Abstract
Injuries to the posterolateral corner (PLC) comprise a significant portion of knee ligament injuries. A high index of suspicion is necessary when evaluating the injured knee to detect these sometimes occult injuries. Moreover, a thorough physical examination and a comprehensive review of radiographic studies are necessary to identify these injuries. In this sense, stress radiographs can help to objectively determine the extent of these lesions. Non-operative and operative treatment options have been reported depending on the extent of the injury. Complete PLC lesions rarely heal with non-operative treatment, and are therefore most often treated surgically. The purpose of this article was to review the anatomy and clinically relevant biomechanics, diagnosis algorithms, treatment and rehabilitation protocols for PLC injuries.
Keywords: Anatomical, Fibular collateral ligament, Knee, Posterolateral, Popliteus reconstruction, Reconstruction
Introduction
The posterolateral corner (PLC) of the knee was once referred to as the dark side of the knee due to the limited understanding of the structures, biomechanics and possible treatment options. A number of studies in recent years have led to a heightened understanding of the PLC, and biomechanically validated reconstruction techniques. Posterolateral corner injuries are commonly associated with ACL or PCL tears, with only 28% of all PLC injuries occurring in isolation (1). Failing to address a PLC injury may compromise concurrent cruciate ligament reconstructions and could furthermore derive in altered knee biomechanics, which ultimately can lead to early degenerative changes of the joint (2-4).
In order to identify PLC injuries, a high index of suspicion is necessary and a detailed physical examination should be performed. Likewise, a comprehensive review of radiographic and magnetic resonance imaging (MRI) studies are helpful to better determine the injured structures precisely (5).
Poor outcomes have been reported for grade III PLC injuries treated non-operatively (resulting in varus and rotational instability of the knee) and thus, a reconstruction is indicated (6, 7). A thorough knowledge of the anatomy is essential for surgical treatment of this pathology, since anatomical reconstruction has demonstrated improved outcomes (8). The purpose of this article was to review the current concepts of PLC including anatomy, biomechanics, diagnosis, treatment options and outcomes reported in the literature.
Anatomy
The three major static stabilizers of the PLC are the fibular (lateral) collateral ligament (FCL), the popliteus tendon (PLT) and the popliteofibular ligament (PFL)(9).
Fibular Collateral Ligament (FCL)
The FCL is the primary Varus stabilizer of the knee(10-12). The femoral attachment of the FCL is in a small bony depression slightly proximal (1.4 mm) and posterior (3.1 mm) to the lateral epicondyle(9). The FCL courses distally to insert on the fibular head (8.2 mm posterior to the anterior margin of the fibular head and 28.4 mm distal to the tip of the fibular styloid), occupying 38% of the width of the fibular head. The majority of the distal attachment is in a bony depression that extends to approximately the distal one-third of the lateral aspect of the fibular head. The remaining distal insertion blends with the peroneus longus fascia (9) [Figure 1].
Figure 1.
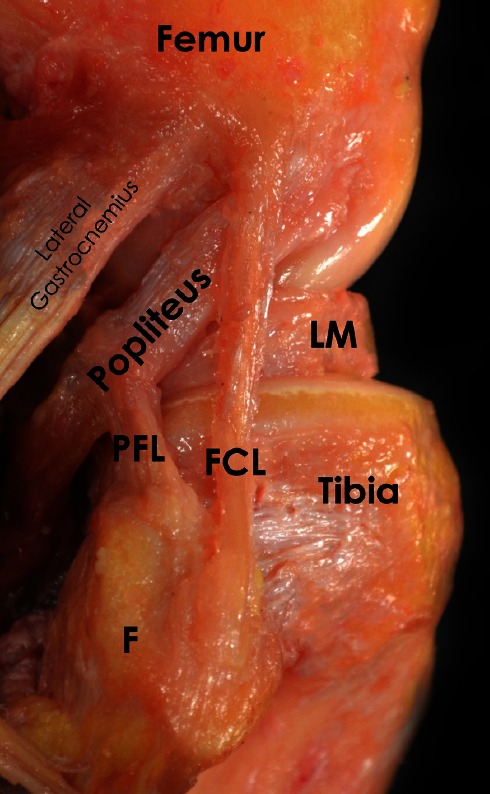
Anatomic dissection of a right knee demonstrating the posterolateral structures of the knee. FCL: Fibular Collateral Ligament; PFL: Popliteofibular Ligament; LM: Lateral Meniscus; F: Fibula.
Popliteus Tendon (PLT)
The femoral insertion of the PLT constitutes the most anterior femoral insertion of the PLC and can be found at an average of 18.5 mm anteriorly (17 – 23 mm) from the FCL attachment with the knee at 70°. After its femoral insertion in the proximal half of the popliteal sulcus, it courses posterodistally in an oblique fashion to insert into the posteromedial tibia. It becomes tendinous in the lateral third of the popliteal fossa and intra-articular as it courses deep to the FCL. The average total length of the popliteus tendon to its musculotendinous junction is 54.5 mm (9).
Popliteofibular Ligament (PFL)
The popliteofibular ligament originates at the musculotendinous junction of the popliteus. The popliteofibular ligament has two divisions (anterior and posterior) that seem to embrace the popliteus musculotendinous junction and insert distally into the posteromedial aspect of the fibular head. The distolateral attachment of the anterior division is located on the anterior downslope of the medial aspect of the fibular styloid process. Similarly, the posterior division attaches at the tip and posteromedial aspect of the fibular styloid process. Overall, the posterior division of the PFL is larger than the anterior division (9) [Figure 2].
Figure 2.
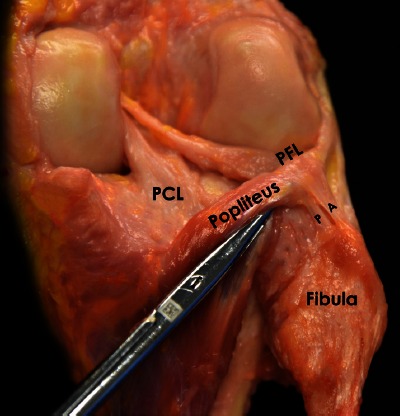
Posterolateral view of an anatomic photograph showing both arms (anterior and posterior) of the popliteofibular ligament in a right knee. A: Anterior; P: Posterior: PFL: Popliteofibular Ligament; PCL: Posterior Cruciate Ligament.
Secondary Stabilizers
Secondary structures help stabilize the knee in a static and dynamic manner. From deep to superficial these structures are:
The lateral capsular thickening of the mid-third lateral capsular ligament, with its components consisting of the meniscofemoral and meniscotibial ligaments, can be found in close relationship with the femur and tibia respectively.
The coronary ligament is the attachment of the posterior aspect of the lateral meniscus to the tibia.
Nearly 14 mm posterior to the femoral FCL attachment, along the supracondylar process, the lateral gastrocnemius tendon arises and courses distally (in intimate relationship with the posterolateral capsule) to fuse with the medial gastrocnemius and the soleus to form the sural triceps. Because it is less frequently injured than the other structures of the posterolateral knee, it can serve as an important landmark during surgical reconstruction.
The fabellofibular ligament is the distal thickening of the capsular arm of the short head of the biceps femoris that extends vertically from the fabella to the fibular styloid. The fabella is a sesamoid bone (or a cartilaginous analogue the rest of the time) that is found within the proximal lateral gastrocnemius tendon in 30% of individuals (13).
The long head of the biceps femoris divides approximately 1 cm proximal to the fibular head into direct and anterior arms. The direct arm inserts onto the posterolateral aspect of the fibular head and the anterior arm has a small insertion site on the more distal and anterior aspect of the fibular head. Both arms enclose the FCL distal attachment (9). A crucial access point when reconstructing the FCL is the anterior arm of the biceps femoris. Furthermore, the common peroneal nerve lies deep to this tendon proximally and emerges posteriorly 1-2 cm proximal to the fibular head. The short head of the biceps tendon also divides into two segments that attach along the posterolateral aspect of the knee, along the lateral aspect of the fibular styloid, medial to the direct arm attachment of the long head (9).
The iliotibial band (ITB) is the most superficial layer of the lateral aspect of the knee. It is a broad layer that attaches onto Gerdy’s tubercle in the anterolateral aspect of the tibia. Numerous peripheral attachments to the patella, the lateral intermuscular septum and the capsule exist (9).
Biomechanics
The PLC structures provide the primary restraint to varus forces of the knee as well as posterolateral rotation of the tibia relative to the femur In cases of cruciate deficient knees, these structures are also important secondary stabilizers to anterior and posterior tibial translation (10-11,14). The FCL is the primary restraint to varus stress across the knee, the rest of the posterolateral structures act as secondary varus stabilizers that has been noted after sectioning of the FCL (15, 16). In regards to tibial external rotation, the FCL and the popliteus complex are the primary restraints, especially between 30° and 40° of flexion and the PCL acts as a secondary restraint (17-18). Thus, combined PCL and PLC injuries are more susceptible to external rotation forces. The PLT is a minor primary stabilizer (the ACL is the main stabilizer in lower flexion angles and ALL in higher flexion angles) in preventing internal rotation (19). A small, yet significant, increase in internal rotational laxity was demonstrated in a popliteus cutting study (15). The other PLC structures are secondary restraints to internal rotation. Lastly, minimal contribution of the PLC has been reported for anteroposterior tibial translation (popliteus tendon), specifically in full extension and with ACL or PCL deficient knees (3, 10, 15).
Evaluation
Typically, patients recall a specific trauma that occurred to their knee. A common mechanism of injury to the PLC is a direct blow to the anteromedial knee (20). However, hyperextension and non-contact varus stress injuries can also damage the PLC (20). Most often, PLC tears are associated with ACL and/or PCL injuries, justifying close examination for a PLC injury for every cruciate ligament tear.
Frequently reported symptoms include pain, perceived side-to-side instability near extension, increased difficulty walking on uneven ground or up and down stairs, ecchymosis and swelling. This instability and difficulty walking can present as a varus thrust gait seen during the initiation of the stance phase (21). It is not uncommon for the patient to complain of paresthesia of the common peroneal nerve distribution or foot drop. Common peroneal nerve injury has been reported to occur in up to one third of PLC injuries (20, 22).
A thorough examination is essential to properly diagnose a PLC injury. When possible, tests that should always be performed include varus stress testing, the dial test, reverse pivot shift test and the external rotation recurvatum test. All tests should be performed bilaterally to compare to the uninjured knee.
The varus stress test is performed both in full extension and at 20-30° of flexion [Figure 3]. The femur is stabilized to the examination table with one hand, which is also used to assess the amount of lateral compartment gapping, while the other hand is used to hold the patient’s foot or ankle and apply a varus force. Lateral compartment gapping in comparison to the contralateral side, with the knee flexed to 30° indicates an injury to the FCL and potentially to the secondary stabilizers of the PLC. If stability is restored when tested in full extension, an isolated injury to the FCL is presumed. However, if the varus instability persists in full extension, a combined FCL, PLC and cruciate ligament injury is assumed.
Figure 3.
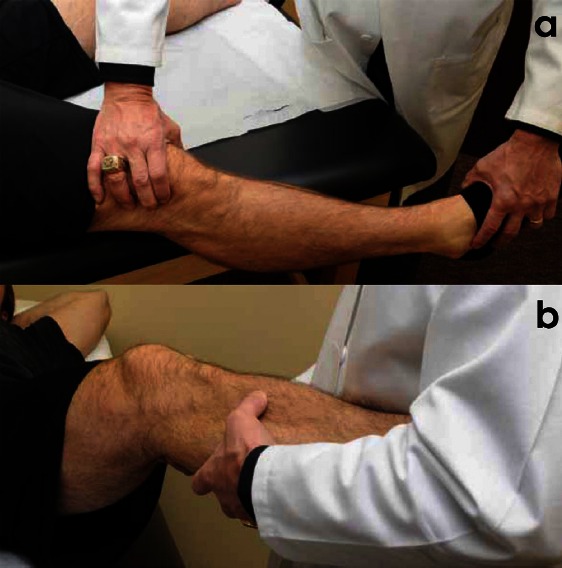
Demonstration of the varus stress test a) performed at 0 degrees of knee flexion and b) showing the test performed at 30 degrees of flexion with an alternative method for patients with larger legs.
Another helpful tool for the examiner is the dial test [Figure 4], which measures external rotation of the tibia relative to the femur. With the patient in the prone or supine position and the knee flexed to 30°, the femur is fixed with one hand while the ankle and foot are externally rotated. An increase of more than 10° of external rotation compared with the contralateral side suggests an injury to the PLC (11). The knee is then flexed to 90°. Because of its role as an important secondary stabilizer, a knee with an intact PCL will see a decrease in external rotation. If, at 90°, there is an increase in external rotation, as compared with 30°, a combined PLC and PCL injury is presumed. A cadaveric biomechanical study by Bae and coworkers demonstrated increased external rotation following sectioning of at least three ligaments of the PCL and PLC complex (23). As a result, they suggested that the dial test might not be sufficiently sensitive to identify one- or two-ligament injuries.
Figure 4.
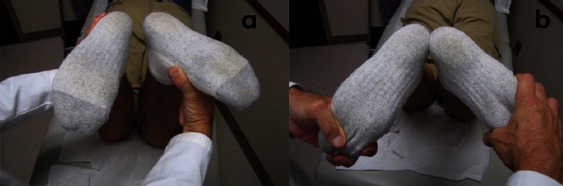
Image of the dial test being performed with the patient prone on the operating table and the knees flexed to a) 90 degrees and b) 30 degrees. Note the increased external rotation of the right leg compared to the left.
The reverse pivot shift [Figure 5] is an essential component of the PLC examination. To perform this test, the patient should lie supine with the knee flexed to near 90°. The joint line is palpated, a valgus load is applied through the knee, an external rotation force is applied to the tibia and the knee is slowly extended. If the previously subluxated lateral tibial plateau reduces at approximately 35° to 40° of flexion, this is a positive test. This reduction is the result of the ITB function changing from knee flexor to knee extender with extension (20). A positive reverse pivot shift has been reported to have a positive predictive value of 68% and a negative predictive value of 89%. Comparison to the contralateral knee is important as a positive test has been reported in 35% of uninjured knees (17, 20, 24).
Figure 5.
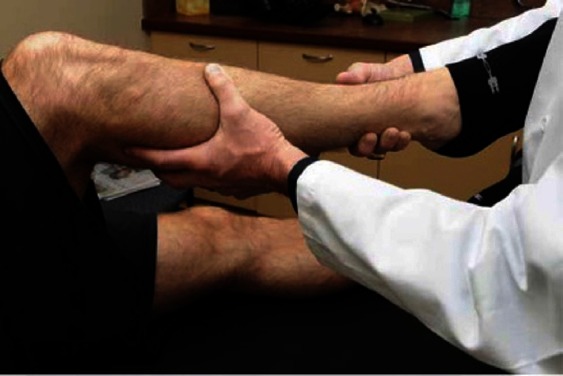
Demonstration of the starting position to perform the reverse pivot shift test on a right knee. The knee is then slowly extended while externally rotating the tibia and placing a valgus force on the knee.
While a wide range of sensitivity has been reported with the external rotation recurvatum test [Figure 6] if done properly, it can still be an important component when investigating a potential PLC injury. To examine recurvatum, the patient should be in the supine position with legs extended and the great toe is grasped and the leg lifted from the table while securing the femur to the table by applying gentle pressure to the anterior distal femur. The height of the heel knee is then measured and compared with the contralateral side. This test was recently been evaluated by LaPrade and colleagues who found it to identify less than 10% of injuries in a series of 134 patients with PLC injuries (25). However, their study revealed that a positive external rotation recurvatum test predicted a combined ACL and PLC injury.
Figure 6.
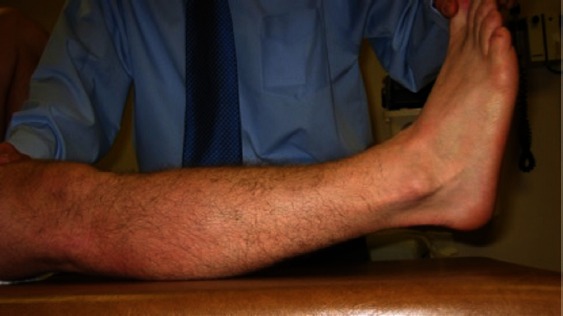
Demonstration of the external rotation recurvatum test being performed on a right leg.
Imaging
If PLC injury is still suspected after a history is obtained and a physical examination is performed, imaging is warranted. Standard anteroposterior (AP), lateral and bent knee patellofemoral (sunrise) view radiographs of the knee should be obtained, but are frequently normal in acute injuries. For chronic PLC injuries, a standing long-leg AP alignment radiograph is a requirement because malalignment needs to be recognized and corrected with a biplanar osteotomy prior to, or at the time of, surgical PLC reconstruction
It is essential to obtain varus stress radiographs for the objective diagnosis of PLC injuries because they have been reported to be a reliable and reproducible objective method to evaluate the severity of these lesions (26). Bilateral varus stress radiographs should be performed at 20° of knee flexion. Lateral compartment gapping is determined by measuring the shortest distance between the subchondral bone surface of the most distal aspect of the lateral femoral condyle and the corresponding tibial plateau. LaPrade et al. has reported that an isolated complete FCL tear has a side-to-side difference of 2.7 to 4.0 mm, while a difference of greater than 4 mm represents an associated grade III PLC injury (26). When available magnetic resonance imaging (MRI) may be performed to assist in the diagnosis of acute lesions, to assess concurrent injuries and to determine the location of the damaged structures (27).
Treatment Rationale
The literature on non-operative treatment of grade I and II is still sparse. Good results have been reported for non-surgical treatment for grade I and II injuries (28, 29). Minimal radiographic changes at 8 year follow-up were reported following the use of an early mobilization protocol (28, 29). However, poor functional outcomes for non-operatively treated grade III PLC injuries with persistent instability and degenerative changes have been reported (8, 30). Increased forces on the PCL and ALC reconstruction grafts have been reported if concurrent PLC injuries are not addressed (2, 3).
Primary repairs for complete FCL and popliteus tendons avulsions without mid-substance injury have been performed within 2-3 weeks of the injury; however, studies have reported poorer outcomes. Stannard et al. evaluated outcomes of repair versus reconstruction after PLC injuries, and reported a 37 % failure rate in the repair group versus 9 % the reconstruction group (31, 5). In another study by Levy et al. a 40% failure rate was reported in the repair group versus 6% in the reconstruction group (32). Acute treatment (within 3 weeks) is reported to have improved outcomes, while treatment after 3 weeks has been reported to have similar outcomes to chronic injuries (33).
In chronic injuries (greater than 6 weeks from injury), lower extremity alignment should be evaluated and corrected prior to ligament reconstruction because failure to address malalignment can lead to increased stress and stretching of the reconstruction grafts and failure.
Several PLC reconstruction techniques have been described in the literature; however, these techniques are based on isometry and not on anatomical footprints of the reconstructed structures. The authors´ preferred reconstruction technique is an anatomical reconstruction of all three static stabilizers of the PLC. This technique has been biomechanically validated to restore native knee biomechanics (34). Improved clinical outcomes have been reported on this technique (35). A lateral hockey stick incision is made extending from the distal femoral shaft along the ITB and extending distally between Gerdy´s tubercle and the fibular head. Dissection is carried down to the IT band, creating a posterior based vascularized skin flap. A neurolysis of the common peroneal nerve is performed and the nerve is protected. A small horizontal incision into the biceps bursa is made to locate the remnant of the FCL which is tagged with a suture (36). Putting tension on the tag stich will help localize the femoral insertion of the FCL because even in grade III injuries a remnant of the FCL can usually be found. The anterior arm of the long head of the biceps femoris tendon is incised longitudinally, and the distal attachment of the FCL is sharply dissected to create room for the reconstruction tunnel on the fibular head. Blunt dissection between the soleus and the lateral head of the gastrocnemius is carried out to identify the musculotendinous junction of the popliteus. A 7 mm fibula tunnel is reamed coursing from the FCL footprint to the posteromedial aspect of the fibular head where the PFL attaches. A 9 mm tibial tunnel is reamed from the flat spot distal and medial to Gerdy´s tubercle, exiting at the musculotendinous junction, which is about 1 cm medial and 1 cm proximal to the fibular tunnel exit point. The IT band is opened along the direction of the fibers; tension is placed on the tag stich on the FCL to localize the femoral attachment of the FCL. The FCL remnant is incised and a guide pin is drilled in this position aiming proximally and anteriorly to avoid potential convergence with the ACL tunnel in concurrent injuries. The popliteus attachment is 18.5 mm distal and anterior to the FCL attachment. A guide pin is drilled at this position parallel to the first one. The femoral tunnels for the popliteus and the FCL are drilled with a 9 mm reamer to 25 mm depth. An Achilles allograft with 9 * 25 mm bone plugs is used for this reconstruction. The grafts are fixed in the femoral tunnels with 7 * 20 mm titanium interference screws. The popliteus graft is passed through the popliteal hiatus, the FCL graft is passed distally over the popliteus graft and under the superficial IT band. It is passed though the fibular tunnel is an anterolateral to posteromedial direction and fixed on the fibula with a 7 * 23 mm bio interference screw with the knee at 20°, neutral rotation and applying a slight valgus force. Both grafts are then passed from posterior to anterior through the tibial tunnel and fixed in the tibia with a 9 * 23 mm bio absorbable interference screw with the knee flexed at 60° and in neutral rotation [Figure 7].
Figure 7.
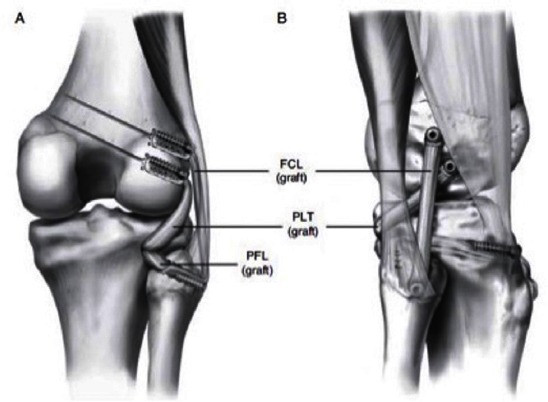
Anatomical reconstruction of the PLC using Achilles allograft. Bone plugs are fixed to the femur with titanium interference screw. After fixing the graft to the fibula (reconstructing the FCL), the graft goes into the tibia tunnel becoming the PFL, and fixed with a biointerference screw together with the popliteus graft, thus all the 3 major static stabilizers of the PLC of the knee are reconstructed.
Postoperative Rehabilitation
Following PLC reconstruction, patients utilize a knee immobilizer and stay non-weight bearing for 6 weeks. Formal rehabilitation begins immediately postoperatively and focuses on restoration of tibiofemoral and patellofemoral range of motion, edema and pain management, and restoration of quadriceps function. Passive range of motion 0-90° for the first 2 weeks and then progressed to full range of motion as tolerated. At 6 weeks, patients are permitted to begin spinning on a stationary bike and begin to wean off crutches. Once full weight bearing, patients begin closed chain strength exercises with training parameters focused on first developing a muscular endurance base before progressing to muscular strength and power development. Isolated hamstring strengthening is limited to avoid stressing the reconstruction until a minimum of 4 months postoperatively. A running progression and speed and agility work may begin once appropriate strength and power characteristics have been developed, typically around 6 months post-surgery. Return to sports or activity is allowed when normal strength, stability, and knee range of motion comparable to the contralateral side has been achieved (usually between 6 to 9 months and based on concurrent cruciate ligament or other ligament surgery).
Outcomes
Studies by Stannard and Levy reported higher reoperation rates in patients treated with repair of the PLC compared to reconstruction (31, 32). Black et al. reported that reconstruction had superior results with respect to failure compared to repair (37). Patient satisfaction was reported to be similar between reconstruction and repair groups (37). In a recent systematic review by Geeslin et al. reported IKDC scores of 78.1 – 91.3 and Lysholm scores of 87.5 – 90.3 in patients operated within 3 weeks (38). Based on objective examination with varus stress examination or radiographs there was an overall failure rate of 19%. In the series of repaired procedures and staged reconstruction of the cruciates, failure rates were as high as 38 %. The authors concluded that the repair of acute grade III PLC injuries and staged treatment of combined cruciate injuries were associated with a substantially higher postoperative PLC failure rate (38). A systematic review by Moulton et al reported a 90% success rate and 10 % failure rate in chronic PLC injuries treated with different reconstruction techniques (18).
The anatomic reconstruction described by LaPrade et al. was reported to reduce the objective laxity on varus stress x-ray from 6.2 mm preoperatively to 0.1 mm side-to-side difference at final follow-up (8). The Cincinnati score improved from 21.9 preoperatively to 81.4 postoperatively while the IKDC subjective outcomes scores increased significantly from 29.1 to 81.5 (8).
Posterolateral corner injuries are not as rare as previously thought. Diagnosing these lesions entails a detailed understanding about the posterolateral structures and function. A high level of suspicion is recommended based on the trauma setting and the patient symptoms. Failure to address this condition may place other reconstruction graft at risk and result in poor outcomes for the patient. Stress radiographs and MRI are a key to precisely define the injury location/s and associate lesions. Operative treatment is recommended for grade III injuries. An early rehabilitation protocol should be initiated as soon as possible to be able to obtain the best outcomes.
References
- 1.Geeslin AG, LaPrade RF. Location of bone bruises and other osseous injuries associated with acute grade III isolated and combined posterolateral knee injuries. Am J Sports Med. 2010;38(12):2502–8. doi: 10.1177/0363546510376232. [DOI] [PubMed] [Google Scholar]
- 2.Harner CD, Vogrin TM, Hoher J, Ma CB, Woo SL. Biomechanical analysis of a posterior cruciate ligament reconstruction. Deficiency of the posterolateral structures as a cause of graft failure. Am J Sports Med. 2000;28(1):32–9. doi: 10.1177/03635465000280011801. [DOI] [PubMed] [Google Scholar]
- 3.LaPrade RF, Resig S, Wentorf F, Lewis JL. The effects of grade III posterolateral knee complex injuries on anterior cruciate ligament graft force. A biomechanical analysis. Am J Sports Med. 1999;27(4):469–75. doi: 10.1177/03635465990270041101. [DOI] [PubMed] [Google Scholar]
- 4.LaPrade RF, Muench C, Wentorf F, Lewis JL. The effect of injury to the posterolateral structures of the knee on force in a posterior cruciate ligament graft: a biomechanical study. Am J Sports Med. 2002;30(2):233–8. doi: 10.1177/03635465020300021501. [DOI] [PubMed] [Google Scholar]
- 5.Scott WN. Insall & Scott Surgery of the Knee. Philadelphia: Elsevier Health Sciences; 2011. [Google Scholar]
- 6.Griffith CJ, Wijdicks CA, Goerke U, Michaeli S, Ellermann J, LaPrade RF. Outcomes of untreated posterolateral knee injuries: an in vivo canine model. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1192–7. doi: 10.1007/s00167-010-1358-z. [DOI] [PubMed] [Google Scholar]
- 7.LaPrade RF, Wentorf FA, Crum JA. Assessment of healing of grade III posterolateral corner injuries: an in vivo model. J Orthop Res. 2004;22(5):970–5. doi: 10.1016/j.orthres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672–83. doi: 10.2106/JBJS.J.01639. [DOI] [PubMed] [Google Scholar]
- 9.LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854–60. doi: 10.1177/03635465030310062101. [DOI] [PubMed] [Google Scholar]
- 10.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69(2):233–42. [PubMed] [Google Scholar]
- 11.Grood ES, Stowers SF, Noyes FR. Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J Bone Joint Surg Am. 1988;70(1):88–97. [PubMed] [Google Scholar]
- 12.Gwathmey FW, Jr, Tompkins MA, Gaskin CM, Miller MD. Can stress radiography of the knee help characterize posterolateral corner injury. Clin Orthop Relat Res. 2012;470(3):768–73. doi: 10.1007/s11999-011-2008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawashima T, Takeishi H, Yoshitomi S, Ito M, Sasaki H. Anatomical study of the fabella, fabellar complex and its clinical implications. Surg Radiol Anat. 2007;29(8):611–6. doi: 10.1007/s00276-007-0259-4. [DOI] [PubMed] [Google Scholar]
- 14.LaPrade RF, Tso A, Wentorf FA. Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am J Sports Med. 2004;32(7):1695–701. doi: 10.1177/0363546503262694. [DOI] [PubMed] [Google Scholar]
- 15.LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543–9. doi: 10.1177/0363546509349493. [DOI] [PubMed] [Google Scholar]
- 16.LaPrade RF. Arthroscopic evaluation of the lateral compartment of knees with grade 3 posterolateral knee complex injuries. Am J Sports Med. 1997;25(5):596–602. doi: 10.1177/036354659702500502. [DOI] [PubMed] [Google Scholar]
- 17.Ranawat A, Baker CL, 3rd, Henry S, Harner CD. Posterolateral corner injury of the knee: evaluation and management. J Am Acad Orthop Surg. 2008;16(9):506–18. [PubMed] [Google Scholar]
- 18.LaPrade RF. Posterolateral knee injuries: anatomy, evaluation, and treatment. Stuttgart: Thieme; 2011. [Google Scholar]
- 19.Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(8):NP22. doi: 10.1177/0363546514562751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPrade RF, Terry GC. Injuries to the posterolateral aspect of the knee. Association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25(4):433–8. doi: 10.1177/036354659702500403. [DOI] [PubMed] [Google Scholar]
- 21.Cooper JM, McAndrews PT, LaPrade RF. Posterolateral corner injuries of the knee: anatomy, diagnosis, and treatment. Sports Med Arthrosc. 2006;14(4):213–20. doi: 10.1097/01.jsa.0000212324.46430.60. [DOI] [PubMed] [Google Scholar]
- 22.Veltri DM, Deng XH, Torzilli PA, Warren RF, Maynard MJ. The role of the cruciate and posterolateral ligaments in stability of the knee. A biomechanical study. Am J Sports Med. 1995;23(4):436–43. doi: 10.1177/036354659502300411. [DOI] [PubMed] [Google Scholar]
- 23.Bae JH, Choi IC, Suh SW, Lim HC, Bae TS, Nha KW, et al. Evaluation of the reliability of the dial test for posterolateral rotatory instability: a cadaveric study using an isotonic rotation machine. Arthroscopy. 2008;24(5):593–8. doi: 10.1016/j.arthro.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Cooper DE. Tests for posterolateral instability of the knee in normal subjects. Results of examination under anesthesia. J Bone Joint Surg Am. 1991;73(1):30–6. [PubMed] [Google Scholar]
- 25.LaPrade RF, Ly TV, Griffith C. The external rotation recurvatum test revisited: reevaluation of the sagittal plane tibiofemoral relationship. Am J Sports Med. 2008;36(4):709–12. doi: 10.1177/0363546507311096. [DOI] [PubMed] [Google Scholar]
- 26.LaPrade RF, Heikes C, Bakker AJ, Jakobsen RB. The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee injuries. An in vitro biomechanical study. J Bone Joint Surg Am. 2008;90(10):2069–76. doi: 10.2106/JBJS.G.00979. [DOI] [PubMed] [Google Scholar]
- 27.LaPrade RF, Gilbert TJ, Bollom TS, Wentorf F, Chaljub G. The magnetic resonance imaging appearance of individual structures of the posterolateral knee. A prospective study of normal knees and knees with surgically verified grade III injuries. Am J Sports Med. 2000;28(2):191–9. doi: 10.1177/03635465000280020901. [DOI] [PubMed] [Google Scholar]
- 28.Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17(1):83–8. doi: 10.1177/036354658901700114. [DOI] [PubMed] [Google Scholar]
- 29.Krukhaug Y, Molster A, Rodt A, Strand T. Lateral ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 1998;6(1):21–5. doi: 10.1007/s001670050067. [DOI] [PubMed] [Google Scholar]
- 30.LaPrade RF, Hamilton CD, Engebretsen L. Treatment or acute and chronic combined anterior cruciate ligament and posterolateral knee ligament injuries. Sports Med Arthrosc. 1997;5(2):91–9. [Google Scholar]
- 31.Stannard JP, Brown SL, Farris RC, McGwin G, Jr, Volgas DA. The posterolateral corner of the knee: repair versus reconstruction. Am J Sports Med. 2005;33(6):881–8. doi: 10.1177/0363546504271208. [DOI] [PubMed] [Google Scholar]
- 32.Levy BA, Dajani KA, Morgan JA, Shah JP, Dahm DL, Stuart MJ. Repair versus reconstruction of the fibular collateral ligament and posterolateral corner in the multiligament-injured knee. Am J Sports Med. 2010;38(4):804–9. doi: 10.1177/0363546509352459. [DOI] [PubMed] [Google Scholar]
- 33.Clancy WG, Jr, Sutherland TB. Combined posterior cruciate ligament injuries. Clin Sports Med. 1994;13(3):629–47. [PubMed] [Google Scholar]
- 34.McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, Laprade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674–81. doi: 10.1177/0363546510361220. [DOI] [PubMed] [Google Scholar]
- 35.LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16–22. doi: 10.2106/JBJS.I.00474. [DOI] [PubMed] [Google Scholar]
- 36.LaPrade RF, Hamilton CD. The fibular collateral ligament-biceps femoris bursa. An anatomic study. Am J Sports Med. 1997;25(4):439–43. doi: 10.1177/036354659702500404. [DOI] [PubMed] [Google Scholar]
- 37.Black BS, Stannard JP. Repair versus reconstruction in acute posterolateral instability of the knee. Sports Med Arthrosc. 2015;23(1):22–6. doi: 10.1097/JSA.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 38.Geeslin AG, Moulton SG, LaPrade RF. A systematic review of the outcomes of posterolateral corner knee injuries, part 1: surgical treatment of acute injuries. Am J Sports Med. 2015;44(2):1–7. doi: 10.1177/0363546515592828. [DOI] [PubMed] [Google Scholar]


