Abstract
Aneurysmal bone cyst (ABC) is a benign expansile bone tumor, most commonly involving the medulla of long bones. ABC rarely arises within the cortex or in the subperiosteal region, radiographically mimicking other conditions, in particular surface osteosarcomathat is low-grade in nature and may go secondary ABC changes, and telangiectatic osteosarcoma. Both of these are sometimes mistaken microscopically for primary ABC. We review the characteristics of ABC cases in our center and report four unusualsurface ABCs arising in the subperiosteal or cortical region of long bones, identified among 38 histologically proven ABCs during a four-year period in our center. The surface ABCs occurred at an older agewith a predilection for diaphysis of femur, tibia, and humerus.
Keywords: Aneurysmal bone cyst, Cortical, Histopathology, Radiography, Subperiosteal
Introduction
A neurysmal bone cysts (ABCs) are benign and expansile bone tumors, occurring in the first two decades of life, comprising about 1% of all primary bone tumors (1). Almost 80% of the lesions occur in skeletally immature patients who are younger than 20 years (2). Aggressive local growth, cortical destruction, and recurrences are common. ABC typically affects the metaphysis of long bones, pelvis, and posterior vertebral elements. However, some cases of ABC involving unusual bones including rib, hand, foot, scapula, clavicle, mandible, and skull have been reported (1-8). The radiographic feature is very distinctive in most cases, characteristically presenting as a lytic and multi-loculated lesion with blowout or expanded character, andan overlying area of cortical disruption (2). Some authors have proposed different classifications for ABCs of long bones based on the radiographic appearances (9-11). The conventional medullary type, being the most common, usually arises symmetrically within the medullary cavity and causes thinning of the bone cortex. Surface ABC, former being called as subperiosteal giant cell tumor or subperiosteal osteoclasia, are less common, with a relative incidence of about 16-17% (11, 12). Surface ABCs, with either subperiosteal or cortical localization have been rarely documented. The aim of this study is to review the characteristics of ABC cases in our center in a period of four years and illustrate features of the surface variants.
Materials and Methods
A retrospective study performed on all histologically proven cases of aneurysmal bone cysts referred to Shafa Yahyaeyan Hospital Pathology Laboratory a specialized center for bone and soft tissue tumors, over the past four years between April 2011 and April 2015. We report four unusual cases of surface ABCs extracted from this series of primary ABCs. Only primary ABCs were included in the study. Surface ABCs were diagnosed based on morphologic classification of ABCs described by Cappana and co-workers (10). In this classification types I-III represent medullary origin and type IV denotes the subperiosteal variety and V describes the subperiosteal type that extends towards the center of bone and is regarded as cortical type. Data analysis performed using SPSS software version 16.0.
Case Reports
A total number of 38 primary ABCs were identified, of which four (10.5%) were considered to originate from bone surface either in subperiosteal or cortical region. Patients had a mean age of 17.5± 9.4 years, ranging from 3 to 44 years. It is most frequent in the second decade of age (55.3% of cases). We observed no sex predominance (M: F ratio=1). 28 lesions (68.5%) were located in long bones with femur being the most frequent site (23.7%) followed by tibia (15.8%), fibula (10.5%), humerus (10.5%), radius (5.3%), and ulna (2.6%). Table 1 demonstrates the demographic and clinical characteristics of the cases. Most cases of long bones involved the metaphysis (81.9%) with only one case being purely epiphyseal (4.5%) and 3 purely diaphyseal (13.6%). All of the cases were treated with curettage procedures at the first presentation. Five cases (13.2%) experienced recurrences. Recurred cases showed a predilection for long bones but it was not statistically different compared to others (P=0.697) [Table 1]. Other characteristics of recurred cases are illustrated in Table 1 in comparison with non-recurred ones.
Table 1.
Clinical, histologic, and demographic characteristics of ABC cases
| Variable | All ABCs | Recurred ABCs | Non-recurred ABCs | Surface ABCs | Intramedullary ABCs | |
|---|---|---|---|---|---|---|
| Age (Mean± SD) | 17.5± 9.4 | 17.6± 10.8 | 17.5± 9.4 | 28.5± 11.1 | 16.2± 8.5 | 0.980a 0.011b |
| Sex No. (%) | ||||||
| Male | 19 (50) | 4 (80) | 15 (45.5) | 3 (75) | 16 (47.1) | 0.170a 0.302b |
| Female | 19 (50) | 1 (20) | 18 (54.5) | 1 (25) | 18 (52.9) | |
| Involved Bone Type No. (%) | ||||||
| Long | 26 (68.4) | 4 (80) | 22 (66.7) | 4 (100) | 22 (64.7) | |
| Small tubular | 4 (10.5) | 0 (0) | 4 (12.1) | 0 (0) | 4 (11.8) | 0.697a 0.356b |
| Flat | 8 (21.1) | 1 (20) | 7 (21.2) | 0 (0) | 8 (23.5) | |
| Radiologic location No. (%) | ||||||
| Intramedullary | 34 (89.5) | 0 (0) | 4 (12.1) | - | - | 0.554a |
| Surface | 4 (10.5) | 5 (100) | 29 (87.9) | - | - | |
| Recurrence No. (%) | ||||||
| Yes | 5 (13.2) | - | - | 0 (0) | 5 (14.7) | 0.554b |
| No | 33 (86.8) | - | - | 4 (100) | 29 (85.3) | |
| Microscopic type No. (%) | ||||||
| Conventional | 36 (94.7) | 4 (80) | 32 (97) | 4 (100) | 32 (94.1) | 0.249a |
| Solid | 2 (5.3) | 1 (20) | 1 (3) | 0 (0) | 2 (5.9) | 0.798b |
| Total | 38 | 5 | 33 | 4 | 34 | |
P-value derived from comparison analyses between recurred and non-recurred groups.
P-value derived from comparison analyses between surface and intramedullary groups.
Surface ABCs were found to occur merely in long bones. Statistical analysis of surface ABCs showed that these patients were significantly older in age (28.5±11.1) compared to those with intramedullary lesions (16.2± 8.5) (P=0.011), and that surface ABCs affect diaphysis more commonly (75%) compared to intramedullary ABCs which often involve the metaphysis (69.2%) with or without extension to epiphysis or diaphysis (P=0.02). Other characteristics of surface ABCs are shown on Table 1. We report clinical, radiological and histomorphological characteristics of surface ABCs as follow:
Case 1
A 19-year-old boy presented with a 5-month history of right lower leg pain in November 2014. Plain radiography showed a rather well-defined lytic cortical lesion in proximal tibia metaphysis [Figure 1]. Spiral computed tomography demonstrated a cortical lytic lesion with irregular borders measuring 30×10 mm in proximal tibia with soft tissue extension which was hyper intense in MR images [Figure 2], and active lesion with increased uptake on bone scan [Figure 3]. The overall findings were in favor of an osteoblastic tumor. He had undergone surgical biopsy at another center with the clinical suspicion of osteosarcoma. His first pathology report was in favor of osteosarcoma and the physician requested consultation by another pathologist. We received the paraffin blocks of his specimen for second opinion. Sections were prepared and stained with hematoxilin and eosin method. Microscopic examination of the slides revealed a benign giant cell-rich lesion, composed of oval to spindle-shaped cells admixed with many osteoclast-like giant cells and some blood-filled cystic structures outlined by fibro connective tissue. Areas of osteoid formation were noted [Figure 4]. The patient was then candidate of bone lesion curettage in the same center. The second specimen also confirmed the same diagnosis. The patient has not experienced any recurrences after the surgery. Post-operative images are not available.
Figure 1.
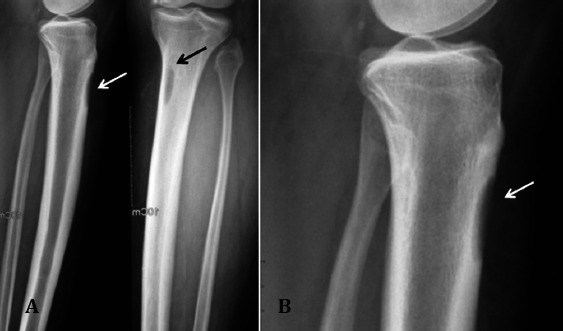
Radiograph. Antroposterior (AP), lateral and magnified radiographs of right proximal tibia show a lytic corticallesion (arrow) at the proximal metaphysis of tibia.
Figure 2.
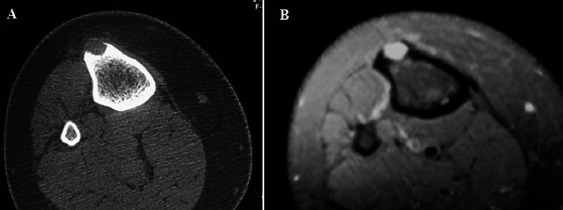
Acorticallytic lesion, hypodense in CT scan (A) and hyper signal in Fat-saturated T2W MRI (B) with a delicate cortical Shell.
Figure 3.
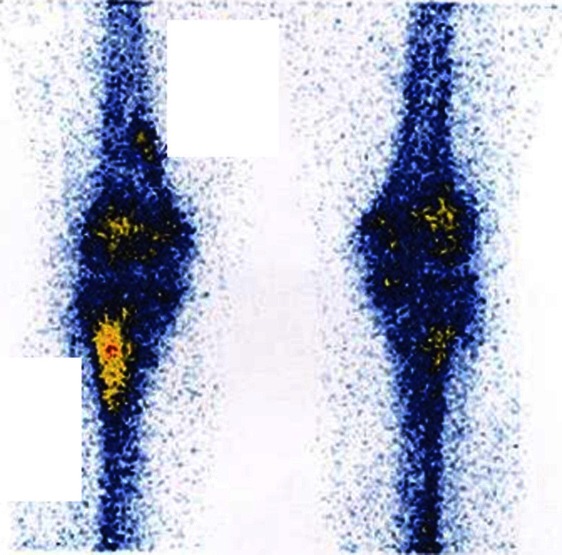
Bone scintigraphy revealedabnormal intense uptake at proximal tibia metaphysis.
Figure 4.
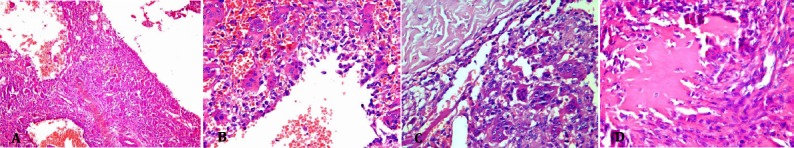
Microscopic slides. Low power (×10) (A) and high power (×40) (B-D) views of the tumor showing blood-filled cystic spaces surrounded by fibrous tissue composed of oval to spindle-shaped cells admixed with many osteoclast-like giant cells, and areas of osteoid formation.
Case 2
A 44-year-old male presented to the hospital with a six-month history of painful swelling of his left upper arm with recent increase in size. There was no history of fever, chills, weight loss, or trauma. Physical examination revealed moderate swelling of the anterio medial aspect of his upper arm with intact motion of the joints. Laboratory tests revealed an erythrocyte sedimentation rate (ESR) of 11 mm/h, C-reactive protein (CRP) of 2.0 mg/l, a white blood cell (WBC) count of 5860/mm3, an alkaline phosphatase (ALP) level of 276 mU/ml, lactic dehydrogenase (LDH) of 354 mU/ml, creative phosphokinase (CPK) of 66 mU/ml, calcium of 9.3 mg/dl, phosphorous of 3.4 mg/dl and parathormonhormone (PTH) of 32 pg/dl. Plain radiography showed a lytic intra cortical bone lesion within the humerus cortex [Figure 5]. CT scanning revealed a cortical lesion protruding from the bone cortex with extra osseous extension forming a prominent soft tissue component surrounded by a thin osseous layer [Figure 6]. Erosion of the cortex did not extend intra osseously to involve the medulla. Tc-MDP bone scintigraphy revealed abnormal areas of increased blood pool and uptake at the middle portion of left humerus [Figure 7].
Figure 5.
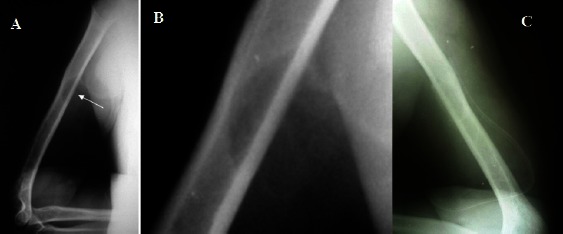
Radiograph. Lateral plain X-ray of the left arm (A) and the magnification view (B) shows a lytic mid-humeral shaft lesion. Post-operative image of the same lesion is shown (C).
Figure 6.
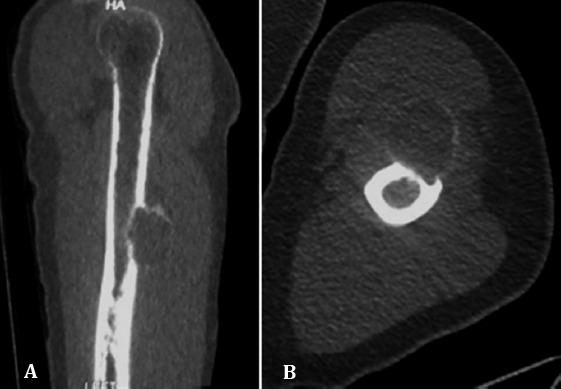
CT. Coronal reformat and axial CT images show intra cortical lesion bulging into adjacent soft tissues surrounded by a distinct new periosteal bone shell.
Figure 7.
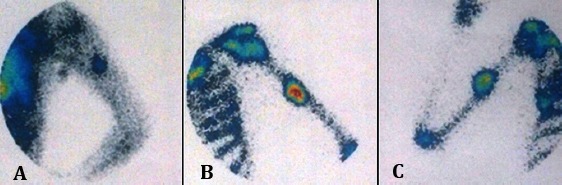
Whole body scan showed active tracer uptake with increased uptake in blood pool (A) and delayed (B)static images at the middle portion of left humerus.
The patient underwent incisional biopsy, confirming the diagnosis of ABC, and subsequently curettage and bone graft. The excised tumor measured 8×2.5×2 cm, consisting of three pieces of honeycombed spongy bone containing multiple blood-filled cystic spaces of varied sizes, scattered within solid bony tissue. Pathological examination of the resected specimen revealed spaces of various sizes containing blood and intervening septae consisting of fibrous tissue [Figure 8]. There was proliferation of osteoclast-like giant cells and stromal spindle-shaped cells and foci of osteoid formation along the septae. As in the incisional biopsy specimens, no atypical cells were detected, and there were no findings suggestive of malignancy. The patient is now disease-free with no recurrences since then. Post-operative plain radiographs are shown in Figure 5.
Figure 8.
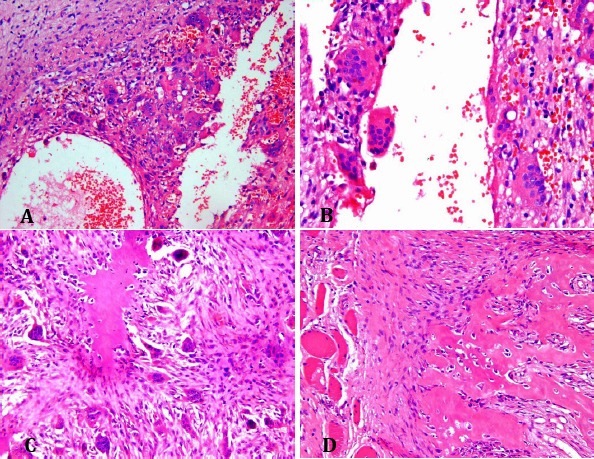
Microscopic slides. Low power (A) and high power (B) views of the tumor, composed of sinusoidal and vascular blood-filled spaces surrounded by fibrous septa consisted of immature bone trabeculae, many osteoclast-like giant cells, and fibroblasts (C). Note the bone shell at the periphery and within the soft tissue (D).
Case 3
A 21-year-old male presented to our hospital with a two-month history of left thigh pain. He mentioned no history of fever or trauma. Physical examination revealed mild tenderness at the anteromedial aspect of left thigh. Conventional radiographs showed a subperiosteal lytic lesion in the middle part of femur shaft with cortical cauterization [Figure 9]. CT scan showed a hypodenseosteolytic lesion with extension to soft tissue but it was rimmed by a peripheral osseous shell [Figure 10]. On magnetic resonance images, a well-defined lobulated mass appeared at left femoral shaft beneath the periosteum, containing multiple internal septations and fluid-fluid levels; T2W sequences revealed peripheral soft tissueedema, all in favor of an aneurysmal bone cyst [Figure 11]. Whole body scan revealed an expanded bone lesion with a rim of increased radiotracer uptake and photopenic center in left femoral mid shaft, accompanied by regional reactive hyperemia, which was suggestive of bone tumoral involvement, i.e. Ewing sarcoma, osteosarcoma, etc. [Figure 12].
Figure 9.
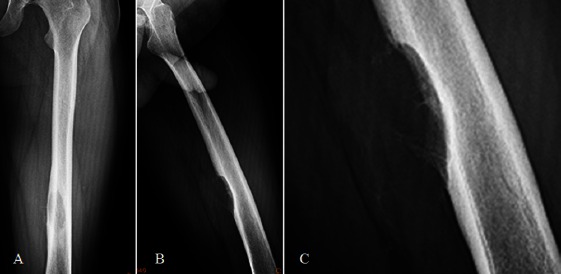
Radiographs. AP (A), lateral (B) and magnified (C) and Post-operative (D) plain radiographs of left femoral shaft show a lytic sub periosteal lesion with cortical cauterization.
Figure 10.
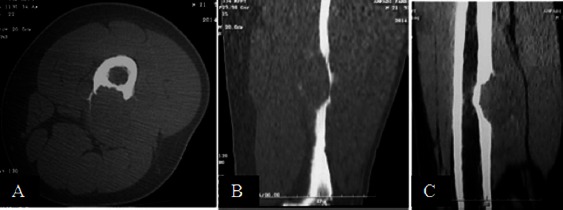
CT scan. Transverse (A), sagittal (B) and coronal (C) reformat CT scan images demonstrate a cortical hypodense lytic lesion sparing the medulla, bulging into surrounding soft tissue, leaving a thin osseous boundary at the periphery.
Figure 11.
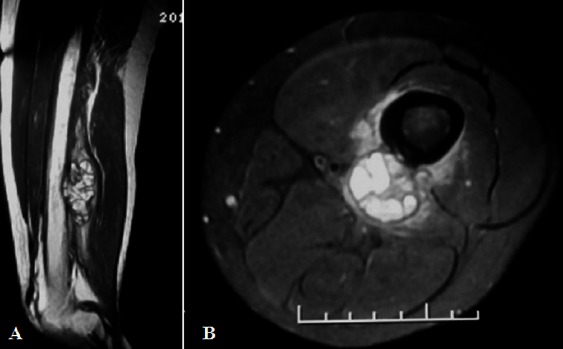
MR. left thigh sagittal T2W (A) and axial T2W-fat saturation (B) MR images reveal a sub periosteal lobulated mass with septationn and fluid-fluid levels.
Figure 12.
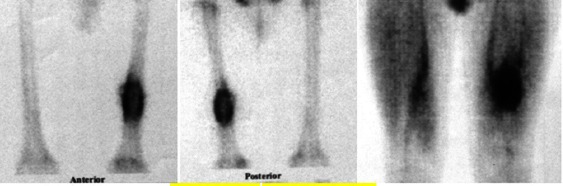
Bone scintigraphy. A bony lesion in left distal third femoral shaft with increased radio tracer uptake at periphery and photopenic center.
The patient undergone bone curettage followed by bone graft. The overall clinical data was in favor of a benign process and no biopsy performed prior to the surgery. Gross evaluation of the received specimen showed multiple pieces of creamy rubbery tissue measuring 9×8×3.5 cm with foci of firm consistency and hemorrhagic cystic cavities, rimmed by a thin bony shell. Microscopically the tumor showed vascularized hypo cellular areas of fibroblastic proliferation interspersed by cystic blood-filled spaces. Degenerated fibromyxoid tissue, some multinucleated osteoclast-like giant cells, and wide strands of reactive bone formation were also noted [Figure 13].
Figure 13.
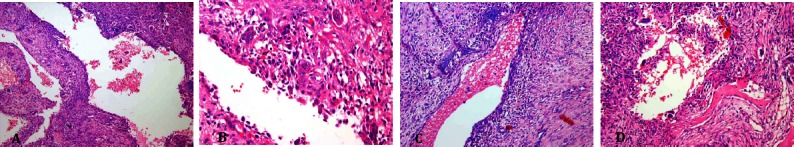
Microscopic slides. Low power (A) and high power (B) views of subperiosteal femur lesion. The tumor is composed ofcystic blood-filled spaces, surrounded by fibroblastic septae, showing degenerated fibro myxoid tissue (C) , some multinucleated osteoclast-like giant cells and strands of osteoid formation (D).
The patient has been remained recurrence-free since then and post-operative radiographs show resolution of the lesion [Figure 9].
Case 4
A 29-year-old female presented with a two-year history of a painful mass with recent increase in size at left proximal leg. Conventional radiographs of left knee revealed a subperiosteal lytic lesion in the proximal tibia shaft [Figure 14].
Figure 14.
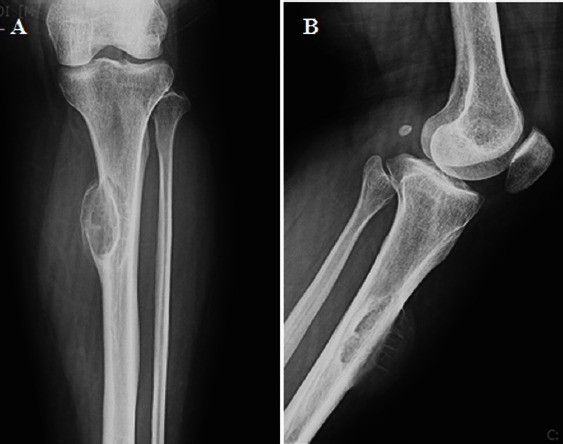
Radiograph. Antero posterior (A), lateral (B) and post-operative (C) radiographs of left knee show a cortical lytic lesion with cortical expansion and sun-ray periosteal reaction.
The patient underwent extended curettage, and we received fragmented specimen including pieces of spongy and hemorrhagic soft tissue admixed with bone fragments, totally measuring 5×4×2 cm. There was no biopsy performed in this patient prior to the surgery, because it was clinically suspected as a benign lesion. On microscopic inspection of the specimen, we found large blood-filled cystic spaces lined by fibroblasts and histiocytes and separated by fibrous septa, alternating with solid areas containing scattered osteoclast like giant cells. Reactive woven bone formation was seen at the periphery of the lesion [Figure 15]. Post-operative plain radiographs shows signs of bone grafting. The patient follow up revealed no recurrences or complications after the surgery [Figure 14].
Figure 15.
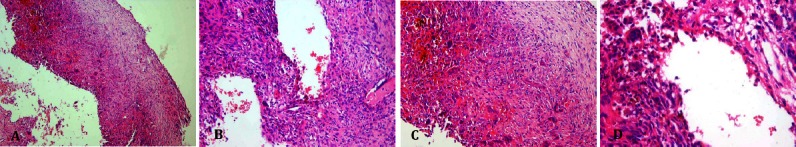
Microscopic slides. Low power and high power views of ABC of tibia, cyst wall (A) and blood- filled spaces lacking endothelial lining (B,C) and containing scattered osteoclast-like giant cells (D).
Discussion
ABC is a benign but locally aggressive bone lesion, which was once considered a reactive lesion, but now has been proven to have a neoplastic nature characterized by rearrangements of the USP6-gene (13, 14). The cause of this strange process in bone is unknown, although there are several examples of ABC apparently arising following a fracture (15). According to our data ABC was most frequent in the second decade of age and over 70% of patients were younger than 20 years at the first presentation. In the long bones, ABC tends to involve the metaphysis. In almost 24% of cases, the lesion was located in the femur. The prevalence of solid ABCs and recurrence rate were 2.6% and 13.2% respectively. Radulescu et al. reported a prevalence of about 3.2% for solid type (16). ABC may share similar clinical symptoms, radiographic features, and age distribution to that of osteosarcoma, especially telangiectatic type that is sometimes difficult to distinguish radiographically and histologically (17) from an aggressive ABC. Telangiectatic osteosarcoma can have a deceptively innocent radiographic appearance with sharply demarcated margins, stimulating a benign bone cyst.
Subperiosteal or cortical origin of ABC is a relatively uncommon finding and has been rarely reported (18-22). Surface ABCs account for a small but clinically important number of ABCs. They can demonstrate an aggressive radiographic appearance and may be differentiated from malignant neoplastic processes, in particular periosteal osteosarcoma which may indeed go secondary ABC changes (23). Other radiographic differential diagnoses of surface ABC include giant cell tumor, sub periosteal hematoma, telangiectatic osteosarcoma, periosteal chondroma, and periosteal chondrosarcoma (9, 24, 25). Areas similar to an ABC are found in various benign conditions, including giant cell tumor, chondroblastoma, chondromyxoid fibroma, and fibrous dysplasia (15). Surface ABCs may exhibit prominent soft tissue component and/or periosteal reaction. On the other hand, surface osteosarcomas are predominantly low-grade tumors and ABCs may be mistaken for low-grade osteosarcomas histologically with delicate woven bone trabeculae formation.
Interestingly, all four reported cases occurred in the three long bones common for conventional type of ABC, i.e. femur, tibia, and humerus, but unlike the medullary type, they occurred at an older age with a mean age of 28 years. The incidence rate of surface ABCs is about 10.5% in our center. Sherman et al. reported an incidence of 9.3% in 1957, while in 2002 Maiya et al. reported an incidence rate of 16% (9, 11). For some unknown reasons, surface ABCs have not been reported to affect flat bones or the spines (11). Taking into account only long bones, Capanna et al. reported an incidence rate of about 27% for surface ABCs (10). The diagnosis of a cortical, subperiosteal, or mixed type ABC relies on imaging showing the relationship of the lesion with the cortex and adjacent soft tissue. Different radiographic classifications of long bone ABCs have been proposed (9-11), with some differences in defining surface variants. However, difficulties occur when applying the classifications to a large cortical ABC extending to the medulla, or a large eccentric medullary one extending to the cortex, which explains a part of different incidence rates claimed by authors using different classifications. For practical purposes, a more precise radiologic classification of ABCs studying a large number of cases is recommended. Presented cases did not show a different behavior from that of the conventional type, histologically. Our survey suggests a similar incidence rate of subperiosteal ABCs in Iranian population to that of reported previously in the literature, mostly occurring in older age groups compared to the conventional type, with a diphyseal location predilection. Nevertheless, the fact that should be kept in mind is that sub periosteal and intra cortical ABCs do occur, though rare, and the clinicians and pathologists must think of it as a differential diagnosis for aggressive cortical lesions in long bones.
The patients were informed that this report and any accompanying images would be submitted for publication and gave their consent.
References
- 1.Wheeless CR. Wheeless’ textbook of orthopaedics. Towson, US: Data Trace Internet Pub; 1996. [Google Scholar]
- 2.Dorfman HD, Czerniak B. Bone tumors. New York: Mosby; 1998. [Google Scholar]
- 3.Apaydin A, Ozkaynak C, Yilmaz S, Akyildiz F, Sindel T, Aydin AT, et al. Aneurysmal bone cyst of metacarpal. Skeletal Radiol. 1996;25(1):76–8. doi: 10.1007/s002560050037. [DOI] [PubMed] [Google Scholar]
- 4.Capote-Moreno A, Acero J, Garcia-Recuero I, Ruiz J, Serrano R, de Paz V. Giant aneurysmal bone cyst of the mandible with unusual presentation. Med Oral Patol Oral Cir Bucal. 2009;14(3):E137–40. [PubMed] [Google Scholar]
- 5.Dossing KV. Aneurysmal bone cyst of the hand. An unusual location in the first phalanges of the first finger. Case report. Scand J Plast Reconstr Surg Hand Surg. 1990;24(2):173–5. doi: 10.3109/02844319009004540. [DOI] [PubMed] [Google Scholar]
- 6.Gold RH, Mirra JM. Case report 234. Aneurysmal bone cyst of left scapula with intramural calcified chondroid. Skeletal radiol. 1983;10(1):57–60. doi: 10.1007/BF00355395. [DOI] [PubMed] [Google Scholar]
- 7.Parashari UC, Khanduri S, Upadhyay D, Bhadury S, Singhal S. Radiologic and pathologic correlation of aneurysmal bone cysts at unusual sites. J Cancer Res Ther. 2012;8(1):103–5. doi: 10.4103/0973-1482.95183. [DOI] [PubMed] [Google Scholar]
- 8.Theron S, Steyn F. Clinical image. An unusual cause of proptosis: aneurysmal bone cyst of the anterior skull base. Pediatr Radiol. 2006;36(9):997. doi: 10.1007/s00247-006-0180-8. [DOI] [PubMed] [Google Scholar]
- 9.Sherman RS, Soong KY. Aneurysmal bone cyst: its roentgen diagnosis. Radiology. 1957;68(1):54–64. doi: 10.1148/68.1.54. [DOI] [PubMed] [Google Scholar]
- 10.Capanna R, Bettelli G, Biagini R, Ruggieri P, Bertoni F, Campanacci M. Aneurysmal cysts of long bones. Ital J orthop Traumatol. 1985;11(4):409–17. [PubMed] [Google Scholar]
- 11.Maiya S, Davies M, Evans N, Grimer J. Surface aneurysmal bone cysts: a pictorial review. Eur Radiol. 2002;12(1):99–108. doi: 10.1007/s003300101009. [DOI] [PubMed] [Google Scholar]
- 12.Vergel De Dios AM, Bond JR, Shives TC, McLeod RA, Unni KK. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69(12):2921–31. doi: 10.1002/1097-0142(19920615)69:12<2921::aid-cncr2820691210>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira AM, Chou MM. USP6-induced neoplasms: the biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum Pathol. 2014;45(1):1–11. doi: 10.1016/j.humpath.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Jacquot C, Szymanska J, Nemana LJ, Steinbach LS, Horvai AE. Soft-tissue aneurysmal bone cyst with translocation t(17;17)(p13;q21) corresponding to COL1A1 and USP6 loci. Skeletal Radiol. 2015;44(11):1695–9. doi: 10.1007/s00256-015-2205-6. [DOI] [PubMed] [Google Scholar]
- 15.Unni KK, Inwards CY, Research MFME. Dahlin’s bone tumors: general aspects and data on 10,165 cases. Philadelphia: Wollters Kluwer Health/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 16.Radulescu R, Badila A, Manolescu R, Sajin M, Japie I. Aneurysmal bone cyst--clinical and morphological aspects. Rom J Morphol Embryol. 2014;55(3):977–81. [PubMed] [Google Scholar]
- 17.Saito T, Oda Y, Kawaguchi K, Tanaka K, Matsuda S, Sakamoto A, et al. Five-year evolution of a telangiectatic osteosarcoma initially managed as an aneurysmal bone cyst. Skeletal Radiol. 2005;34(5):290–4. doi: 10.1007/s00256-004-0865-8. [DOI] [PubMed] [Google Scholar]
- 18.Slavotinek JP, Wicks A, Spriggins AJ. Subperiosteal aneurysmal bone cyst with associated bone marrow oedema: an unusual appearance. Australas Radiol. 2003;47(4):475–8. doi: 10.1046/j.1440-1673.2003.01226.x. [DOI] [PubMed] [Google Scholar]
- 19.Malfair D, Munk PL, O’Connell JX. Subperiosteal aneurysmal bone cysts: 2 case reports. Cana Assoc Radiol J. 2003;54(5):299–304. [PubMed] [Google Scholar]
- 20.Burnstein MI, De Smet AA, Hafez GR, Heiner JP. Case report 611: Subperiosteal aneurysmal bone cyst of tibia. Skeletal Radiol. 1990;19(4):294–7. doi: 10.1007/BF00191676. [DOI] [PubMed] [Google Scholar]
- 21.Bloch C, Peck HM. Aneurysmal bone cyst with doculmented subperiosteal origin. J Mt Sinai Hosp N Y. 1968;35(2):192–5. [PubMed] [Google Scholar]
- 22.Schoedel K, Shankman S, Desai P. Intracortical and subperiosteal aneurysmal bone cysts: a report of three cases. Skeletal radiology. 1996;25(5):455–9. doi: 10.1007/s002560050114. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S, Hayakawa K, Takeno K, Baba H, Meir A. Parosteal aneurysmal bone cyst of the humerus with birdcage-like ossification on three-dimensional CT scanning: a case report. Joint Bone Spine. 2009;76(6):705–7. doi: 10.1016/j.jbspin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Woertler K, Brinkschmidt C. Imaging features of subperiosteal aneurysmal bone cyst. Acta Radiol. 2002;43(3):336–9. doi: 10.1080/j.1600-0455.2002.430318.x. [DOI] [PubMed] [Google Scholar]
- 25.Okada K, Masuda H, Shozawa T, Arai M. A small aneurysmal bone cyst restricted to the cortical bone of the femur resembling so-called subperiosteal giant cell tumor or subperiosteal osteoclasia. Acta Pathol Jpn. 1989;39(8):539–44. doi: 10.1111/j.1440-1827.1989.tb01521.x. [DOI] [PubMed] [Google Scholar]


