Abstract
Objectives:
To investigate the molecular epidemiology of pneumococcal isolates in Chongqing, China.
Methods:
In this cross-sectional study, 51 invasive Streptococcus pneumoniae (S. pneumoniae) strains were from children with invasive pneumococcal disease (IPD) and 32 carriage strains from healthy children from January 2010 to December 2013 at the Children’s Hospital of Chongqing Medical University, Chongqing, China. Multilocus sequence typing was used to identify the sequence types (STs). Capsular serotypes were determined by multiplex polymerase chain reaction. Drug susceptibility and resistance was determined by minimum inhibitory concentrations.
Results:
In this study, 11 serotypes were identified among the 83 S. pneumoniae clinical isolates tested. Prevalent serotypes were 19A (20.4%), 6A/B (20.4%), 19F (15.7%), 14 (14.5%), and 23F (10.8%). Serotype 19F was the most frequent carriage strain, and serotype 19A was the most frequent invasive strain. The ST983 was the most prevalent ST for carriage strains, and ST320 was the most prevalent ST for invasive strains. For gene analysis, psaA (99.5%) and piaA (98.6%) were present and much conserved in all pneumococci tested. The cps2A and pcsB genes were more frequent in invasive isolates than carriage strains. Antimicrobial resistance rates of invasive pneumococcal isolates to erythromycin, penicillin, meropenem, cefotaxime, and clindamycin were higher than the carriage isolates from children.
Conclusion:
Our epidemiological evidence shows that 19A, 6A/B, 19F, 14, and 23F remain the most prevalent serotypes, which can be targeted by PCV13. Genotypes and drug resistance varied between carriage and invasive strains. The PsaA and PiaA may be good protein vaccine candidates.
Streptococcus pneumoniae (S. pneumoniae) is an important bacterial pathogen to cause invasive pneumococcal diseases (IPDs) in children, including bacteremia and meningitis. It has been estimated that more than 1.6 million children die from S. pneumoniae infections worldwide every year.1 The increasing resistance to different antibiotics of S. pneumoniae in recent years is a new challenge for public health management worldwide.2 This situation seems much more worrying in many Asian countries,3 especially in China.4 Heptavalent pneumococcal conjugate vaccine (PCV)7, composed of capsular polysaccharide antigens from serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F has been demonstrated to have high effectiveness in controlling IPD in the developed areas.5,6 It has been reported, however, that only 60% of the pneumococcal isolates could be covered by PCV7 in Asia.7 The PCV7 has not been commonly used due to its high price in China. However, there are reports that non-vaccine type strains have a high prevalence in population with IPDs in China.8,9 The PCV13 has recently been introduced in China; however, due to the lack of epidemic data, its coverage rate is still unknown in most regions of China. The S. pneumoniae commonly colonizes the respiratory tract asymptomatically, which may cause IPD when the balance between pneumococci and host immunity is broken. Transmission of S. pneumoniae occurs through the respiratory droplets, and is more commonly associated with healthy individuals who carry the organism in the upper respiratory tract.10 Although previous studies suggested that the phenotype, genotype, serotype, and virulence factors were related with the pathogenesis,11,12 it has not been investigated regarding the difference at the DNA sequence level of virulence factor between the invasive and carriage pneumococcal strains. Serotypes of carriage isolates may vary from geographic regions, vaccine policies, and over time.13 The prevalence of the nasopharyngeal carriage of S. pneumoniae is related to invasive pneumococcal diseases.14 There are more than 90 pneumococcal serotypes, but few serotypes account for most of the invasive diseases. The surveillance regarding the serotype distribution of nasopharyngeal carrier isolates in healthy children is necessary for a better management of IPDs, and understanding the information regarding the coverage of novel vaccines. So far, only a few studies have been performed to evaluate the nasopharyngeal carriage pattern of S. pneumoniae among children in mainland China. This study attempts to present the updated molecular epidemiology evidence and pathogenic features of the pneumococcal strains isolated from young children in Chongqing, China between 2010 and 2013. The phenotype, genotype, and serotype of invasive pneumococcal strains were compared with those of nasopharyngeal carriage strains.
Methods
Bacterial strains
In this cross-sectional study, all invasive S. pneumoniae strains were obtained from children less than 11 years old diagnosed with IPD by laboratory testing and professional clinicians based on typical clinical manifestation and imaging examination at the Children’s Hospital of Chongqing Medical University, Chongqing, China from January 2010 to December 2013. Healthy children under 6 years old (n=789) who attended the community health center for national immunization (pneumococcal vaccines were not included in the national immunization program), or at kindergarten were also enrolled for the isolation of nasopharyngeal carriage strains of S. pneumoniae. Children with respiratory tract infections, chronic diseases, congenital cranio-facial anomalies, or those who had received antibiotics within 2 weeks prior to study entry were excluded. Nasopharyngeal specimens were collected by a trained technician. Nasopharyngeal swab specimens were collected with a cotton-tipped wooden swab, and immediately plated onto sheep blood agar and incubated at 37°C with 5% CO2. Based on the typical colony morphology of S. pneumoniae, which include umbilical fossa, pale, translucent, grass green lysis, isolates were collected after 20-24 hours of incubation, and were further confirmed using the Optochin sensitivity test, bile solubility test, and automatic bacterial identified system phoenix100 (Becton, Dickinson Co., USA). All participants were truly informed (from their guardians where necessary) prior to their participation in the study; the above protocol was approved by the Clinical Research Ethics Committee of the affiliated Children’s Hospital of Chongqing Medical University, and informed consent was obtained from all participants. This study was conducted according to the principles of Helsinki Declaration.
Serotyping
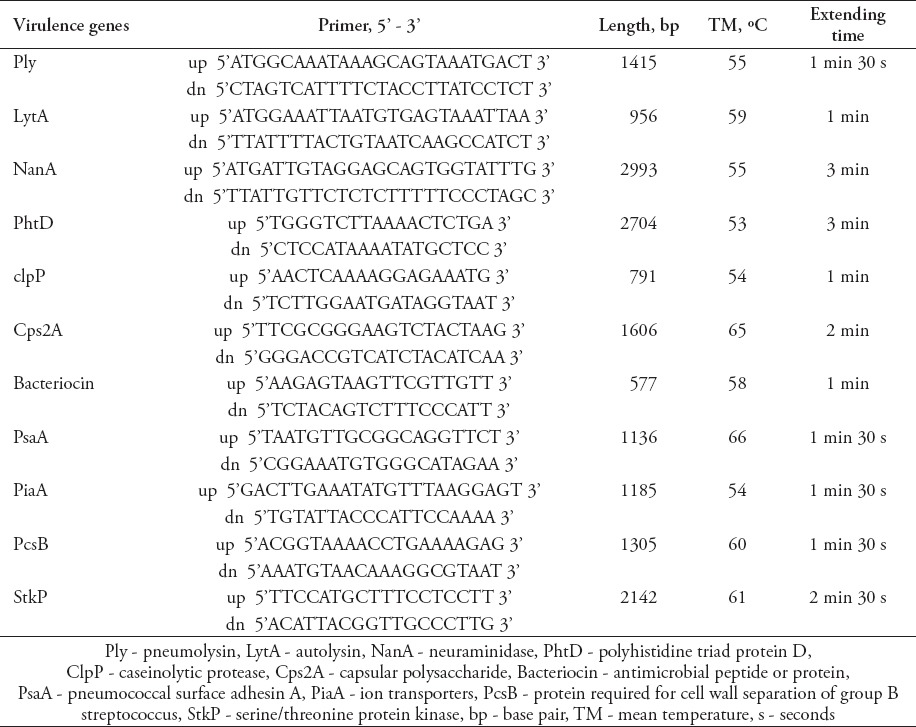
The serotype of each pneumococcal isolate was determined by multiplex polymerase chain reaction (PCR) amplification using a previously published method,15 and the quellung reaction16 with the sera of different reactivity from the Statens Serum Institut (Copenhagen, Denmark). The genomic DNA was extracted from bacteria using a bacterial DNA extraction kit (DP302-02; TIAGEN, Beijing, China). The amplification was performed in a 25 µl reaction volume with 30 thermal cycles, and other conditions were listed in Table 1.
Table 1.
Primers and amplification conditions used to amplify the 11 virulence genes.
Multilocus sequence typing
The sequence types (STs) were determined by multi-locus sequence typing (MLST). Internal fragments (approximately 550-600 base pair [bp]) of the aroE, gdh, gki, recP, spi, xpt, and ddl genes were amplified by PCR using previously described methods.17 The sequences of each of the 7 loci were obtained by comparing with those of all known alleles at these loci and with the STs in the pneumococcal MLST website database (http://spneumoniae.mlst.net). The STs were analyzed for genetic relatedness using the eBURST v3 program (http://eburst.mlst.net).
Detection of virulence genes
Eleven known virulence factors including pneumolysin (Ply), autolysin (lytA), neuraminidase (nanA), polyhistidine triad protein D (PhtD), caseinolytic protease (clpP), capsular polysaccharide (cps2A), bacteriocin (antimicrobial peptide or protein), pneumococcal surface adhesin A (psaA), ion transporters (piaA), protein required for cell wall separation of group B streptococcus (pcsB), and serine/threonine protein kinase (stkP) were sequenced. The primers were designed according to the S. pneumoniae genomes available on the United States National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). The primer sequences were listed in Table 1. The 11 virulence factors were amplified by PCR and sequenced to reveal any mutations, which were carried out by HuaDa Genomics Company (Beijing, China).
Antimicrobial susceptibility testing
Antimicrobial susceptibility patterns were determined by automatic bacteria identification/susceptibility system Phoenix100 (BD, USA), and used to assess the antibiotic susceptibility of all 12 isolated antibiotics according to the guidelines established by the Clinical and Laboratory Standards Institute (CLSI).18 The CLSI 2013 criteria_ENREF_3919 for minimum inhibitory concentrations (MICs) were applied to classify susceptible, intermediate, and resistant isolates. The separate interpretive breakpoints for non-meningeal, meningeal were used to define cefotaxime and Cefepime resistance: MIC 2 µg/ml (meningeal) and ≥4 µg/ml (non-meningeal). For parenteral penicillin resistance: MIC ≥0.12 µg/ml (meningeal), and ≥8 µg/ml (non-meningeal). As to the exact MIC of vancomycin, it would only be given when the MIC value is greater than 1 µg/ml, otherwise, the MIC of vancomycin would be given as <1 µg/ml by analytical platform. The S. pneumoniae ATCC 49619 was used as a quality control strain during susceptibility testing.
Statistical analysis
Data were collected and classified. The detection rate of virulence factor and nonsensitive percentage of antibiotic was calculated. Numeration data were described by positive example and rate, and then analyzed using the X2 test or Fisher’s exact test (2-tailed). All analyses were performed using the Statistical Package for Social Sciences software for Windows version 10 (SPSS Inc., Chicago, IL, USA). P<0.05 were considered statistically significant.
Results
General properties of pneumococcal isolates
A total of 83 isolates including 51 invasive strains and 32 carriage strains were obtained from children in Chongqing. Among the 51 isolates of invasive S. pneumoniae, 32 strains (62.8%) were isolated from blood, 9 strains (17.7%) from cerebrospinal fluid, 7 strains (17.7%) from pleural effusion, 2 strains (13.7%) from pus, and one strain (2%) from bone marrow. Among the 51 strains, 30 strains (58.8%) were from males, and 21 (41.2%) strains were from females. Among the 789 healthy children attending the study, 44 subjects had a vaccination history of PCV, and 745 subjects do not have vaccination history of pneumococcal vaccines. In addition, 32 carriage strains that were mentioned above were isolated from the nasopharyngeal cavity of healthy children. The carriage rate of S. pneumoniae was 4.1%. Among the 32 S. pneumoniae carriages, 18 isolates were from infants under 2 years old, whereas 14 were from preschoolers 2 years old and above. Fifteen isolates were from boys, whereas 17 isolates were from girls.
Serotypes of the pneumococcal isolates
Serotype distribution was diverse and widely divergent among the carriage and invasive isolates (Tables 2-5). The 83 isolates were composed of 11 STs. Prevalent serotypes were 19A (20.4%), 6A/B (20.4%), 19F (15.7%), 14 (14.5%), and 23F (10.8%). Nine STs were identified in 51 invasive isolates. Most serotypes were 19A (25.5%), followed by serotype 14 (19.6%), 6A/6B (19.6%), 23F (13.7%), 19F (6.0%) and 1 (5.9%). In addition, there is only one isolate for serotypes 33/33B, 17F, and 11/11A (2%). Seven serotypes were identified in 32 of the carriage isolates. Most serotypes were 19F (25%), followed by 6A/B (22%), 19A (13%), 15 (9%), 6C (6%), 14 (6%), and 23F (6%). Four carriage isolates were non-typeable. Serotypes 14, 6A/6B, 23F, 19F and 19A were detected in both strain types. However, several serogroups among the invasive strains have never been identified in carriage strains, such as serogroups 1, 33/33B, 17F, and 11/11A. The serogroups 15 and 6C found in the carriage strains were rarely isolated as invasive groups.
Table 2.
Results of sequence types (STs) and serotypes of 51 strains of invasive Streptococcus pneumoniae (S. pneumoniae).
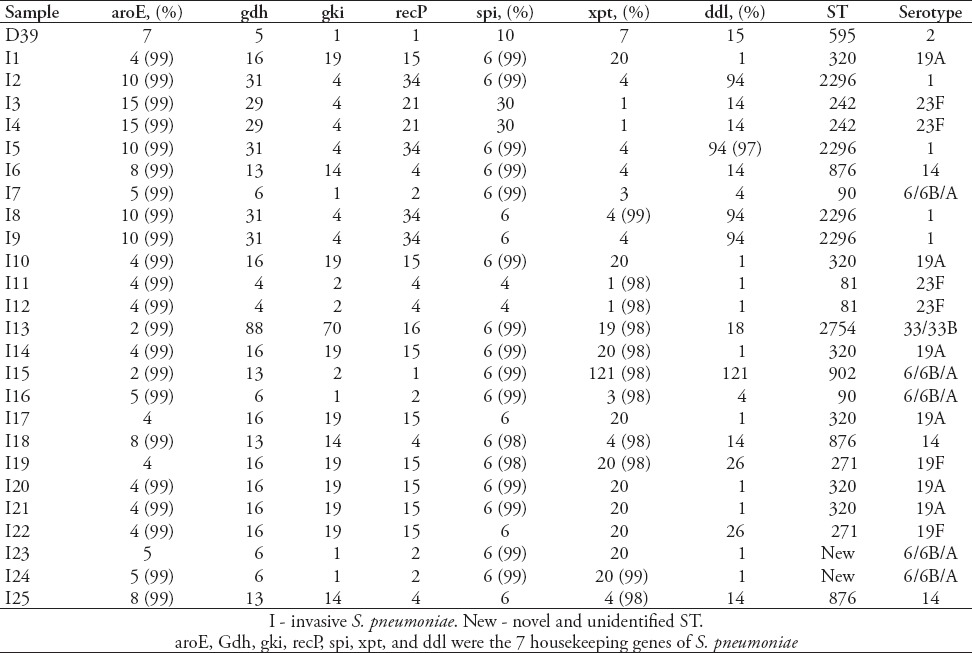
Table 3.
Results of sequence types (STs) and serotypes of 51 strains of invasive Streptococcus pneumoniae (S. pneumoniae) (continued).
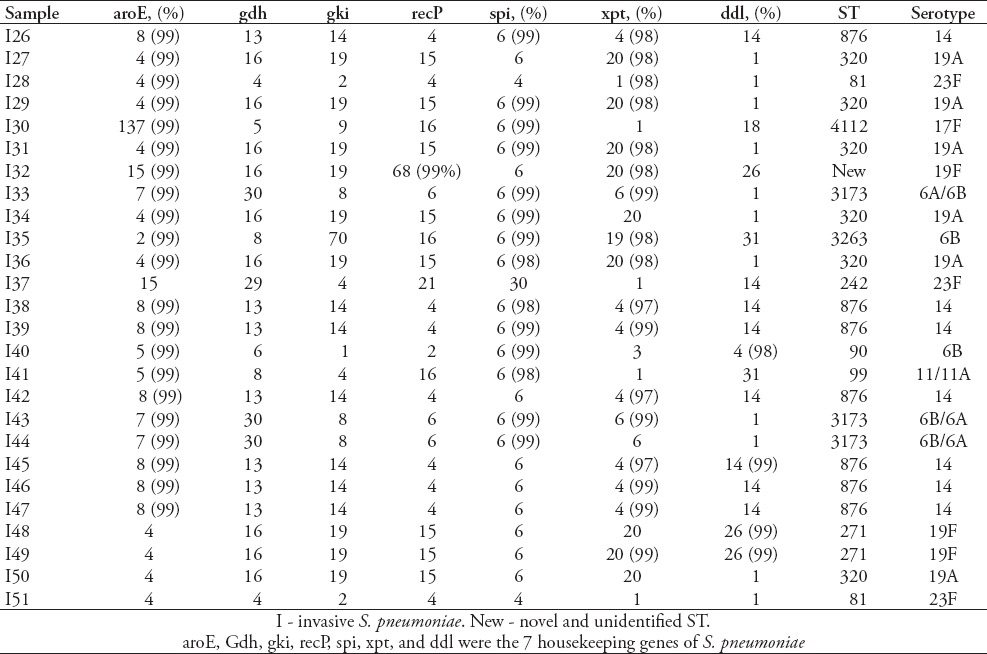
Table 4.
Results of sequence types (STs) and serotypes of 32 strains of carriage Streptococcus pneumoniae (S. pneumoniae).
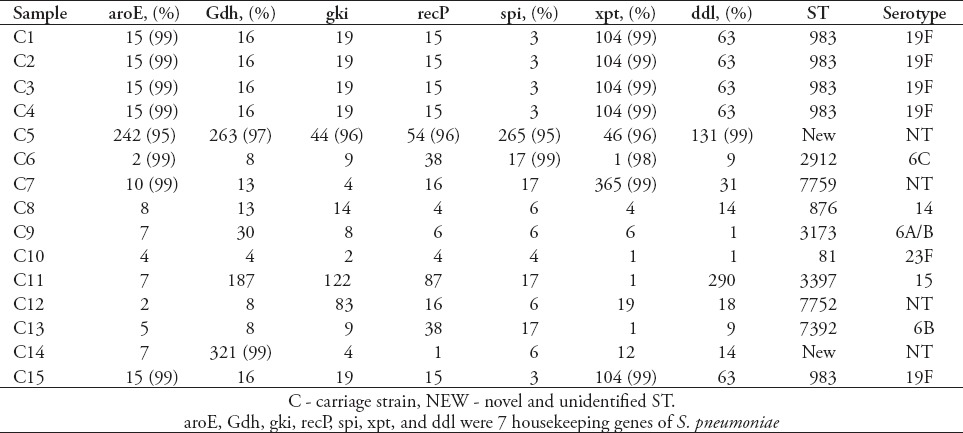
Table 5.
Results of sequence types (STs) and serotypes of 32 strains of carriage Streptococcus pneumoniae (S. pneumoniae) (continued).
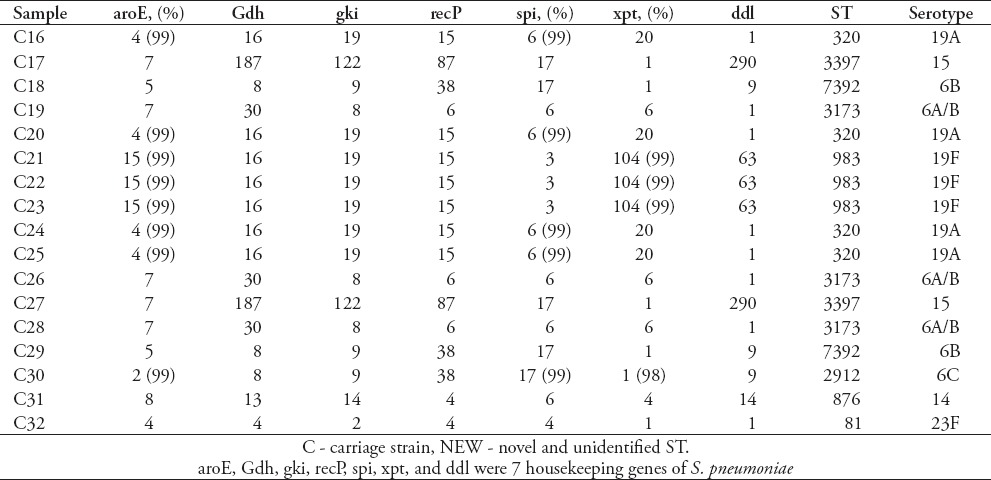
The MLST
Eighty-three isolates were characterized using MLST, and 18 known STs were identified, 5 of which have not been previously recorded in the online pneumococcal database (http://spneumoniae.mlst.net). A strong association between the ST and serotype of isolates was observed (Tables 2-5). Isolates of closely related STs (clone complexes) almost invariably had the same serotype. In invasive isolates, ST320 and ST876 were the most frequent STs (serotypes 19A and 14) (23.5%), followed by ST876 (19.6%), ST2296 (7.8%), ST90 (7.8%), ST81 (7.8%), and ST271 (7.8%), T242 (5.9%), and ST3173 (5.9%), ST2754 (2%), ST902 (2%), ST4112 (2%), ST3263 (2%), and new STs account for 5.8%. Nine STs were identified in 32 of the carriage isolates. The ST983 (serotypes 19F [28.6%]) was the primary ST for carriage strain, followed by ST2912, ST7759, ST3397, ST7752, ST7392, ST876, ST81 and ST3173 (for all), and new STs account for 14.3%. Diversity comparison of STs showed that ST7752, ST7392, ST983, ST2912, ST7759 and ST3397 were not presented in invasive isolates, and ST2296, ST90, ST271, ST242, ST2754, ST902, ST4112 and ST3263 were rarely observed in carriage isolates. In addition, eBURST analyses using the stringent 6/7 identical loci definition grouped the 18 STs into 2 clonal complexes, and 16 singletons expressing a high level of genetic diversity among the isolates.
Detection of virulence genes
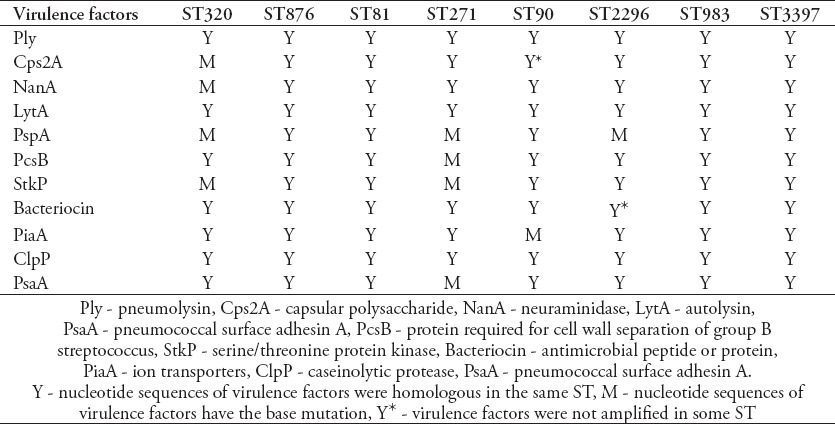
Virulence factors are important candidate targets for the design of next-generation protein vaccines. The conservation of the protein antigens in epidemic strains is taken as an important index to decide whether this antigen is conserved enough to protect broad protection against pneumococcal infections. Eleven virulence factors were amplified by PCR and sequenced. Results showed the prevalence rates of cps2A (90.2%), bacteriocin (82.4%), and phtD (94.1%). The other 8 virulence factors could be amplified from all the invasive isolates. For the carriage strains, the prevalence rate for the virulence factors of pcsB was 57.1%, cps2A - 57.1%, clpP - 78.6%, bacteriocin - 71.4%, phtD - 78.6%, nanA - 78.6%, stkP - 85.7%, ply - 85.7%, lytA - 85.7%, psaA - 100%, and piaA - 100%. The prevalence of cps2A was significantly different between invasive (90.2%) and carriage (57.1%) isolates (p=0.029). Similarly, the prevalence of pcsB was significantly different between invasive (100%) and carriage (57.1%) isolates (p=0.008) (Figure 1). The conservation from DNA sequence level of the virulence factors, including psaA was 99.5%, piaA - 98.6%, clpP - 98.5%, ply - 97.8%, stkP - 96.7%, lytA - 96.3%, pcsB - 88.7%, phtD - 87.5%, cps2A - 83.7%, nanA - 83%, and bacteriocin was 66.3% in the invasive isolates. In general, a strong association between the ST and the DNA sequence of virulence factors was observed in this collection of isolates (Table 6). The DNA sequence of virulence factors was almost invariable in the same STs. In our study, ST271 and ST320 were mainly detected in invasive strains, while were rarely observed in the carriage S. pneumoniae. The virulence factor were conservation in most STs. Interestingly, the base sequence of virulence factor in ST271 and ST320 have more mutations than other STs.
Figure 1.
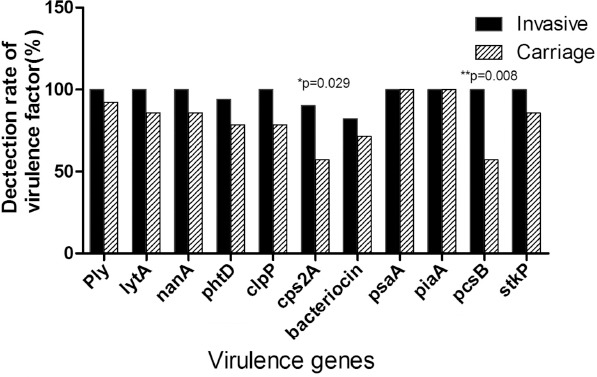
Individual prevalence of the 11 virulence genes in invasive and carriage pneumococcal strains.
Table 6.
The association of sequence types (STs) and virulence factors.
Drug resistance test
The antibiotic resistance patterns of invasive and carriage isolates are summarized in Table 7. Drug resistance data showed that antimicrobial resistance of the invasive pneumococcal strains was generally higher than carriage strains. All 83 isolates were completely sensitive to vancomycin, linezolid, levofloxacin, and moxifloxacin at the current applied breakpoints. Penicillin’s nonsusceptibility was displayed by 58.8% of the invasive isolates. Moreover, the nonsusceptibility of the invasive isolates to trimethoprim/sulfamethoxazole was 62.8%, tetracycline - 84.3%, clindamycin - 94.1%, and erythromycin was 100% (Table 7). The nonsusceptibility rates of invasive strains to erythromycin (p=0.001), penicillin (p=0.001), meropenem (p=0.018), cefotaxime (p=0.007), and clindamycin (p=0.034) were significantly higher than the carriage strains. Only non-susceptibility to chloramphenicol (p=0.005) was significantly lower in invasive strains than carriage strains (Figure 2).
Table 7.
Susceptibility testing of Streptococcus pneumoniae strains isolated from children younger than 6 years old.
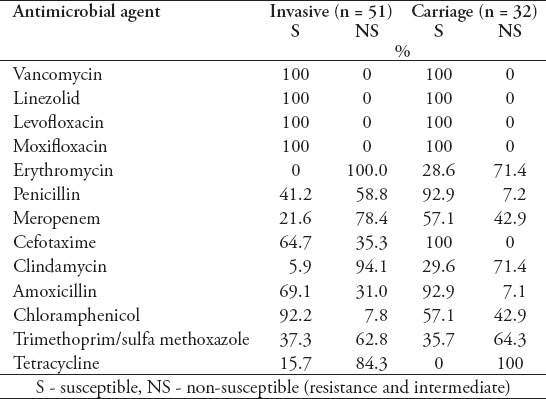
Figure 2.
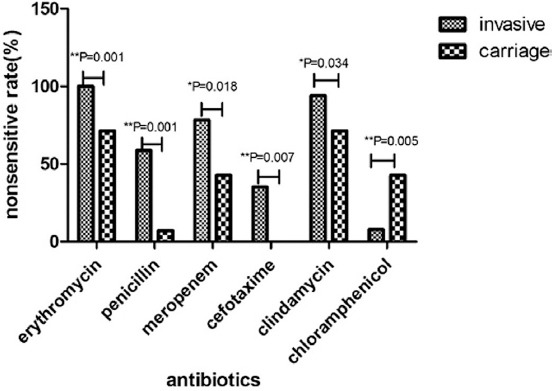
Antimicrobial resistance between carriage and invasive pneumococcal isolates.
Discussion
The capsule is the main virulence determinant of S. pneumoniae. Only a few capsular types tend to be associated with invasive diseases, which may partly attribute to the differential ability of the capsular type variants in resisting phagocytosis.20,21 Serotypes of invasive isolates may vary from region to region and over time.22-24 Our study indicates that serotype 19A is the most prevalent invasive strain among children in Chongqing. This observation is similar to that of a study reported before in mainland China.9 In contrast, serotype 14 was the most common invasive isolates reported among children in India.25 The PCV7 coverage rate against invasive strains (62.7%) reported in this study is also similar to those of some Asian countries, such as Denmark and Singapore <65%).26-28 However, this figure is lower than those observed in the United States (87%),29 Canada, Europe (78-94%),30 and Spain (72.3%).31 Some researchers explained that the low percentage of PCV7-related serotypes may be due to the herd immunity effect, as reported in the United States,32 and United Kingdom.33 However, unlike the situation of high vaccine inoculation rate of PCV7 in the United States and United Kingdom, the prevalence of pneumococcal strains reflects the real circulating pneumococcal strains in Chongqing since the vaccine inoculation rate is only 4.4% in this region, which is quite the same as that in Singapore.26 The PCV13 extends the serotype coverage, and serotypes 19F, 14, 6A, 6B, 23F, 18C, 9V, 4, 1, 5, 7F, 3, and 19A were included. The present finding showed that this vaccine could cover 94.1% (48/51) of the invasive isolates, and hence indicates the potential value of PCV13 in protecting pneumococcal infections in China, specifically in Chongqing city.
The MLST analysis reveals a significant diversity among all strains in terms of their STs. Our data show that ST320 is the most prevalent clone among the invasive isolates, whereas ST983 was the most prevalent clone among the carriage isolates in Chongqing, China. A strong association between the serotype and ST among carriage and invasive populations was observed.34 Although previous studies17 have shown that the pneumococcal serotype has a dominant role in determining its invasiveness, the genotype can affect the invasiveness of S. pneumoniae. Serotype 19F was identified to have 2 STs, ST271 and ST983 in the present study. The ST271 was only found in invasive serotype 19F, whereas ST983 was only found in the carriage strains. This phenomenon was also observed for serotype 6 A/B. Besides, 2 STs in our study including ST 90 and ST 2912 were associated with multiple serotypes indicating a history of serotype switching, which has been noted in West Africa.35 The gene spectrums of 14.5% isolates were matched with those in pneumococcal molecular epidemiology network (PMEN) database. These isolates could be defined as the corresponding PMEN cloning plants or its mutant strains. In Chongqing, the most prevalent PMEN cloning was Spain23F-1.
Pneumococcal isolates with lower susceptibility to antibiotics and non-susceptible strains are increasing. This increase in the prevalence of drug-resistant pneumococci may result from the frequent and unnecessary use of antimicrobial drugs. Our study shows that drug resistance was higher in invasive strains than carriage strains, except for chloramphenicol, which carriage strains have a higher resistant rate than the invasive strains (p=0.005). The increase in chloramphenicol resistance may reflect the abusive use of this drug in communities. The incidence reported in this study is consistent with that previously reported in China.8 The nonsusceptibility rate to penicillin is higher in China than in the United States and several other Asian countries.36-38 Unlike the figure reported in the Western countries, macrolide nonsusceptibility of invasive strains is high in Chongqing.39 The major reasons for this high level of macrolide resistance may be the widespread use of macrolides in clinical practice and clone spread of macrolide-resistant strains in China cities. Thus, the empirical use of macrolides alone for the treatment of pneumococcal infections can be an inappropriate option in China. The surveillance of the antibiotic resistance of carriage strains is useful to guide the empirical treatment against IPD.
Although PCVs are effective against IPDs, they are not widely accepted in undeveloped countries due to their high cost and limited serotype coverage. An alternative strategy is to design a protein-based pneumococcal vaccine and some protein candidates were protective against pneumococcal infections, either alone or in combinations.40-42 The PsaA and PiaA have been demonstrated to decrease the number of bacteria in the lungs of mice challenged with S. pneumoniae, and increase the survival rate in a mouse pneumococcal lethal intranasal challenge model.43 In our study from the aspect of antigen conservation, it shows that PsaA and PiaA are likely to be important protein vaccine candidates, and are worthy of further investigation. Notably, our study shows that cps2A and pcsB were less apt to be detected in carriage strains than in invasive strains, which suggests the positive effect of cps2A and pcsB of the invasive strains to combat with the host. The base sequence of virulence factors in ST271 and ST320, which were apt to be determined in invasive isolates have more mutations than in other STs. We hypothesize that these genetic mutations might strengthen the virulence of strains to cause invasive pneumococcal disease. In addition, the genetic mutations of invasive S. pneumoniae might be a result of “the survival of the fittest”. Accordingly, those mutations might involve in the genes of virulence factor.
Nasopharyngeal colonization with S. pneumoniae is asymptomatic in most infants with carriage rates that vary from 3.8-90%.13,44,45 Our study shows a very low nasopharyngeal carrier rate (4.1%) for pneumococci in Chongqing children. This low carrier rate observed in our study may be due to the hot climate in Chongqing, sample collection time or specimen processing methods.
This study has several limitations. First, the number of carriage strains was limited. Secondly, potential primer divergence implies that a PCR negative result does not necessarily indicate the absence of proteins. Thirdly, this study was performed in Chongqing, which may not represent the whole situation of China. Fourthly, some serotypes and STs have not been identified so far, and further studies are needed to identify them in the future.
In conclusion, pneumococcal infection remains an important burden in children population. Our study shows prevalent serotypes among the 83 S. pneumoniae clinical isolates were 19A, 6A/B, 19F, 14, and 23F. Of them, serotype 19A was the most prevalent invasive isolates, and serotype 19F was the most prevalent carriage isolates. The potential coverage for PCV13 against invasive strains is 94.1%. Drug resistance varied among different serotypes and between invasive and carriage strains. The Cps2A and PcsB may be partly responsible for the increased virulence of invasive strains with a higher attack rate. The PsaA and PiaA are highly conserved among the pneumococcal clinical isolates, which shall be important candidates to be considered in protein based vaccines.
Footnotes
Ethical Consent.
All manuscripts reporting the results of experimental investigations involving human subjects should include a statement confirming that informed consent was obtained from each subject or subject’s guardian, after receiving approval of the experimental protocol by a local human ethics committee, or institutional review board. When reporting experiments on animals, authors should indicate whether the institutional and national guide for the care and use of laboratory animals was followed.
References
- 1.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Wierzbowski AK, Karlowsky JA, Adam HJ, Nichol KA, Hoban DJ, Zhanel GG. Canadian Antimicrobial Resistance Alliance. Evolution and molecular characterization of macrolide-resistant Streptococcus pneumoniae in Canada between 1998 and 2008. J Antimicrob Chemother. 2014;69:59–66. doi: 10.1093/jac/dkt332. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56:1418–1426. doi: 10.1128/AAC.05658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng Q, Zhang T, Ding Y, Tao Y, Lin Y, Wang Y, et al. Molecular characterization and antimicrobial susceptibility of Streptococcus pneumoniae isolated from children hospitalized with respiratory infections in Suzhou, China. PLoS One. 2014;9:e93752. doi: 10.1371/journal.pone.0093752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanquet G, Kissling E, Fenoll A, George R, Lepoutre A, Lernout T, et al. Pneumococcal serotypes in children in 4 European countries. Emerg Infect Dis. 2010;16:1428–1439. doi: 10.3201/eid1609.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito S, Principi N. Impacts of the 13-valent pneumococcal conjugate vaccine in children. J Immunol Res. 2015;2015:591580. doi: 10.1155/2015/591580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, Yu SJ, Gao W, Yao KH, Shen AD, Yang YH. Serotype distribution and antibiotic resistance of 140 pneumococcal isolates from pediatric patients with upper respiratory infections in Beijing 2010. Vaccine. 2011;29:7704–7710. doi: 10.1016/j.vaccine.2011.07.137. [DOI] [PubMed] [Google Scholar]
- 8.Xue L, Yao K, Xie G, Zheng Y, Wang C, Shang Y, et al. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates that cause invasive disease among Chinese children. Clin Infect Dis. 2010;50:741–744. doi: 10.1086/650534. [DOI] [PubMed] [Google Scholar]
- 9.Zhao C, Zhang F, Chu Y, Liu Y, Cao B, Chen M, et al. Phenotypic and genotypic characteristic of invasive pneumococcal isolates from both children and adult patients from a multicenter surveillance in China 2005-2011. PLoS One. 2013;8:e82361. doi: 10.1371/journal.pone.0082361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill PC, Townend J, Antonio M, Akisanya B, Ebruke C, Lahai G, et al. Transmission of Streptococcus pneumoniae in rural Gambian villages: a longitudinal study. Clin Infect Dis. 2010;50:1468–1476. doi: 10.1086/652443. [DOI] [PubMed] [Google Scholar]
- 11.Browall S, Norman M, Tangrot J, Galanis I, Sjostrom K, Dagerhamn J, et al. Intraclonal variations among Streptococcus pneumoniae isolates influence the likelihood of invasive disease in children. J Infect Dis. 2014;209:377–388. doi: 10.1093/infdis/jit481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai F, Talekar SJ, Klugman KP, Vidal JE. Expression of virulence-related genes in the nasopharynx of healthy children. PLoS One. 2013;8:e67147. doi: 10.1371/journal.pone.0067147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, Wanjiru E, et al. Rates of acquisition and clearance of pneumococcal serotypes in the nasopharynges of children in Kilifi District, Kenya. J Infect Dis. 2012;206:1020–1029. doi: 10.1093/infdis/jis447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogaert D, De Groot R, Hermans P. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 15.Resti M, Moriondo M, Cortimiglia M, Indolfi G, Canessa C, Becciolini L, et al. Community-acquired bacteremic pneumococcal pneumonia in children: diagnosis and serotyping by real-time polymerase chain reaction using blood samples. Clin Infect Dis. 2010;51:1042–1049. doi: 10.1086/656579. [DOI] [PubMed] [Google Scholar]
- 16.Siira L, Kaijalainen T, Lambertsen L, Nahm MH, Toropainen M, Virolainen A. From Quellung to multiplex PCR, and back when needed, in pneumococcal serotyping. J Clin Microbiol. 2012;50:2727–2731. doi: 10.1128/JCM.00689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donkor ES, Stabler RA, Hinds J, Adegbola RA, Antonio M, Wren BW. Comparative phylogenomics of Streptococcus pneumoniae isolated from invasive disease and nasopharyngeal carriage from West Africans. BMC Genomics. 2012;13:569. doi: 10.1186/1471-2164-13-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wayne P. Methods for Dilution Antimicrobial Susceptibility Tests f or Bacteria That Grow Aerobically; Approved Standard - Ninth Edition. CLSI Document M07-A9. Wayne (Pennsylvania): Clinical and Laboratory Standards Institute. 2012. Available from: antimicrobianos.com.ar/ATB/wp-content/.../03-CLSI-M07-A9-2012.pdf .
- 19.Wayne P. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI Document M100-S24. Wayne (Pennsylvania): Clinical and Laboratory Standards Institute. 2014. Available from: http://ncipd.org/control/images/NCIPD_docs/CLSI_M100-S24.pdf .
- 20.Melin M, Trzciński K, Antonio M, Meri S, Adegbola R, Kaijalainen T, et al. Serotype-related variation in susceptibility to complement deposition and opsonophagocytosis among clinical isolates of Streptococcus pneumoniae. Infect Immun. 2010;78:5252–5261. doi: 10.1128/IAI.00739-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguinagalde L, Corsini B, Domenech A, Domenech M, Camara J, Ardanuy C, et al. Emergence of amoxicillin-resistant variants of Spain9V-ST156 pneumococci expressing serotype 11A correlates with their ability to evade the host immune response. PLoS One. 2015;10:e0137565. doi: 10.1371/journal.pone.0137565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaiswal N, Singh M, Das RR, Jindal I, Agarwal A, Thumburu KK, et al. Distribution of serotypes, vaccine coverage, and antimicrobial susceptibility pattern of Streptococcus pneumoniae in children living in SAARC countries: a systematic review. PLoS One. 2014;9:e108617. doi: 10.1371/journal.pone.0108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bewick T, Sheppard C, Greenwood S, Slack M, Trotter C, George R, et al. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax. 2012;67:540–545. doi: 10.1136/thoraxjnl-2011-201092. [DOI] [PubMed] [Google Scholar]
- 24.Saha SK, Naheed A, El Arifeen S, Islam M, Al-Emran H, Amin R, et al. Surveillance for invasive Streptococcus pneumoniae disease among hospitalized children in Bangladesh: antimicrobial susceptibility and serotype distribution. Clin Infect Dis. 2009;48(Suppl 2):S75–S81. doi: 10.1086/596544. [DOI] [PubMed] [Google Scholar]
- 25.Balaji V, Jayaraman R, Verghese VP, Baliga PR, Kurien T. Pneumococcal serotypes associated with invasive disease in under five children in India and implications for vaccine policy. Indian J Med Res. 2015;142:286–292. doi: 10.4103/0971-5916.166588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jauneikaite E, Mary Carnon Jefferies J, William Vere Churton N, Tzer Pin Lin R, Lloyd Hibberd M, Charles Clarke S. Genetic diversity of Streptococcus pneumoniae causing meningitis and sepsis in Singapore during the first year of PCV7 implementation. Emerg Microbes Infect. 2014;3:e39. doi: 10.1038/emi.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho PL, Chiu SS, Ang I, Lau YL. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae before and after introduction of 7-valent pneumococcal conjugate vaccine, Hong Kong 1995-2009. Vaccine. 2011;29:3270–3275. doi: 10.1016/j.vaccine.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Harboe ZB, Valentiner-Branth P, Ingels H, Rasmussen JN, Andersen PH, Bjerre CC, et al. Pediatric invasive pneumococcal disease caused by vaccine serotypes following the introduction of conjugate vaccination in Denmark. PLoS One. 2013;8:e51460. doi: 10.1371/journal.pone.0051460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendes RE, Hollingsworth RC, Costello A, Jones RN, Isturiz RE, Hewlett D, Jr, et al. Noninvasive Streptococcus pneumoniae serotypes recovered from hospitalized adult patients in the United States in 2009 to 2012. Antimicrob Agents Chemother. 2015;59:5595–5601. doi: 10.1128/AAC.00182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Serres G, Pilishvili T, Link-Gelles R, Reingold A, Gershman K, Petit S, et al. Use of surveillance data to estimate the effectiveness of the 7-valent conjugate pneumococcal vaccine in children less than 5 years of age over a 9 year period. Vaccine. 2012;30:4067–4072. doi: 10.1016/j.vaccine.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Ochoa-Gondar O, Gómez-Bertomeu F, Vila-Córcoles A, Raga X, Aguirre C, Utrera J, et al. [Prevalence of serotypes causing invasive pneumococcal disease in the region of Tarragona, Spain 2006-2009: vaccine-serotype coverage for the distinct antipneumococcal vaccine formulations] Rev Esp Quimioter. 2015;28:29–35. Spanish. [PubMed] [Google Scholar]
- 32.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. J Infect Dis 2010; 201: 32-41. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigo C, Bewick T, Sheppard C, Greenwood S, Macgregor V, Trotter C, et al. Pneumococcal serotypes in adult non-invasive and invasive pneumonia in relation to child contact and child vaccination status. Thorax. 2014;69:168–173. doi: 10.1136/thoraxjnl-2013-203987. [DOI] [PubMed] [Google Scholar]
- 34.Pfaller MA, Farrell DJ, Sader HS, Jones RN. AWARE Ceftaroline Surveillance Program (2008-2010): trends in resistance patterns among Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in the United States. Clin Infect Dis. 2012;55(Suppl 3) doi: 10.1093/cid/cis561. [DOI] [PubMed] [Google Scholar]
- 35.Donkor ES, Adegbola RA, Wren BW, Antonio M. Population biology of Streptococcus pneumoniae in West Africa: multilocus sequence typing of serotypes that exhibit different predisposition to invasive disease and carriage. PLoS One. 2013;8:e53925. doi: 10.1371/journal.pone.0053925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siripongpreeda N, Hattasingh W, Amornvipas P, Eampokalap B, Sakoolgnam S, Pancharoen C, et al. Frequency and clinical course of invasive pneumococcal disease caused by penicillin-resistant and penicillin-sensitive Streptococcus pneumoniae in Thai children. J Med Assoc Thai. 2010;93(Suppl 5):S1–S5. [PubMed] [Google Scholar]
- 37.Shah AS, Knoll MD, Sharma PR, Moisi JC, Kulkarni P, Lalitha MK, et al. Invasive pneumococcal disease in Kanti Children’s Hospital, Nepal, as observed by the South Asian Pneumococcal Alliance network. Clin Infect Dis. 2009;48(Suppl 2):S123–S128. doi: 10.1086/596490. [DOI] [PubMed] [Google Scholar]
- 38.Phongsamart W, Srifeungfung S, Chatsuwan T, Nunthapisud P, Treerauthaweeraphong V, Rungnobhakhun P, et al. Changing trends in serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae causing invasive diseases in Central Thailand 2009-2012. Hum Vaccin Immunother. 2014;10:1866–1873. doi: 10.4161/hv.28675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godfroid F, Hermand P, Verlant V, Denoël P, Poolman JT. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect Immun. 2011;79:238–245. doi: 10.1128/IAI.00378-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min X, Zhang X, Wang H, Gong Y, Li M, Xu W, et al. Protection against pneumococcal infection elicited by immunization with glutamyl tRNA synthetase, polyamine transport protein D and sortase A. Vaccine. 2012;30:3624–3633. doi: 10.1016/j.vaccine.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 41.Harfouche C, Filippini S, Gianfaldoni C, Ruggiero P, Moschioni M, Maccari S, et al. RrgB321, a fusion protein of the three variants of the pneumococcal pilus backbone RrgB, is protective in vivo and elicits opsonic antibodies. Infect Immun. 2012;80:451–460. doi: 10.1128/IAI.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Wang H, Zhang S, Zeng L, Xu X, Wu K, et al. Mucosal immunization with recombinant fusion protein DnaJ-DeltaA146Ply enhances cross-protective immunity against Streptococcus pneumoniae infection in mice via interleukin 17A. Infect Immun. 2014;82:1666–1675. doi: 10.1128/IAI.01391-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu JH, Dai WJ, Chen B, Fan XY. Mucosal immunization with PsaA protein, using chitosan as a delivery system, increases protection against acute otitis media and invasive infection by Streptococcus pneumoniae. Scand J Immunol. 2015;81:177–185. doi: 10.1111/sji.12267. [DOI] [PubMed] [Google Scholar]
- 44.Ricketson LJ, Wood ML, Vanderkooi OG, MacDonald JC, Martin IE, Demczuk WH, et al. Trends in asymptomatic nasopharyngeal colonization with Streptococcus pneumoniae after introduction of the 13-valent pneumococcal conjugate vaccine in Calgary, Canada. Pediatr Infect Dis J. 2014;33:724–730. doi: 10.1097/INF.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 45.Kumar KL, Ashok V, Ganaie F, Ramesh AC. Nasopharyngeal carriage, antibiogram and serotype distribution of Streptococcus pneumoniae among healthy under five children. Indian J Med Res. 2014;140:216–220. [PMC free article] [PubMed] [Google Scholar]


