quartile 3 (8.3–9.2) | 5 | −12, 23 | 11 | 0.2, 22 |
| Quartile 4 (9.2–11.6) | 10 | −7, 28 | 17 | 6, 28 |
| IQR (3.2) | 3 | −8, 14 | 9 | 2, 16 |
| NO2, ppb | ||||
| Quartile 1 (3.7–7.1) | 0 | Referent | 0 | Referent |
| Quartile 2 (7.1–13.7) | −11 | −28, 7 | 10 | −1, 22 |
| Quartile 3 (13.7–20.7) | 0 | −19, 18 | 12 | 1, 24 |
| Quartile 4 (20.7–41.4) | −4 | −23, 14 | 18 | 6, 30 |
| IQR (13.6) | 3 | −9, 15 | 13 | 6, 20 |
| Multipollutant model | ||||
| IQR (3.2) increase in PM2.5 | −3 | −17, 10 | 7 | −2, 15 |
| IQR (13.6) increase in NO2 | 5 | −9, 18 | 8 | −1, 17 |
Abbreviations: CI, confidence interval; IQR, interquartile range; NO2, nitrogen dioxide; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 µm; ppb, parts per billion.
a Adjusted for maternal age, parity, prepregnancy body mass index, and infant sex.
Figure 1.
Associations between levels of adipokines and prenatal exposure to pollutants, adjusted for birth weight z score, Maternal-Infant Research on Environmental Chemicals Study, Canada, 2008–2011. A) Nitrogen dioxide (NO2) and umbilical cord blood leptin levels; B) particulate matter with an aerodynamic diameter less than or equal to 2.5 µm (PM2.5) and cord blood leptin levels; C) nitrogen dioxide and cord blood adiponectin levels; D) PM2.5 and cord blood adiponectin levels. Associations were adjusted for maternal age, parity, prepregnancy body mass index, birth weight z score, and infant sex. Adjustments for birth weight z score were fitted using a natural cubic spline with 3 degrees of freedom. Dotted lines, 95% confidence intervals. ppb, parts per billion.
Figure 2.
Associations between levels of adipokines and prenatal exposure to pollutants, without adjustment for birthweight z score, Maternal-Infant Research on Environmental Chemicals Study, Canada, 2008–2011. A) Nitrogen dioxide (NO2) and cord blood leptin levels; B) particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5) and cord blood leptin levels; C) NO2 and cord blood adiponectin levels; D) PM2.5 and cord blood adiponectin levels. Associations were adjusted for maternal age, parity, prepregnancy body mass index, and infant sex. Dotted lines, 95% confidence intervals. ppb, parts per billion.
Some associations were found for adiponectin levels (Table 2). We observed 11% (95% CI: 4, 17) higher adiponectin levels for each IQR increase in PM2.5. Statistically significant differences were also observed when comparing the 3 upper quartiles of PM2.5 exposure with the lowest (quartile 1) exposure quartile. Higher levels of adiponectin were found for study participants in higher quartiles (quartiles 2–4) compared with the lowest quartile (quartile 1) of nitrogen dioxide exposure and increased monotonically across quartiles. The latter finding is supported by the graphical representations of the natural cubic spline of nitrogen dioxide on adiponectin levels, as shown in Figures 1 and 2. In models not adjusting for birth weight z score, associations between PM2.5 (per IQR increase, 9%, 95% CI: 2, 16) or nitrogen dioxide (per IQR increase, 13%, 95% CI: 6, 20), and cord blood adiponectin levels decreased slightly but remained statistically significant (Table 3). In addition, multipollutant models (adjusting for PM2.5 and nitrogen dioxide) showed that only nitrogen dioxide remained independently and significantly positively associated with levels of leptin and adiponectin when adjusting for birth weight z score (Tables 2 and 3).
Figures 3 and 4 show results of the analyses for leptin and adiponectin, respectively, stratified by infant sex and adjusted for birth weight z score. No clear patterns can be identified from these figures, although the association between air pollution and adiponectin appeared greater among female infants than among male infants. In addition, results for effect modification by infant sex were not statistically significant for any of the associations (P for interaction > 0.28; results not shown). In the sensitivity analyses, similar estimates were observed for all associations when excluding study participants with gestational diabetes and impaired glucose tolerance (results not shown). Additional adjustment for annual household income in the multivariable models provided results similar to the ones obtained in the main analysis, suggesting that this variable, a proxy for socioeconomic status, had little influence on the estimates (Web Table 1, available at http://aje.oxfordjournals.org/). Results for trimester-specific periods of exposure to PM2.5 and nitrogen dioxide revealed that estimates were similar to those obtained for pregnancy average exposures (Table 4). In addition, exposures to PM2.5 (Pearson correlation coefficients ranged from 0.72 to 0.75) and nitrogen dioxide (Pearson correlation coefficients ranged from 0.59 to 0.90) were highly correlated between trimesters (results not shown).
Figure 3.
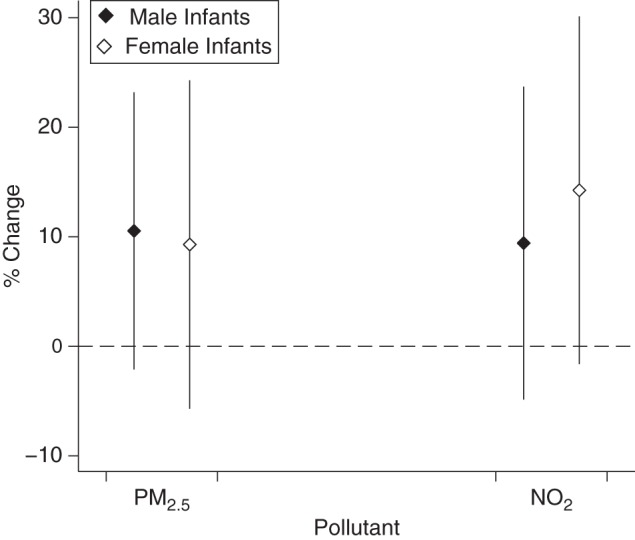
Associations between percentage change in cord blood leptin levels and an interquartile-range increase in exposure to particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5) and nitrogen dioxide (NO2) during pregnancy, by infant sex, Maternal-Infant Research on Environmental Chemicals Study, Canada, 2008–2011. Associations were adjusted for maternal age, parity, prepregnancy body mass index, and birth weight z score. Bars, 95% confidence interval.
Figure 4.
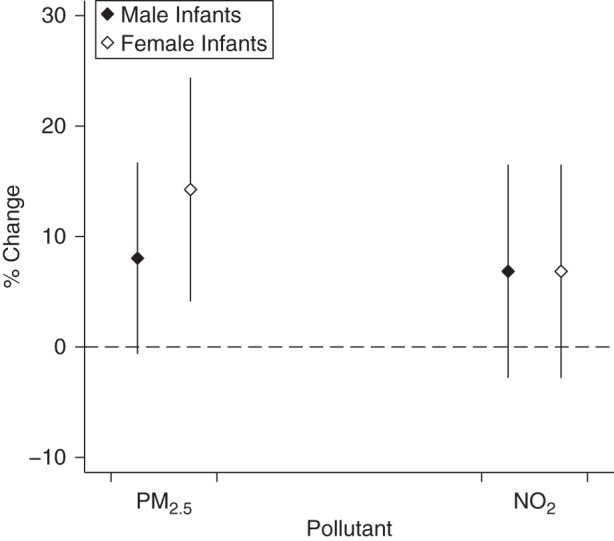
Associations between percentage change in cord blood adiponectin levels and an interquartile-range increase in exposure to particulate matter with an aerodynamic diameter less than or equal to 2.5 µm (PM2.5) and nitrogen dioxide (NO2) during pregnancy, by infant sex, Maternal-Infant Research on Environmental Chemicals Study, Canada, 2008–2011. Associations were adjusted for maternal age, parity, prepregnancy body mass index, and birth weight z score. Bars, 95% confidence intervals.
Table 4.
Associationsa Between Percentage Change in Cord Blood Leptin and Adiponectin Levels and an Interquartile-Range Increase in PM2.5 or Nitrogen Dioxide by Trimester of Pregnancy, Maternal-Infant Research on Environmental Chemicals Study, Canada, 2008–2011
| Pollutant and Trimester of Pregnancy | IQR | Leptin |
Adiponectin |
||
|---|---|---|---|---|---|
| % Change | 95% CI | % Change | 95% CI | ||
| PM2.5, µg/m3 | |||||
| First trimester | 3.4 | 12 | 3, 22 | 13 | 7, 19 |
| Second trimester | 3.4 | 9 | 0, 19 | 7 | 0, 13 |
| Third trimester | 3.3 | 7 | 0, 13 | 8 | 2, 15 |
| NO2, ppb | |||||
| First trimester | 13.1 | 12 | 2, 22 | 10 | 3, 16 |
| Second trimester | 12.1 | 11 | 1, 21 | 9 | 2, 15 |
| Third trimester | 12.3 | 10 | 1, 21 | 10 | 3, 16 |
Abbreviations: CI, confidence interval; IQR, interquartile range; NO2, nitrogen dioxide; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 µm; ppb, parts per billion.
a Adjusted for maternal age, parity, prepregnancy body mass index, birth weight z score, and infant sex.
DISCUSSION
Our study presents novel findings for ambient levels of air pollution and fetal metabolic function using measurements of leptin and adiponectin in cord blood. We found evidence that greater prenatal exposure to outdoor PM2.5 and nitrogen dioxide was associated with higher cord blood levels of adiponectin. Greater exposure to air pollution during the whole pregnancy was associated with higher levels of cord blood leptin when adjusting for birth weight z score, but no associations were observed when birth weight was not accounted for in the models. We found no evidence of significant effect modification by infant sex, although the associations between air pollution and adiponectin appeared greater in female infants than in male infants.
Prenatal exposure to air pollution is associated with low birth weight (15). Our findings raise questions regarding the role of air pollution in fetal growth, because high leptin levels in cord blood are usually correlated with higher birth weight (6). Our findings suggest positive associations between air pollution markers and cord blood leptin levels when adjusting for birth weight z score. However, no associations were observed when birth weight was removed from the models. In the present study, birth weight z score was used as a surrogate for fetal fat mass (12–14) because the latter is a determinant of cord blood leptin (35). Recent evidence suggests that a lower birth weight z score is associated with a higher proportion of abdominal fetal fat mass (36). Because adjusting for birth weight z score has the limitation that it does not distinguish between fat mass and skeletal growth, future investigations that can adjust solely for fetal fat mass could offer an even further refined ability to disentangle the relationships between air pollution and cord blood leptin levels. In addition, air pollution exposure during pregnancy has been previously associated with decreased fetal growth measures (17, 18). Therefore, prenatal exposure to air pollution could potentially affect skeletal growth and, simultaneously, adipose tissues, suggesting that air pollution could affect fetal growth through 2 different pathways. The biological mechanisms underlying these potential pathways require further clarification.
To our knowledge, no previous epidemiologic studies have investigated the association between exposure to air pollution during pregnancy and cord blood adipokine levels. The scientific literature has evaluated mainly the association between perinatal exposure to air pollution and measures of adiposity, which are known to be positively correlated with adipokine levels (37). One previous laboratory study showed that prenatal exposure to polycyclic aromatic hydrocarbons, a family of air pollutants generated during incomplete combustion, was associated with weight gain and increases in fat mass in offspring mice. This occurred through an alteration of adipose gene expression and DNA methylation in genes important to adipocyte differentiation after exposure to polycyclic aromatic hydrocarbons among pregnant dams (20). In an epidemiologic study conducted in New York, New York, Rundle et al. (19) reported results in line with these findings, showing that children of mothers in the highest category of exposure to polycyclic aromatic hydrocarbons during gestation had an increased risk of being obese at 5 and 7 years of age. In another laboratory analysis, investigators found that early life exposure to PM2.5 in mice was associated with increased weight gain due to increases in measures of adiposity (21). We found only 1 epidemiologic study of the association between air pollution and blood leptin levels, and those authors reported a positive association between annual mean ambient black carbon exposure and leptin levels in older adults (32).
In a few studies, investigators have shown that cord blood leptin and adiponectin levels are associated with obesity-related outcomes during childhood (9, 11). In addition, it appears that children with higher cord blood adiponectin levels have a rapid decrease in adiponectin levels during infancy (11). This might be of concern, because lower adiponectin levels have been associated with both metabolic syndrome and type 2 diabetes mellitus (38, 39). In light of these findings, it is essential to better understand prenatal factors that can affect cord blood adipokine levels, which might have adverse long-term health consequences.
In models that adjusted for both PM2.5 and nitrogen dioxide, we found that nitrogen dioxide remained independently and significantly positively associated with levels of leptin and adiponectin. It is important to note that the 2 exposure models of air pollution used in this study had very different spatial scales and were only moderately correlated (r = 0.49), likely reflecting different aspects of residential exposure to air pollution. Nitrogen dioxide is a strong marker of local traffic-related air pollution, and PM2.5 reflects a more heterogeneous mixture of regional pollution. This finding could also be attributed to differences in degree of exposure measurement error or exposure variability. Therefore, the validity of the findings based on the multipollutant model can be questioned, and this area requires further clarification.
The strengths of our study include the use of a study population of pregnant women for whom a broad range of covariate data were available and who were located across Canada, which allowed the exploration of different exposure levels. In addition, cord blood leptin and adiponectin data were available for a relatively large proportion of the initial study population. The use of spatiotemporal models for nitrogen dioxide and PM2.5 allowed us to calculate average exposures over the duration of pregnancy with a minimal amount of missing data.
Limitations of our study include potential misclassification of exposure to the air pollutants. Air pollution measures were assigned to the population-weighted geographic coordinates of the 3-digit postal codes in which mothers lived during pregnancy and were not based on the actual residential address. In addition, we did not have information about work locations or time spent at home, which could have improved the accuracy of exposure estimates (40). In our study, these systematic nondifferential exposure assessment errors likely resulted in underestimation of associations. We also relied on self-reported prepregnancy weight and height, which might have resulted in underestimates of BMI. Given the small sample size, this study may have had insufficient statistical power to detect any interactions. The generalizability of our findings might be limited because the subjects in our cohort were mostly white, had higher socioeconomic status, had lower BMI, and were less likely to smoke than members of the general population.
In conclusion, we found that exposure to outdoor air pollution during pregnancy was associated with higher cord blood adiponectin levels. Significant associations were seen between air pollution markers and cord blood leptin levels in models adjusted for birth weight z score but not in models unadjusted for birth weight z score. Our findings add evidence to the growing body of literature examining the association between prenatal environmental exposures and fetal metabolic development. Future studies should clarify the relationships between prenatal exposure to air pollution, adipokine levels at birth, fetal fat mass, and the evolution of weight and metabolic status during childhood.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Air Health Effects Division, Health Canada, Ottawa, Ontario, Canada (Eric Lavigne, Markey Johnson, Liu Sun); Perinatal Epidemiology Research Unit, Dalhousie University, Halifax, Nova Scotia, Canada (Jillian Ashley-Martin, Linda Dodds); Population Studies Division, Health Canada, Ottawa, Ontario, Canada (Tye E. Arbuckle, Mandy Fisher); College of Public Health and Human Sciences, Oregon State University, Corvallis, Oregon (Perry Hystad); Department of Sociology, University of New Brunswick, Fredericton, New Brunswick, Canada (Dan L. Crouse); Yale School of Public Health, Yale University, New Haven, Connecticut (Adrienne S. Ettinger); University of Montreal, Montreal, Quebec, Canada (Gabriel D. Shapiro; Maryse F. Bouchard); Centre Hospitalier Universitaire Sainte-Justine Research Centre, Montreal, Quebec, Canada (Gabriel D. Shapiro); Endocrinology and Genomics, Laval University Medical Center, Quebec City, Quebec, Canada (Anne-Sophie Morisset); Faculty of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada (Shayne Taback); Department of Obstetrics and Gynecology, McGill University, Montreal, Quebec, Canada (Patricia Monnier); Laval University, Quebec City, Quebec, Canada (Renée Dallaire); and Department of Obstetrics and Gynecology, University of Sherbrooke, Sherbrooke, Quebec, Canada (William D. Fraser).
This study was supported by a grant from the Canadian Diabetes Association (grant OG-2-11-3424). The Maternal-Infant Research on Environmental Chemicals (MIREC) Study was funded by the Chemicals Management Plan of Health Canada, the Canadian Institutes of Health Research (grant MOP-81285), and the Ontario Ministry of the Environment.
We thank the MIREC Study Group, the MIREC Biobank, and the MIREC research staff for their dedication. We also thank Drs. Dave Stieb and Scott Weichenthal for providing comments on an earlier version of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. J Am Med Assoc. 2014;3118:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 suppl):S50–S55. [DOI] [PubMed] [Google Scholar]
- 3.Newbold RR, Padilla-Banks E, Jefferson WN et al. Effects of endocrine disruptors on obesity. Int J Androl. 2008;312:201–208. [DOI] [PubMed] [Google Scholar]
- 4.Tsai PJ, Yu CH, Hsu SP et al. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol (Oxf). 2004;611:88–93. [DOI] [PubMed] [Google Scholar]
- 5.Cortelazzi D, Corbetta S, Ronzoni S et al. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf). 2007;663:447–453. [DOI] [PubMed] [Google Scholar]
- 6.Karakosta P, Chatzi L, Plana E et al. Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2011;252:150–163. [DOI] [PubMed] [Google Scholar]
- 7.Mantzoros CS, Rifas-Shiman SL, Williams CJ et al. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;1232:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retnakaran R, Ye C, Hanley AJ et al. Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birth weight among women without gestational diabetes mellitus. CMAJ. 2012;18412:1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volberg V, Heggeseth B, Harley K et al. Adiponectin and leptin trajectories in Mexican-American children from birth to 9 years of age. PLoS One. 2013;810:e77964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuda J, Yokota I, Iida M et al. Serum leptin concentration in cord blood: relationship to birth weight and gender. J Clin Endocrinol Metab. 1997;825:1642–1644. [DOI] [PubMed] [Google Scholar]
- 11.Cinaz P, Sen E, Bideci A et al. Plasma leptin levels of large for gestational age and small for gestational age infants. Acta Paediatr. 1999;887:753–756. [DOI] [PubMed] [Google Scholar]
- 12.Ashley-Martin J, Dodds L, Arbuckle TE et al. Maternal blood metal levels and fetal markers of metabolic function. Environ Res. 2015;136:27–34. [DOI] [PubMed] [Google Scholar]
- 13.Ashley-Martin J, Dodds L, Arbuckle TE et al. A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ Health. 2014;13:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gossai A, Lesseur C, Farzan S et al. Association between maternal urinary arsenic species and infant cord blood leptin levels in a New Hampshire pregnancy cohort. Environ Res. 2015;136:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dadvand P, Parker J, Bell ML et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;1213:267–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stieb DM, Chen L, Eshoul M et al. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. [DOI] [PubMed] [Google Scholar]
- 17.van den Hooven EH, Pierik FH, de Kluizenaar Y et al. Air pollution exposure during pregnancy, ultrasound measures of fetal growth, and adverse birth outcomes: a prospective cohort study. Environ Health Perspect. 2012;1201:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estarlich M, Ballester F, Aguilera I et al. Residential exposure to outdoor air pollution during pregnancy and anthropometric measures at birth in a multicenter cohort in Spain. Environ Health Perspect. 2011;1199:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rundle A, Hoepner L, Hassoun A et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;17511:1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Z, Zhang H, Maher C et al. Prenatal polycyclic aromatic hydrocarbon, adiposity, peroxisome proliferator-activated receptor (PPAR) γ methylation in offspring, grand-offspring mice. PLoS One. 2014;910:e110706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Yavar Z, Verdin M et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;3012:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbuckle TE, Fraser WD, Fisher M et al. Cohort profile: the Maternal-Infant Research on Environmental Chemicals research platform. Paediatr Perinat Epidemiol. 2013;274:415–425. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins R, Peters P. PCCF+ Version 5K User's Guide. Automated Geographic Coding Based on the Statistics Canada Postal Code Conversion Files, Including Postal Codes Through May 2011. Ottawa, Ontario, Canada: Health Analysis Division, Statistics Canada; 2012. [Google Scholar]
- 24.Beckerman BS, Jerrett M, Serre M et al. A hybrid approach to estimating national scale spatiotemporal variability of PM2.5 in the contiguous United States. Environ Sci Technol. 2013;4713:7233–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stieb DM, Chen L, Beckerman BS et al. Associations of pregnancy outcomes and PM2.5 in a national Canadian study. Environ Health Perspect. 2016;1242:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christakos GA. Bayesian maximum-entropy view to the spatial estimation problem. Math Geol. 1990;227:763–776. [Google Scholar]
- 27.Hystad P, Setton E, Cervantes A et al. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect. 2011;1198:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hystad P, Villeneuve PJ, Goldberg MS et al. Exposure to traffic-related air pollution and the risk of developing breast cancer among women in eight Canadian provinces: a case-control study. Environ Int. 2015;74:240–248. [DOI] [PubMed] [Google Scholar]
- 29.Johnson M, Macneill M, Grgicak-Mannion A et al. Development of temporally refined land-use regression models predicting daily household-level air pollution in a panel study of lung function among asthmatic children. J Expo Sci Environ Epidemiol. 2013;233:259–267. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. BMI Classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html Published January 1, 2006 Updated August 7, 2015 Accessed August 7, 2015.
- 31.Institute of Medicine and National Research Council, Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. http://www.ncbi.nlm.nih.gov/books/NBK32813/ Accessed February 26, 2016. [Google Scholar]
- 32.Wang Y, Eliot MN, Kuchel GA et al. Long-term exposure to ambient air pollution and serum leptin in older adults: results from the MOBILIZE Boston study. J Occup Environ Med. 2014;569:e73–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:262–263. [Google Scholar]
- 34.Shapiro GD, Dodds L, Arbuckle TE et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environ Int. 2015;83:63–71. [DOI] [PubMed] [Google Scholar]
- 35.Clapp JF 3rd, Kiess W. Cord blood leptin reflects fetal fat mass. J Soc Gynecol Investig. 1998;56:300–303. [PubMed] [Google Scholar]
- 36.Carlsen EM, Renault KM, Nørgaard K et al. Newborn regional body composition is influenced by maternal obesity, gestational weight gain and the birthweight standard score. Acta Paediatr. 2014;1039:939–945. [DOI] [PubMed] [Google Scholar]
- 37.Marchini G, Fried G, Ostlund E et al. Plasma leptin in infants: relations to birth weight and weight loss. Pediatrics. 1998;1013:429–432. [DOI] [PubMed] [Google Scholar]
- 38.Cruz M, García-Macedo R, García-Valerio Y et al. Low adiponectin levels predict type 2 diabetes in Mexican children. Diabetes Care. 2004;276:1451–1453. [DOI] [PubMed] [Google Scholar]
- 39.Shaibi GQ, Cruz ML, Weigensberg MJ et al. Adiponectin independently predicts metabolic syndrome in overweight Latino youth. J Clin Endocrinol Metab. 2007;925:1809–1813. [DOI] [PubMed] [Google Scholar]
- 40.Nethery E, Leckie SE, Teschke K et al. From measures to models: an evaluation of air pollution exposure assessment for epidemiological studies of pregnant women. Occup Environ Med. 2008;659:579–586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.