Abstract
Objectives:
To investigate the seroprevalence of coexisting autoantibodies among type 1 diabetes mellitus (T1DM) patients, and to look for possible correlations with age at diagnosis, diabetes duration, and glycemic control.
Methods:
This is a cross-sectional study conducted at Aseer Central Hospital, Abha, Kingdom of Saudi Arabia from March 2013 to June 2014. A total of 202 T1DM patients were screened for serum anti-thyroglobulin (TG), anti-thyroid peroxidase (TPO), anti-tissue transglutaminase (aTTG), anti-endomysial (EMA), and anti-cyclic citrullinated peptide (anti-CCP) antibodies along with glycated hemoglobin, and biometric data.
Results:
From the 202 T1DM patients (96 males, and 106 females) (mean age: 11.3 years), 33 (16.3%) were positive for thyroid autoantibodies. Specifically, 19 (9.4%) were positive for TG and 25 (12.8%) were positive for TPO, and 11 were double positive. There were 21 (10.4%) patients that showed a double positive for both aTTG-IgA and EMA, and only one case of T1DM was positive for anti-CCP. No significant correlations were noticed between the presence of autoantibodies and the age at diagnosis, diabetes duration, body mass index, and glycemic control.
Conclusion:
The prevalence of thyroid and celiac disease autoantibodies is high among T1DM patients, while anti-CCP remains low and might be weakly associated with T1DM in the southwestern region of Saudi Arabia. No significant correlation between the age at T1DM diagnosis, duration, and glycemic control, and the presence of autoantibodies was found.
Type 1 diabetes mellitus (T1DM) is one of the most common autoimmune disorders in children and adolescents, characterized by immune auto-reactivity towards insulin-producing β-cells, leading to their destruction. Coexistence of autoimmune diseases, such as celiac disease and autoimmune thyroid diseases with T1DM is well known.1,2 Reports showed approximately 1-10% prevalence of celiac disease, and up to 30% of thyroid autoimmune diseases among patients with T1DM.3,4 The presence of autoantibodies in the serum of patients is the best indicator for estimating the occurrence of clinically apparent autoimmune thyroid diseases.5 Autoimmune thyroiditis is the most common autoimmune disease associated with T1DM.2 The main serum thyroid autoantibodies in autoimmune thyroid diseases are anti-thyroid peroxidase (TPO), anti-thyroglobulin (TG), and anti-thyroid stimulating receptor (TSH) antibodies.3,6 The presence of thyroid autoantibodies in T1DM patients has been reported to be 4 times higher than in normal populations, and increases the risk of developing autoimmune thyroiditis 18 folds in comparison with patients with no antibodies.2,5 In a follow-up study, approximately half of the TIDM patients with elevated anti-TPO developed autoimmune thyroiditis within 3-4 years.7 The International Society for Pediatric and Adolescent Diabetes (ISPAD) recommended investigating anti-thyroid autoantibodies after T1DM diagnosis, in addition to other annual checks in asymptomatic individuals.3 The T1DM patients are significantly more likely to develop celiac disease than non-diabetic subjects.8,9 Two main antibodies have been confirmed for use as a diagnostic tool for celiac disease screening; anti-tissue transglutaminase (aTTG), and anti-endomysial antibodies (EMA). These tests have high sensitivity rate, and patients with high positive titer for aTTG together with positive EMA and HLA DQ2 or DQ8 is most likely to have celiac disease, and diagnosis can be confirmed without intestinal biopsy in the presence of clinical suspicion.10-12 In contrast to celiac disease and autoimmune thyroiditis, rheumatic disease screening is limited, and the suggested association is based on individual cases, or familial studies.13,14 The T1DM autoantibodies among different ethnic groups have been assessed and were shown to be varied.15 There is a shortage of data on the prevalence of autoimmune thyroid diseases among T1DM patients in the southern region of Saudi Arabia. The aim of the current study is to investigate the prevalence of thyroid, celiac and rheumatoid disease autoantibodies among T1DM patients in the Aseer region and to look for possible correlation with different factors such as age at diagnosis, diabetes duration and glycemic control.
Methods
A total of 202 children with T1DM attending the Diabetes Center at Aseer Central Hospital, the main tertiary hospital in Aseer region in southwest Saudi Arabia were enrolled in the study from March 2013 to June 2014. Approval for the study was obtained from the institutional research ethical committee. Consents for the research proposal and objectives were provided by the patients’ parents, or by the adult patients themselves. The patients underwent clinical examination followed by blood sampling. Sera were then tested for the following antibodies: aTTG; EMA; anti-cyclic citrullinated peptide (anti-CCP); TG, and TPO antibodies in addition to the level of glycated hemoglobin (HbA1c).
The aTTG immunoglobulin (Ig) A antibody
Anti-tissue transglutaminase IgA antibodies were measured in T1DM patients serum samples using commercially available indirect enzyme-linked immunosorbent assay (ELISA) kits (IMTEC, Marine Germany GmbH, Hamburg, Germany). Briefly, serum samples were diluted 1:101 using sample diluents and 100 µl of each of serum sample, controls and calibrators (in duplicate) were added to appropriate ELISA plate wells, and the plates were incubated for 45 minutes at room temperature. At the end of the incubation period, the plates were washed manually 3 times, and 100 µl of enzyme-conjugate mixture was added to all wells and incubated for 30 minutes at room temperature. Then the plates were washed 3 times as previously described, and 100 µl of substrate mixture was added to all wells, and the plates were incubated in the dark for 15 minutes at room temperature. The reaction was stopped by addition of 100 µl stop solution (0.5 M sulfuric Acid [H2SO4]) and the plates were bichromatically read at 450 nm and 620 nm using ELISA reader, HumaReader (HUMAN Diagnostics, Wiesbaden, Germany). The levels of antibodies in the patient’s sera were calculated using an ELISA point to point generated standard curve.
The TG and TPO IgG antibodies
Testing for TG and TPO antibodies was carried out using commercially available indirect ELISA kits (IMTEC, Germany). For both tests, serum samples were diluted 1:101 using sample diluent and 100 µl of each serum sample, controls and calibrators (in duplicate) were added to appropriate ELISA plate wells, and well A1 was used as a blank (100 µl of sample diluent). The plates were incubated at room temperature for one hour, then washed manually 3 times with 300 µl of diluted wash buffer followed by addition of 100 µl of conjugate solution to all wells and incubated for 30 minutes at room temperature. At the end of the incubation period, the plates were washed 3 times, and 100 µl of substrate mixture was added to all wells, and plates were incubated in the dark for 10 minutes at room temperature. The reaction was stopped by addition of 100 µl stop solution (0.5 M H2SO4), and the plates were bichromatically read at 450 nm and 620 nm using ELISA reader Humareader (Human, Germany). The levels of antibodies in patients’ sera were calculated using an ELISA point-to-point generated standard curve.
The anti-CCP IgG antibody
Anti-CCP antibodies were screened using commercially available indirect ELISA kits (IMTEC, Hamburg, Germany). Serum samples were diluted 1:50 using sample diluent and 100 µl of each serum samples, controls and calibrators (in duplicate) were added to appropriate ELISA plate wells, and well A1 was used as a blank (100 µl of sample diluent). The plates were incubated at room temperature for one hour, manually washed 3 times with 300 µl of diluted wash buffer, followed by addition of 100 µl of conjugate solution to all wells and incubated for 30 minutes at room temperature. At the end of the incubation period, the plates were washed 3 times, and 100 µl of substrate mixture was added to all wells, and plates were incubated in the dark for 30 minutes at room temperature. The reaction was stopped by the addition of 100 µl stop solution (0.5 M H2SO4) and the plates were bichromatically read at 450 nm and 620 nm using ELISA reader, Humareader (HUMAN Diagnostics, Wiesbaden, Germany). The levels of antibodies in patient’s sera were calculated using an ELISA point to point generated standard curve.
Anti-endomysial IgA antibody
Anti-endomysial IgG antibody was screened in patients samples using commercially available indirect immunofluorescent test (IMMCO Diagnostics, Buffalo, USA). Patients serum samples were diluted 1:2.5 using sample diluents, and one drop (approximately 50 µl) of controls and diluted patients serum samples were added to appropriate wells on slides and incubated for 30 minutes in a humidity chamber at room temperature. At the end of the incubation period, the slides were washed for 10 minutes in a plastic staining jar using reconstituted washing buffer with gentle agitation from time to time. Subsequently, one drop of fluorescein isothiocyanate (FITC)-anti-IgG conjugate was added to all wells and incubated for 30 minutes in a humidity chamber at room temperature. Then the slides were washed one more time as previously described, and one drop of mounting medium was added and a cover slip applied. The results were read under a fluorescent microscope model Eclipse 50i (Nikon, Tokyo, Japan) at a total magnification of 200x and 400x.
Collected patients’ data and results of antibodies and HbA1c testing and disease duration, age at diagnosis, and biometric data were analyzed using the Statistical Package for Social Sciences Program version 16 (SPSS Inc, Chicago, IL, USA). P<0.05 were considered significant using independent paired t- and Chi square tests.
Results
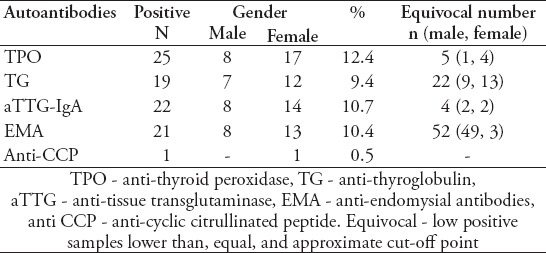
The study involved 202 patients with 96 males (47.5%) and 106 females (52.5%) with T1DM who had been screened for aTTG, EMA, TPO, TG, and anti-CCP antibodies with age range of one to 21 years, and mean age of 11.3 ± 3.9 years. The mean age at T1DM diagnosis was 7 ± 3.7 years (range: one to 17 years). The duration since diagnosis ranged from 0-13 years with a mean of 4.7 ± 3 years. Thirty-three patients (16.3%) with T1DM showed either anti-TPO or anti-TG positive antibodies. The total number of patients with positive anti-TPO antibodies was 25 (12.8%) with 8 males and 17 females, while there were 19 positive anti-TG antibody patients (9.4%), and 12 of them were females. Eleven patients (5.4%) showed a double positive for both anti-TPO or anti-TG antibodies, 7 of them were female and 4 were male (Table 1). The age group with the highest level of autoimmune thyroid antibodies was 10-15 years (11 cases) followed by the 5-10 year age group (7 cases). Twenty-one (10.4%) of 202 T1DM patients showed a positive result for both aTTG-IgA and EMA (13 females and 8 males). Three patients showed low positive results for both tests (low titer for aTTG-IgA and weak positivity for EMA). One patient showed a low positive result for aTTG only, and 2 showed a low positive for EMA antibody alone. All low or weak positive results were not considered as valid positives and were regarded as equivocal (Table 1). The anti-CCP IgG antibody was positive in only one patient with T1DM and when repeated after a 6-month interval was four times higher in titer quantity than the first test. She was 16-years old and had no other positive results for other autoantibodies investigated.
Table 1.
Autoantibodies among type 1 diabetes mellitus patients.
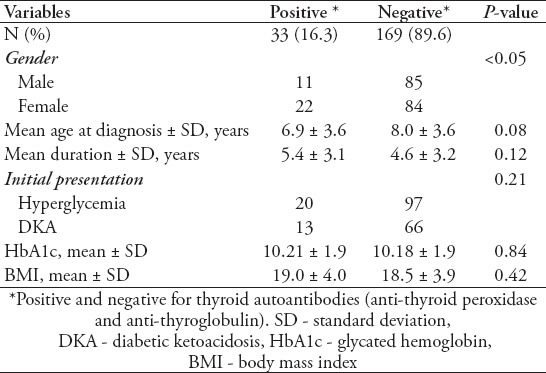
For possible correlations between the presence of autoantibodies and age at diagnosis and diabetic duration, both factors were analyzed along with HbA1c, to monitor the effect of the presence of autoantibodies on glycemic control. No significant variations were found in the initial presentation between positive and negative thyroid and celiac antibodies among T1DM patients. A total of 59.8% of patients in the control non-thyroid T1DM group presented with only hyperglycemia, and 40.2% presented with diabetic ketoacidosis (DKA), and 60.6% (20 of 33) of thyroid positive patients presented with hyperglycemia versus 39.4% (13 of 66) who presented with DKA. No significant variations were noticed in glycemic control, duration of diabetes, and age at presentation in patients with positive autoantibodies for both celiac and thyroid diseases in comparison to negative autoantibodies T1DM patients. Details of initial presentation, disease duration, glycemic control, and body mass index are shown in Tables 2 & 3.
Table 2.
Comparison of type 1 diabetes mellitus patients with positive and negative celiac autoantibodies.
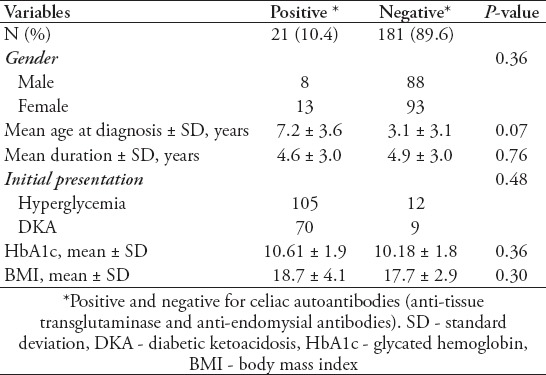
Table 3.
Comparison of type 1 diabetes mellitus patients with positive and negative thyroid autoantibodies.
Discussion
This study reports the high prevalence of autoantibodies in both thyroid and celiac diseases associated with T1DM in patients from the southern region of Saudi Arabia. For thyroid disease, approximately 16.3% patients presented with at least one antibody, 12.4% were significantly positive for anti-TPO and 9.4% for anti-TG. The prevalence of thyroid autoantibodies is similar to the prevalence measured in the Western (Jeddah; 14%)16 but higher than Central KSA (Riyadh; 8.1%).17 The prevalence of T1DM reported in Jordan (9.2%),18 Egypt (12%),19 and Sudan (7.3%)20 showed some variations, and a much higher prevalence was reported in studies of thyroid autoantibodies in T1DM children from Kuwait (24.6%),21 and Libya (31.4%).22 Global variations in prevalence have been shown in different studies. A European study showed positivity of thyroid autoantibody as 18.7% among 219 T1DM patients,7 while a large-cohort multicenter study in Germany and Austria showed that the prevalence of thyroid autoantibodies was 21.6% among T1DM patients.23 This could be explained by genetic differences or environmental factors, such as iodine deficiency, which could have an effect on the thyroid function in some populations. Additionally, autoantibody results have proved to be significantly different depending on the age of the group and the duration of the diabetes.16,23 The Saudi Food and Drug Association (SFDA) recommended iodine supplements to salt to reduce the risk of thyroid dysfunction. In the present study, doubtful low positive results were excluded, since including them alters the prevalence to 21.3%. In contrast to low positive titer, which can be detected in the normal population, high titer of thyroid autoantibodies is significantly related to thyroid autoimmune disorders.24 In general, a high number of T1DM patients have associated autoimmune thyroid diseases and monitoring the thyroid function in this group is required.
From the total number of T1DM patients, 10.4% were double positive for celiac disease for both aTTG and EMA. In contrast to thyroid autoantibodies, which can be in a sub-clinical status for a long time, aTTG and EMA are highly sensitive and specific antibodies and mostly associated with some stage of intestinal abnormality.10-12 All patients with positive EMA in a follow-up study developed celiac disease at some point in the future.25 Both antibodies were recommended for celiac screening in T1DM patients and are a significant predictor of celiac disease.3 A meta-analysis review of data for 26,605 patients estimated a worldwide prevalence of biopsy proven celiac disease among T1DM to be 6%. However, a wide variation was noted as in France it was 1.6%, USA 4.6-7.0%, Italy 3.6-6.6%, Sweden 9-9.7%, and 3.3-4.0% in the United Kingdom.26 This variation could be attributed to the duration of the disease and the age at diagnosis, in addition to genetic susceptibility.27 There was no previous data for celiac disease prevalence in the southern region in Saudi Arabia. Our study showed that celiac disease prevalence in T1DM is 10.4%, which is similar to other national studies. Recent studies of celiac disease among T1DM patients showed similar prevalence in Riyadh with 11.3%, and 11.2% in Jeddah.28,29
Anti-CCP results showed a low prevalence (one in 202 cases), and there might be a weak association of this antibody with T1DM. The prevalence of a rheumatoid arthritism (RA)-specific antibody was 0.5%, which is similar to the normal population ratio of anti-CCP.13 The anti-CCP is one of the most important serological tests for RA diagnosis (specificity; 96-98% and sensitivity approximately 68%), and is superior for detection of RA in the early stages rather than other RA serological markers.30 Data available on the association between T1DM and RA, and specifically anti-CCP are limited. Most of the articles that found any sort of association were limited to reported cases of juvenile RA or familial association of T1DM with thyroid diseases and rheumatoid arthritis.14 Weak association between T1DM and RA has been reported previously with either rheumatic factors or anti-CCP, confirming our findings.13
No correlations between the presence of thyroid and celiac autoantibodies and factors, such as gender, age at diagnosis, and duration of diabetes were observed. However, females showed a 2-fold frequency (22 female versus 11 males). The specific gender factor makes a significant difference in thyroid antibody levels during puberty.31 No significant effect was found between the presence of these autoantibodies and diabetic control or BMI, which could be due to the status of sub-clinical thyroid disease. In contrast to hyperthyroidism, glycemic control is not often much affected by hypothyroidism.3 Both TPO and TG, the autoantibodies measured in this study, were associated highly with hypothyroidism. We found that celiac disease patients were similar in glycemic control to non-celiac T1DM patients. Celiac disease is known to be linked more with hypoglycemic attacks with unexplained reduction of insulin doses and is not necessarily associated with poor growth or poor diabetic control.3 Presence of thyroid and celiac diseases autoantibodies might not be the major factor affecting the glycemic control in T1DM. One of the factors most affecting glycemic control and body weight is the pubertal age due to changes in eating habit, lifestyle and decreased care from parents.16
The limitations of the study are the need for proven biopsy diagnosis of celiac cases, and measurement and follow-up of positive thyroid patients in order to find out the precise prevalence of thyroid and celiac diseases among those patients. It seems that there was no previous data on both autoantibodies among T1DM in southern region. Additionally, reports regarding the prevalence of anti-CCP among T1DM are very limited in literature.
In conclusion, the prevalence of thyroid and celiac disease autoantibodies is high among patients with T1DM in the southern region of Saudi Arabia, and is similar to the prevalence in the central and western areas. Anti-CCP prevalence is low and might be weakly associated with T1DM. No significant correlation was found between the age of the patient when diagnosed with T1DM, duration of the disease and glycemic control, and the presence of thyroid and celiac diseases autoantibodies.
Acknowledgment
The author gratefully acknowledges Dr. Ayed A. Shati and Dr. Ali Alsuheel from the Department of Child Health, College of Medicine, King Khalid University for their help in samples collections, Dr. Fatiha H. Benahmed, Mr. Ali H. Al-Waylee from the Laboratory Department of Aseer Central Hospital, and Mr. Riyadh A. Moosa from the Department of Microbiology and Clinical Parasitology, College of Medicine, King Khalid University for their assistance in laboratory work, and Sami Alshehri and Abdullah Asiri (Medical Students) for their contribution in the current work.
Footnotes
References
- 1.Tsirogianni A, Pipi E, Soufleros K. Specificity of islet cell autoantibodies and coexistence with other organ specific autoantibodies in type 1 diabetes mellitus. Autoimmun Rev. 2009;8:687–691. doi: 10.1016/j.autrev.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki E. Type 1 diabetes and autoimmunity. Clin Pediatr Endocrinol. 2014;23:99–105. doi: 10.1297/cpe.23.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kordonouri O, Klingensmith G, Knip M, Holl RW, Aanstoot HJ, Menon PS, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Other complications and diabetes-associated conditions in children and adolescents. Pediatr Diabetes. 2014;15(Suppl 20):270–278. doi: 10.1111/pedi.12183. [DOI] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Latif KA, Murphy MB, Lambeth HC, Stentz F, Bush A, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care. 2003;26:1181–1185. doi: 10.2337/diacare.26.4.1181. [DOI] [PubMed] [Google Scholar]
- 5.Kakleas K, Paschali E, Kefalas N, Fotinou A, Kanariou M, Karayianni C, et al. Factors for thyroid autoimmunity in children and adolescents with type 1 diabetes mellitus. Ups J Med Sci. 2009;114:214–220. doi: 10.3109/03009730903276381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakleas K, Soldatou A, Karachaliou F, Karavanaki K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM) Autoimmun Rev. 2015;14:781–797. doi: 10.1016/j.autrev.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Kordonouri O, Deiss D, Danne T, Dorow A, Bassir C, Gruters-Kieslich A. Predictivity of thyroid autoantibodies for the development of thyroid disorders in children and adolescents with Type 1 diabetes. Diabet Med. 2002;19:518–521. doi: 10.1046/j.1464-5491.2002.00699.x. [DOI] [PubMed] [Google Scholar]
- 8.Larsson K, Carlsson A, Cederwall E, Jonsson B, Neiderud J, Jonsson B, et al. Annual screening detects celiac disease in children with type 1 diabetes. Pediatr Diabetes. 2008;9:354–359. doi: 10.1111/j.1399-5448.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- 9.Pham-Short A, Donaghue KC, Ambler G, Chan AK, Craig ME. Coeliac disease in Type 1 diabetes from 1990 to 2009: higher incidence in young children after longer diabetes duration. Diabet Med. 2012;29:e286–e289. doi: 10.1111/j.1464-5491.2012.03720.x. [DOI] [PubMed] [Google Scholar]
- 10.Husby S, Koletzko S, Korponay-Szabo IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins HR, Murch SH, Beattie RM. Diagnosing coeliac disease. Arch Dis Child. 2012;97:393–394. doi: 10.1136/archdischild-2011-301198. [DOI] [PubMed] [Google Scholar]
- 12.Vivas S, Ruiz de Morales JG, Riestra S, Arias L, Fuentes D, Alvarez N, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol. 2009;15:4775–4780. doi: 10.3748/wjg.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desplat-Jégo S, Deharveng I, Baronne R, Valéro R, Bégu-Le Corroller A, Vialettes B. Antibodies to cyclic citrullinated peptides (anti-CCP) in Type 1 diabetes mellitus. Diabet Med. 2010;27:725–727. doi: 10.1111/j.1464-5491.2010.02959.x. [DOI] [PubMed] [Google Scholar]
- 14.Liao KP, Gunnarsson M, Kallberg H, Ding B, Plenge RM, Padyukov L, et al. Specific association of type 1 diabetes mellitus with anti-cyclic citrullinated peptide-positive rheumatoid arthritis. Arthritis Rheum. 2009;60:653–660. doi: 10.1002/art.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin P, Huang G, Lin J, Yang L, Xiang B, Zhou W, et al. High titre of antiglutamic acid decarboxylase autoantibody is a strong predictor of the development of thyroid autoimmunity in patients with type 1 diabetes and latent autoimmune diabetes in adults. Clin Endocrinol (Oxf) 2011;74:587–592. doi: 10.1111/j.1365-2265.2011.03976.x. [DOI] [PubMed] [Google Scholar]
- 16.Al-Agha AE, Alafif MM, Abd-Elhameed IA. Glycemic control, complications, and associated autoimmune diseases in children and adolescents with type 1 diabetes in Jeddah, Saudi Arabia. Saudi Med J. 2015;36:26–31. doi: 10.15537/smj.2015.1.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullah MA, Salman H, Bahakim H, Gad al Rab MO, Halim K, Abanamy A. Antithyroid and other organ-specific antibodies in Saudi Arab diabetic and normal children. Diabet Med. 1990;7:50–52. doi: 10.1111/j.1464-5491.1990.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 18.Radaideh A, El-Khateeb M, Batieha AM, Nasser AS, Ajlouni KM. Thyroid function and thyroid autoimmunity in patients with type 1 diabetes mellitus. Saudi Med J. 2003;24:352–355. [PubMed] [Google Scholar]
- 19.Omara M, Rizq M, El-Kafoury A, Kilany D. Screening for thyroid disease among children and adolescents with type 1 diabetes mellitus. Alexandria Journal of Medicine. 2015;50:77–82. [Google Scholar]
- 20.Magzoub MM, Abdel-Hameed AA, Bottazzo GF. Prevalence of islet cell and thyrogastric autoantibodies in Sudanese patients with type 1 diabetes. Diabet Med. 1994;11:188–192. doi: 10.1111/j.1464-5491.1994.tb02018.x. [DOI] [PubMed] [Google Scholar]
- 21.Al-Khawari M, Shaltout A, Qabazard M, Al-Sane H, Elkum N. Prevalence of thyroid autoantibodies in children, adolescents and young adults with type 1 diabetes in kuwait. Med Princ Pract. 2015;24:280–284. doi: 10.1159/000381547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghawil M, Tonutti E, Abusrewil S, Visentini D, Hadeed I, Miotti V, et al. Autoimmune thyroid disease in Libyan children and young adults with type 1 diabetes mellitus. Eur J Pediatr. 2011;170:983–987. doi: 10.1007/s00431-010-1386-1. [DOI] [PubMed] [Google Scholar]
- 23.Kordonouri O, Klinghammer A, Lang EB, Gruters-Kieslich A, Grabert M, Holl RW. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care. 2002;25:1346–1350. doi: 10.2337/diacare.25.8.1346. [DOI] [PubMed] [Google Scholar]
- 24.Rho MH, Kim DW, Hong HP, Park YM, Kwon MJ, Jung SJ, et al. Diagnostic value of antithyroid peroxidase antibody for incidental autoimmune thyroiditis based on histopathologic results. Endocrine. 2012;42:647–652. doi: 10.1007/s12020-012-9695-y. [DOI] [PubMed] [Google Scholar]
- 25.Glastras SJ, Craig ME, Verge CF, Chan AK, Cusumano JM, Donaghue KC. The role of autoimmunity at diagnosis of type 1 diabetes in the development of thyroid and celiac disease and microvascular complications. Diabetes Care. 2005;28:2170–2175. doi: 10.2337/diacare.28.9.2170. [DOI] [PubMed] [Google Scholar]
- 26.Elfstrom P, Sundstrom J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40:1123–1132. doi: 10.1111/apt.12973. [DOI] [PubMed] [Google Scholar]
- 27.Frohlich-Reiterer EE, Kaspers S, Hofer S, Schober E, Kordonouri O, Pozza SB, et al. Anthropometry, metabolic control, and follow-up in children and adolescents with type 1 diabetes mellitus and biopsy-proven celiac disease. J Pediatr. 2011;158:589–593. doi: 10.1016/j.jpeds.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 28.Al-Hussaini A, Sulaiman N, Al-Zahrani M, Alenizi A, El Haj I. High prevalence of celiac disease among Saudi children with type 1 diabetes: a prospective cross-sectional study. BMC Gastroenterol. 2012;12:180. doi: 10.1186/1471-230X-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saadah OI, Al-Agha AE, Al Nahdi HM, Bokhary RY, Bin Talib YY, Al-Mughales JA, et al. Prevalence of celiac disease in children with type 1 diabetes mellitus screened by anti-tissue transglutaminase antibody from Western Saudi Arabia. Saudi Med J. 2012;33:541–546. [PubMed] [Google Scholar]
- 30.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in Type 1 diabetes: systematic review and meta-analysis. Diabet Med. 2014;31:126–135. doi: 10.1111/dme.12318. [DOI] [PubMed] [Google Scholar]


