Abstract
Background:
The aim of this study is to find out the spatial and temporal expression of TGF-b1 during the tendon healing, after application of Platelet Rich Plasma (PRP).
Methods:
A patellar tendon defect model in rabbits was used for this purpose. 48 skeletally mature New Zealand White rabbits, weighing 3.5 kg, were used for this study. Equal numbers of animals from both groups were sacrificed at 4 different time points (1st, 2nd, 3rd, and 4th week). A full thickness patellar tendon substance in the right limb of each animal was excised from its central portion during the operation. PRP with a gel form was applied and filled the tendon defect in PRP group. No PRP was applied in the tendon defect of controls. Histological sections with hematoxylin-eosin and immunohistochemical sections with an anti-TGF-b1 primary antibody were made for the evaluation of the results.
Results:
A differentiation of the healing process was observed in the PRP group in comparison with the control group. TGF-b1 expression was detected in various cell populations (inflammatory cells, endothelial cells, macrophages, and tenocytes). Both cytoplasmic and nuclear expressions were present. The larger amounts of immunoexpression were localized in epitenon and in the repair site. PRP group showed stronger and more extensive staining at 1st and 2nd week (P<0.0001), whereas control group showed more extensive staining at the 3rd and 4th week (P<0.0001).
Conclusions:
Our study demonstrates that locally application of PRP result in an alteration of TGF-b1 expression during the healing of a patellar tendon defect.
Keywords: Platelet-Rich Plasma, Patellar tendon defect, Rabbits, Tendon healing, TGF-b1
Introduction
Platelet Rich Plasma (PRP) is defined as a portion of the plasma fraction of autologous blood having a platelet concentration above baseline. As such, PRP contains not only a high level of platelets but also the full complement of clotting factors and secretory proteins (1). Due to the increased concentration and release of a severe number of growth factors, PRP can potentially enhance the recruitment and proliferation of tenocytes, stem cells, and endothelial cells.
One of the growth factors included in PRP is transforming growth factor b (TGF-b). TGF-b is a well-known cytokine that regulates various processes in tendon healing. It modulates the inflammatory responses in early healing stages, participates in the intricate control of angiogenesis, regulates the proteoglycan deposition, and stimulates the production of collagens by tendon fibroblasts (2).
The effect of PRP in tendon and ligament healing has been studied mainly on a biomechanical basis (3-5). We have recently demonstrated the influence of PRP on angiogenesis, on the expression of Insulin-like Growth Factor I (IGF-I) (6-8), and on the mechanical properties of the injured tendons during healing (9). After the encouraging results, we decided to proceed in the immunohistochemical study of the temporal and spatial expression of TGF-b1, in an attempt to understand better the way that PRP acts on tendon healing. For this purpose, we have used the same patellar tendon defect model in rabbits.
Materials and Methods
Animals
Sample size was determined using an 80% power, significance level 0.05, an initial estimate of the standard deviation equal to 17 so as to detect a difference of 30 in the mean. The desired sample size for each group was equal to 6 (EpiInfo, version 6). The calculations refer to every week. After approval from the regional ethical board (nr T44/77), 48 skeletally mature New Zealand White rabbits were used for this study. The average weight was 3.5 kg, and institutional guidelines for the care and treatment of laboratory animals were adhered to. Randomization of the animals was done without stratification for sex and the rabbits were housed one per cage with food and water available ad libidum. 24 animals (24 limbs) received the PRP, and 24 animals (24 limbs) served as untreated control group. Six animals (6 limbs) from PRP group and 6 animals (6 limbs) from control group were sacrificed at 4 different time points (1st, 2nd, 3rd, and 4th week). PRP Fast, kindly provided by Bioteck, was used for the preparation of PRP.
PRP preparation- Surgical procedure
Initially, we have used five rabbits as donors in order to measure some important parameters in the PRP gel. A standard hemocytometer was used to measure platelet counts and commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D System, Inc.,USA) were used to quantify the concentration of TGF-β1, according to the manufacturer’s instructions. Our experimental model was based in the study of Anaguchi et al. (10). PRP preparation and surgical procedure are described in details in a previous published study (6). During the operation, a full thickness patellar tendon substance in the right limb of each animal was excised from its central portion. In PRP group, PRP with a gel form was applied and filled the tendon defect. The same procedure was performed in the right limbs in the control group, without the application of PRP into the patellar tendon defect. We chose not to treat the one limb with PRP and the other limb of the same animal as control because of the potential passage of the growth factors in the systemic circulation. In such a case, PRP’s growth factors would affect the control site. No immobilization was applied after surgery, and the rabbits were allowed unrestricted daily activities in their cages.
Histology
After 1, 2, 3, and 4 weeks the animals were sacrificed with an overdose of intracardiac injection of 10% KCl solution under general anaesthesia. The entire patellar tendon was then removed and dissected free from other tissues. Then, each tissue was fixed in a 10% buffered formalin solution and cast in a paraffin block. The tendon was sectioned parallel to the longitudinal axis, and stained with hematoxylin and eosin. From each tendon, 6 paraffin sections were made. Of them, 3 sections were subjected to microscopic examination, while the other 3 were immunostained with an anti-TGF-β primary antibody. All sections were analyzed by a single pathologist, who was blinded of the treatment groups.
Immunohistochemistry
Section 4 μm in thickness were cut from the paraffin blocks and placed on charged poly-L-lysine coated glass slides, heated overnight at 37oC, deparaffinized in xylene, and rehydrated through a graded series of ethanol. Antigen retrieval was achieved by treating slides in citrate buffer solution 10 mM at pH 6.0 for 15 min. Then the slides were immersed in 3% hydrogen peroxide for 20 min at room temperature to block endogenous peroxidase activity. To block non-specific binding sites, the slides were treated with 10% goat serum for 10 min at room temperature. Thereafter, the slides were incubated with anti-TGF-β1 primary antibody (clone MCA 797, AbD Serotec, Oxford, UK) at a dilution of 1:80 for 30 min at room temperature. Envision detection system was used as the second step of technique (Dako, Glostrup, Denmark). The bound antibodies were visualized using 3,3’ diaminobenzidine tetrahydrochloride (DAB) as chromogen. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted in diphenylxylene for quantitative analysis. In each batch of staining, positive controls with high TGF-β1 expression for the antibody used, whereas substitution of an isotype-matched irrelevant antibody in place of the primary antibody was used as negative control.
Image analysis
Immunohistochemistry was quantified by the use of image analysis (11). Five representative figures from the epitenon and the repair sites (were the strongest expression of TGF-β1 was detected) were captured from each slide with the assistance of a Nikon Eclipse 80i microscope, equipped with a Nikon DS-2MV digital camera (Nikon Corp, Tokyo, Japan). Each figure viewed in 200x original magnification and stored as uncompressed BMP image in a resolution of 1600*1200 pixels. Then, with the appropriate software, Image ProPlus v5.1 (Media Cybernetics, MD, USA) we evaluated the density and extend of brown diaminobenzidine (DAB) staining. Values for color density have a range from 0 (black) to 255 (white). Positive and negative control slides were used for the optimal separation of brown and blue stained areas.
Statistical Analysis
All results are expressed as mean ± SD. Significant differences among groups were evaluated using the Mann-Whitney U test. A difference of P<0.05 was considered to be statistically significant (SPSS 12.0, Chicago, Illinois).
Results
Platelet count and TGF-b1 concentrations
Platelet concentration (mean±SD) in whole blood and PRP was 531 ± 138 * 103, and 4,248 ± 1,132 * 103 respectively. Furthermore, the concentration of TGF-b1 was 52.14 ± 13.32 ng/ml in whole blood and 199.37 ± 36.62 ng/ml in PRP.
Histology
At 1st week the endotenon had a normal appearance in all time-points, while the epitenon was thickened from 2 to 5-6 cell layers. Thickening was more obvious near the repair site. A granular tissue was filling the gap with intense mononuclear infiltratings and newelly formed vessels. Additionally, collagen fibers were admixed with plumbed tenocytes which also lost the normal orientation. Fibroblast like cells became more obvious in the defect site at 2nd week, and there was inconsistent neoformation of blood vessels and rare presence of collagen fibers and fibrosis. At 3rd week the formed tissue remains more immature in the control group, by the meaning of that its synthesis is less compact and there is absence of tenocyte longitudinal orientation. On the other hand, in PRP group the tissue is denser with less elastic fibers remaining [Figure 1]. Finally, at 4th week there was no clearly visible border between the healed site and the proximal tendon in PRP group. In the control group, sparse cellularity was still present.
Figure 1.

Representative figures from control group (A) and PRP group (B) at 3rd week. Epitenon thickening is present in control group with severe amounts of granular tissue and vessels in the parenchyma. At the same time, only a central portion of granular tissue is present, the cellularity is sparse and the tenocytes demonstrate better orientation in PRP group.
Immunohistochemistry
TGF-β1 expression was detected in various cell populations. In the early phases of regeneration, it was found mainly in inflammatory cells, endothelial cells, macrophages from the granular tissue which filled the surgical gap and in irregularly shaped tenocytes. Later, the above cells were suppressed as healing proceeded, and TGF-β1 was detected mainly in tenocytes. Both cytoplasmic and nuclear expressions were present. The larger amounts of immunoexpression were localized in epitenon and in the repair site.
PRP group showed stronger and more extensive staining at 1st and 2nd week (P<0.001). At the 3rd and 4th week control group showed more extensive staining (P<0.001). The tissue levels of TGF-β1 as they quantified with the image analysis, in PRP group were at the peak level from the 1st week, remained high at 2nd week and started to decline at 3rd week. Specimens from this group at the 4th week showed only weak to absent staining. Respectively, control group had the peak level at 2nd week, remained the same at 3rd week and at 4th week slightly declined [Table 1; Figure 2, 3A-H].
Table 1.
Image analysis results of color density (mean, SD) of the area positively stained with TGF-b1. Note that large values of the color density indicate low TGF-b1 expression and conversely
| Week | Control Mean color density (SD) | PRP Mean color density (SD) | P* |
|---|---|---|---|
| 1 | 101.9 (16.7) | 56.2 (17.5) | <0.0001 |
| 2 | 86.1 (15.1) | 67.7 (11.2) | <0.0001 |
| 3 | 89.8 (8.2) | 106.5 (17.1) | <0.0001 |
| 4 | 114.3 (18.6) | 181.9 (25.9) | <0.0001 |
PRP platelet-rich plasma
P<0.05 indicates a significant difference between the two groups
Figure 2.

Presentation of the expression of TGF-b1 in PRP and control group. Note that large values of the color density indicate low TGF-b1 expression and conversely.
Figure 3.
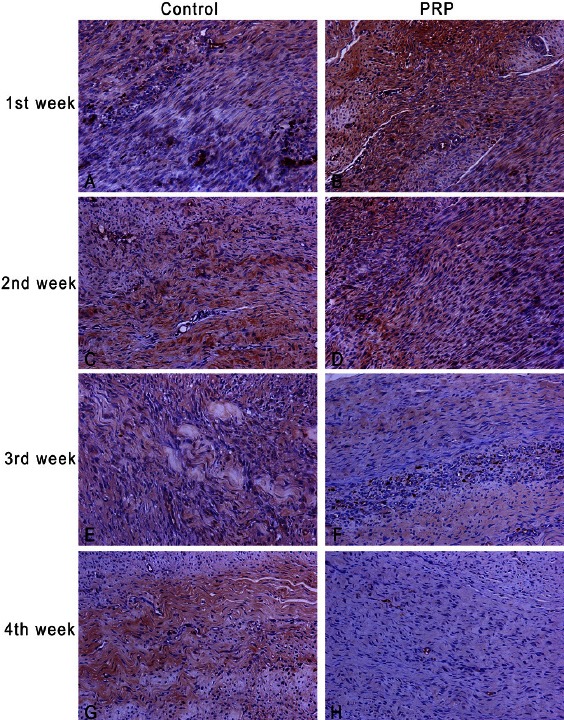
Immunoexpression of TGF-β1 during healing process in control group and PRP group, original magnification 200x. First column refers to control group and the second to PRP group. Staining was detected both in epitenon cells and in the repair site. More dense staining in PRP group at 1st and 2nd week (B,D) compared to control group (A,C). Control group has more extensive staining at 3rd and 4th week (E,G) than PRP group (F,H). Notice that at 4th week control group still presents higher TGF-β1 expression.
Discussion
The role of TGF-b on the physiological healing process has been well documented. Natsu-ume et al. in a rat patellar tendon model showed that tendon healing may be accompanied by extensive changes in the expression of TGF-b over the whole length of the tendon tissue (12). Furthermore, Chan et al. demonstrated that the interaction of TGF-b isoforms exists in the regulation of collagen synthesis in tendon fibroblasts, and that their effects may be further complicated by uneven spatial distribution of TGF-b and TGF-b receptors in healing tendons (13). Both studies revealed that TGF-b levels remained high for at least 8 weeks (12, 13).
The effect of the administration of exogenous TGF-b in the mechanical properties of the healed tendon has been also documented. Yamazaki et al. showed that exogenous administration of TGF-b1 in ACL replacement using flexor tendon autograft, significantly increased the bonding strength of the graft to the tunnel wall at 3 weeks (14). Furthermore, Anaguchi et al. showed that the application of TGF-beta1 significantly increases the tangent modulus and the tensile strength of the fibrous tissue regenerated in the patellar tendon after resecting the central portion (10).
The positive influence of PRP on the mechanical properties of the healed tendons and ligaments has been also showed in previous studies (3-5, 15). On the other hand, in only one study was demonstrated that locally injected PRP in an injured rat patellar tendon act as an activator of circulation-derived cells for enhancement of the initial tendon healing process (2). However, the role of growth factors under the influence of PRP in the tendon healing process is not documented. In our study we demonstrate the temporal and spatial expression of TGF-b after application of PRP. Unfortunately, there are no studies in the national literature concerning the expression of TGF-b1 during tendon healing after application of PRP.
Our results show an immunohistochemically expression of TGF-b1 in the whole early phase of healing in both PRP and control group. These results are in agreement with previous studies (12, 13). The spatial expression of TGF-b1 in inflammatory cells, endothelial cells, macrophage, and tenocytes has been also previously described. Platelets may be a source of extracellular TGF-b1 immediately after injury because the a-granules of platelets contain TGF-b1 and their degranulation releases immunoreactive TGF-b1 (16, 17). Subsequently, TGF-b1 expression mostly corresponded with the cellular distribution. This suggests that TGF-b1 is thereafter synthesized by the cells in the wound site (12). The extracellular distribution of TGF-b1 was not demonstrated in our study because the first time point was in day 7 after injury, where TGF-b1 was already intracellularly expressed.
Furthermore, we demonstrated a significant increased expression of TGF-b1 in PRP group during the first 2 weeks of healing in comparison with controls, and a significant decreased expression during the two last weeks. It seems that the large concentration of platelets in PRP result in the overexpression of TGF-b1 in the healed tendon. Local administration of PRP has also changed the peak time of TGF-b1 and this probably has influenced the whole healing process. We cannot explain the reason of reduction of TGF-b1. It is possible that the acceleration of the healing process in PRP group comprise the signal for TGF-b1 suppression, in antithesis with the controls where the healing progress more slowly. This in turn may play a very important role in the formation of adhesions. It is believed that the suppression of TGF-b1 results in decreasing adhesion formation (18, 19). PRP might influence the later stages of healing by suppression of TGF-b1 and lead to reduction of scar proliferation and increase of functionality.
In conclusion, we have demonstrated that locally application of PRP result in an alteration of TGF-b1 expression during the healing of a patellar tendon defect. Our results, in combination to our previous findings that PRP enhance angiogenesis, alter the expression of IGF-I, and improves the mechanical properties of the healed tendons, may yield novel insights into the process of tendon healing, and provide a rational basis for the application of PRP in tendon defects (6-9).
Acknowledgements
Authors would like to thank Periklis Kakias and Vissaris Kakias for their assistance in the technical part.
References
- 1.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–8. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kajikawa Y, Morihara T, Sakamoto H, Matsuda K, Oshima Y, Yoshida A, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215(3):837–45. doi: 10.1002/jcp.21368. [DOI] [PubMed] [Google Scholar]
- 3.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93–9. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 4.Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24(4):820–30. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 5.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 6.Lyras D, Kazakos K, Verettas D, Polychronidis A, Simopoulos C, Botaitis S, et al. Immunohistochemical study of angiogenesis after local administration of platelet-rich plasma in a patellar tendon defect. Int Orthop. 2010;34(1):143–8. doi: 10.1007/s00264-009-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyras DN, Kazakos K, Verettas D, Polychronidis A, Tryfonidis M, Botaitis S, et al. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30(11):1101–6. doi: 10.3113/FAI.2009.1101. [DOI] [PubMed] [Google Scholar]
- 8.Lyras DN, Kazakos K, Agrogiannis G, Verettas D, Kokka A, Kiziridis G, et al. Experimental study of tendon healing early phase: Is IGF-1 expression influenced by platelet rich plasma gel? Orthop Traumatol Surg Res. 2010;96(4):381–7. doi: 10.1016/j.otsr.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Lyras DN, Kazakos K, Verettas D, Botaitis S, Agrogiannis G, Kokka A, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009;129(11):1577–82. doi: 10.1007/s00402-009-0935-4. [DOI] [PubMed] [Google Scholar]
- 10.Anaguchi Y, Yasuda K, Majima T, Tohyama H, Minami A, Hayasi K, et al. The effect of transforming growth factor-beta on mechanical properties of the fibrous tissue regenerated in the patellar tendon after resecting the central portion. Clin Biomech (Bristol, Avon) 2005;20(9):959–65. doi: 10.1016/j.clinbiomech.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Zafirellis K, Agrogiannis G, Zachaki A, Gravani K, Karameris A, Kombouras C. Prognostic significance of VEGF expression evaluated by quantitative immunohistochemical analysis in colorectal cancer. J Surg Res. 2008;147(1):99–107. doi: 10.1016/j.jss.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Natsu-ume T, Nakamura N, Shino K, Toritsuka Y, Horibe S, Ochi T. Temporal and spatial expression of transforming growth factor-beta in the healing patellar ligament of the rat. J Orthop Res. 1997;15(6):837–43. doi: 10.1002/jor.1100150608. [DOI] [PubMed] [Google Scholar]
- 13.Chan KM, Fu SC, Wong YP, Hui WC, Cheuk YC, Wong MW. Expression of transforming growth factor beta isoforms and their roles in tendon healing. Wound Repair Regen. 2008;16(3):399–407. doi: 10.1111/j.1524-475X.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki S, Yasuda K, Tomita F, Tohyama H, Minami A. The effect of transforming growth factor-beta1 on intraosseous healing of flexor tendon autograft replacement of anterior cruciate ligament in dogs. Arthroscopy. 2005;21(9):1034–41. doi: 10.1016/j.arthro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Anitua E, Sanchez M, Nurden AT, Zalduendo M, de la Fuente M, Orive G, et al. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res A. 2006;77(2):285–93. doi: 10.1002/jbm.a.30585. [DOI] [PubMed] [Google Scholar]
- 16.Wakefield LM, Smith DM, Flanders KC, Sporn MB. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988;263(16):7646–54. [PubMed] [Google Scholar]
- 17.Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, et al. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987;84(17):6020–4. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105(1):148–55. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg Am. 2002;27(4):615–20. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]


