Abstract
Aims
Current statin guidelines in Europe and Canada advocate achieving a fixed LDL target or the attainment of a ≥50% reduction in low-density lipoprotein cholesterol (LDLC), while current US guidelines advocate the use of statin therapies that reduce LDLC by <50% (moderate intensity) or ≥50% (high intensity). Data are limited, however, linking the achievement of these % reduction thresholds to subsequent cardiovascular outcomes particularly for contemporary high-intensity regimens.
Methods and results
In a randomized trial of 17 082 initially healthy men and women with median baseline LDLC of 108 mg/dL (interquartile range 94–119), we (i) used waterfall plots to assess the variability in LDLC response to rosuvastatin 20 mg daily and (ii) evaluated the impact of reaching ≥50% reductions in LDLC on risk of developing the first cardiovascular events. Among rosuvastatin allocated participants, 3640 individuals (46.3%) experienced an LDLC reduction ≥50%; 3365 individuals (42.8%) experienced an LDLC reduction >0 but <50%; and 851 individuals (10.8%) experienced no reduction or an increase in LDLC compared with baseline. These % LDLC reductions directly related to the risks of first cardiovascular events; at trial completion, incidence rates for the primary endpoint were 11.2, 9.2, 6.7, and 4.8 per 1000 person-years for those in the placebo, no LDLC reduction, LDLC reduction <50%, and LDLC reduction ≥50% groups, respectively. Compared with placebo, the multivariable adjusted hazard ratios for sequentially greater on-treatment per cent reductions in LDLC were 0.91 (95%CI 0.54–1.53), 0.61 (95%CI 0.44–0.83), and 0.43 (95%CI 0.30–0.60) (P < 0.00001). Similar relationships between % reduction and clinical outcomes were observed in analyses focusing on non-HDLC or apolipoprotein B.
Conclusions
As documented for low- and moderate-intensity regimens, variability in % LDLC reduction following high-intensity statin therapy is wide yet the magnitude of this % reduction directly relates to efficacy. These data support guideline approaches that incorporate % reduction targets for statin therapy as well as absolute targets, and might provide a structure for the allocation of emerging adjunctive lipid-lowering therapies such as PCSK9 inhibitors should these agents prove broadly effective for cardiovascular event reduction.
Keywords: LDLC, Statin therapy, Apolipoprotein B, Guidelines, Prevention, PCSK9
See page 1380 for the editorial comment on this article (doi:10.1093/eurheartj/ehw102)
Introduction
Current statin guidelines in Europe and Canada advocate achieving a fixed low-density lipoprotein cholesterol (LDLC) target or attaining a ≥50% reduction in LDLC,1,2 while current US guidelines advocate the use of statin therapies that reduce LDLC by <50% (moderate intensity) or ≥50% (high intensity).3 Both of these approaches have intuitive clinical appeal, yet data are limited linking the achievement of a ≥50% per cent reduction in LDLC with subsequent cardiovascular outcomes particularly for contemporary high-intensity statin regimens. As Boekholdt and colleagues have recently shown, the variability in per cent reduction in LDLC following statin therapy is very wide,4 an observation that may impact on future guidelines as emerging adjunctive LDLC-lowering agents such as PCSK9 inhibitors become more widely available. We thus sought to address in greater detail the relationship of per cent reduction in LDLC with clinical outcomes in a contemporary randomized trial of rosuvastatin 20 mg when compared with placebo in the primary prevention of cardiovascular events.
Methods
Study population
This manuscript describes a secondary data analysis of the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. In brief, JUPITER enrolled 17 802 asymptomatic women ≥60 years and men ≥50 years who had LDLC <130 mg/dL, hsCRP> = 2.0 mg/L, and triglycerides <500 mg/dL.5 Critical exclusion criteria included any prior history of cardiovascular disease, diabetes, or use of lipid-lowering therapy. At randomization, all participants were assigned either to rosuvastatin 20 mg daily or to matching placebo and were followed prospectively for a period of up to 5 years for the occurrence of first ever cardiovascular events.
Laboratory measures
Study measurements were performed in a central laboratory on blood samples collected at baseline and follow-up after participants fasted for at least 8 h. For the purpose of the current analyses, paired samples were assayed at baseline and at the 1-year post-randomization visit. An enzymatic process (cholesterol esterase) with a calorimetric endpoint was used to assess total cholesterol, triglycerides were measured with an enzymatic hydrolysis procedure to obtain a calorimetric endpoint value, and high-density lipoprotein cholesterol (HDLC) was measured in the resulting supernatant after heparin-manganese precipitation of apolipoprotein B containing proteins. Low-density lipoprotein cholesterol concentrations were either calculated by using the Friedewald equation when triglycerides were <400 mg/dL or measured directly when triglycerides were ≥400 mg/dL. A high-sensitivity (Behring) nephelometer was used to measure hsCRP, and the concentration of apolipoprotein B (apo B) was measured via immunonephelometry using a Behring nepheolometric assay. Concentrations of non-HDLC were calculated by subtracting HDLC from total cholesterol.
Outcomes
As pre-specified in the JUPITER protocol,5 the trial primary outcome was defined as the first occurrence of non-fatal myocardial infarction, non-fatal stroke, hospitalization for unstable angina, arterial revascularization, or cardiovascular death. Myocardial infarction, stroke, and cardiovascular death were confirmed using standardized criteria. Episodes of unstable angina were confirmed by the presence of ischaemic chest pain at rest or with minimal exertion occurring within the preceding 48 h requiring hospitalization and the presence of objective evidence of ischaemia. Arterial revascularizations were defined as coronary artery bypass graft surgery, at least one percutaneous transluminal intervention, or bypass grafting of a peripheral artery or carotid artery. All endpoints were adjudicated by an independent endpoint committee of physician reviewers unaware of randomized treatment assignment.
Statistical analyses
All statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA). Median per cent reductions in LDLC, non-HDLC, and apo B were calculated in response to statin treatment, and waterfall plots were used to graphically illustrate the intra-individual variation in this response. As current US guidelines define high-intensity statin therapy as agents that lower LDLC by on average ≥50%,3 we used this threshold in our primary analyses. Thus, four study groups were defined for initial analysis: those allocated to placebo; those allocated to rosuvastatin who experienced an LDLC reduction ≥50%; those allocated to rosuvastatin who experienced an LDLC reduction of >0% but <50%; and those allocated to rosuvastatin who experienced either no reduction in LDLC or an increase in LDLC when compared with baseline values. Cholesterol reduction groups were compared using χ2 analysis for categorical characteristics and Kruskal–Wallis one-way analysis of variance for continuous measures. For each study group, we calculated incident event rates (per 1000 person-years) and 95% confidence intervals (CIs) for the trial primary endpoint. Cox proportional hazard models were used to estimate relative hazards and 95% CIs for the comparison of cumulative incidence of cardiovascular events in each statin-treated group, compared with those allocated to placebo. On an a priori basis, multivariable adjusted hazard ratios and 95% CIs were also computed after adjusting for those variables found on regression analysis to have a significant impact on on-treatment LDLC levels; importantly, this adjustment included baseline LDLC.
To address whether alternative lipid measures might affect results, we repeated all the above analyses separately for non-HDLC and apo B. In sensitivity analyses designed to address whether cut-point selection influenced our results, we repeated our analyses using tertiles of per cent lipid reduction rather than the pre-specified ≥50% threshold. Further, to address whether effects were consistent across specific on-treatment LDLC windows, we performed an additional sensitivity analysis limited to those with on-treatment LDLC levels between 50 and 75 mg/dL. Finally, to address if effects were consistent among those allocated to rosuvastatin alone, similar Cox proportional hazard models were also used to estimate relative hazards and 95% CIs for the comparison of cumulative incidence of cardiovascular events in each group, eliminating those allocated to placebo.
Trial registration
The JUPITER trial is registered with ClinicalTrials.gov, number NCT00239681.
Results
Reflecting the JUPITER trial inclusion criteria, the range of lipid levels at study entry was relatively narrow; at study initiation, the median baseline and interquartile range values for LDLC, non-HDLC, and apo B were 108 (94–119), 134 (118–147), and 109 (95–122) mg/dL, respectively.
Overall in the JUPITER trial, random allocation to rosuvastatin 20 mg resulted in a 50% median reduction in LDLC. However, individual variability in per cent LDLC reduction was wide ranging from modest increases to reductions exceeding 80%. In multivariable analyses, the significant predictors of lower on-treatment lipid levels included lower baseline lipid levels, male gender, Caucasian ancestry, higher age, higher body mass index, and lower levels of hsCRP (all P-values <0.05) (Supplementary material online, Table S1).
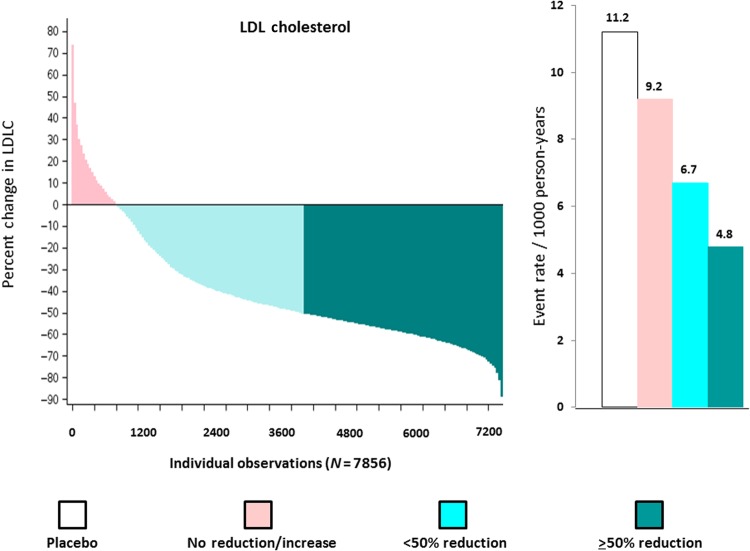
As shown in Figure 1 (left) among those allocated to rosuvastatin 20 mg, 3640 individuals (46.3%) experienced an LDLC reduction ≥50% (dark green); 3365 individuals (42.8%) experienced an LDLC reduction >0 but <50% (light green); and 851 individuals (10.8%) experienced no reduction or an increase in LDLC (pink). As also shown in Figure 1 (right), the magnitude of % reductions in LDLC was directly related to the incidence rates of first cardiovascular events observed during the trial follow-up period. At trial completion, incidence rates for the JUPITER primary endpoint were 11.2 per 1000 for the placebo group and 9.2, 6.7, and 4.8 per 1000 for those with no LDL reduction, LDL reductions <50%, and LDL reductions ≥50%, respectively. When compared with placebo, the hazard ratios for these three groups according to on-treatment % reductions in LDLC were 0.91 (95%CI 0.54–1.53), 0.61 (95%CI 0.44–0.83), and 0.42 (95%CI 0.30–0.60) (P-trend <0.00001). After adjustment for covariates predictive of the change in lipid levels (including baseline LDLC), these multivariable adjusted hazard ratios were: 0.86 (95% CI 0.50–1.49), 0.61 (95%CI 0.44–0.83), and 0.41 (95% CI 0.29–0.58) (P-trend <0.00001). In analysis limited to 3665 participants who achieved on-treatment LDLC levels >50 mg/dL but <75 mg/dL, similar findings were observed. Specifically, in this subgroup, incidence rates for the primary trial endpoint were 18.4, 19.8, 5.4, and 4.1 per 1000 person-years for those in the no LDLC reduction, LDLC reduction <50%, and LDLC reduction ≥50% groups, respectively (P = 0.001) (Table 1).
Figure 1.
Waterfall plot for individual trial participants allocated to rosuvastatin 20 mg for the per cent change in low-density lipoprotein cholesterol (left) and concordant incident event rates (per 1000 person-years) for the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin primary endpoint (right). Data are shown for the placebo group (white bars) and for those allocated to rosuvastatin who had no reduction or an increase in low-density lipoprotein cholesterol (pink), a >0 but <50% reduction in low-density lipoprotein cholesterol (light green), and a ≥50% reduction in low-density lipoprotein cholesterol (dark green).
Table 1.
Hazard ratios and incidence rates (per 1000 person-years) for first cardiovascular events according to magnitude of per cent reduction in cholesterol while on statin therapy in the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin trial
| N | Events | Rate (95% CI) | HR (95% CI) | HR (adjusted)a (95% CI) | |
|---|---|---|---|---|---|
| LDLC | |||||
| Placebo | 7743 | 189 | 11.2 (9.7–12.9) | 1.00 | 1.00 |
| No reduction/increase | 851 | 15 | 9.2 (5.6–15.3) | 0.91 (0.54–1.53) | 0.86 (0.50–1.49) |
| >0 but <50% reduction | 3365 | 49 | 6.7 (5.1–8.9) | 0.61 (0.44–0.83) | 0.61 (0.44–0.83) |
| ≥50% reduction | 3640 | 40 | 4.8 (3.5–6.6) | 0.42 (0.30–0.60) | 0.41 (0.29–0.58) |
| P (all groups) | <0.000001 | <0.000001 | |||
| P (rosuvastatin allocated only) | 0.010 | 0.022 | |||
| Non-HDLC | |||||
| Placebo | 7847 | 190 | 11.1 (9.6–12.8) | 1.00 | 1.00 |
| No reduction/increase | 838 | 16 | 10.0 (6.1–16.3) | 0.99 (0.60–1.66) | 1.04 (0.62–1.74) |
| >0 but <50% reduction | 4887 | 64 | 6.0 (4.7–7.6) | 0.54 (0.41–0.71) | 0.53 (0.40–0.70) |
| ≥50% reduction | 2185 | 26 | 5.2 (3.6–7.7) | 0.46 (0.31–0.70) | 0.44 (0.29–0.67) |
| P (all groups) | <0.000001 | <0.000001 | |||
| P (rosuvastatin allocated only) | 0.046 | 0.056 | |||
| Apolipoprotein B | |||||
| Placebo | 7786 | 188 | 11.0 (9.6–12.8) | 1.00 | 1.00 |
| No reduction/increase | 580 | 14 | 11.9 (7.0–20.1) | 1.14 (0.66–1.97) | 1.15 (0.67–2.00) |
| <50% reduction | 5807 | 74 | 5.7 (4.6–7.2) | 0.51 (0.39–0.67) | 0.50 (0.38–0.65) |
| ≥50% reduction | 1440 | 14 | 4.7 (2.8–7.9) | 0.43 (0.25–0.75) | 0.41 (0.24–0.71) |
| P (all groups) | <0.000001 | <0.000001 | |||
| P (rosuvastatin allocated only) | 0.024 | 0.041 | |||
aAdjusted estimates control for baseline lipid levels, gender, ethnicity, smoking status, hypertension, family history of coronary heart disease, body mass index, and age.
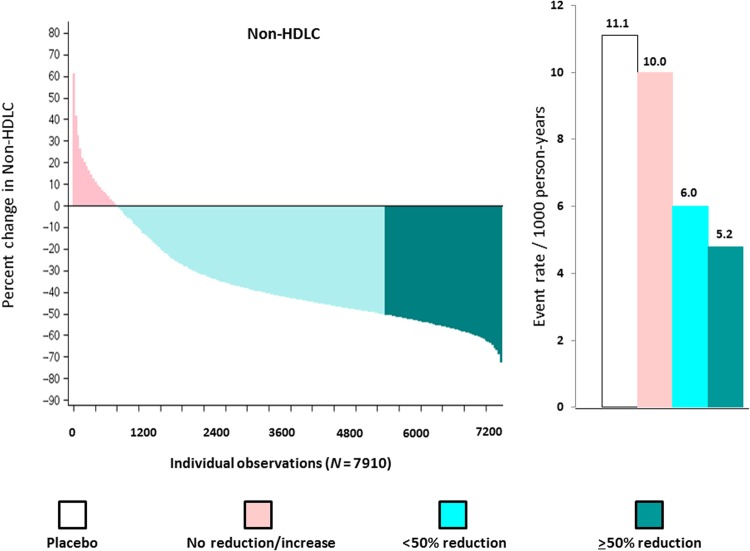
While allocation to rosuvastatin 20 mg resulted in a median per cent reduction in non-HDLC of 44%, similar wide variability in individual response was observed. As shown in Figure 2 (left) among those allocated to rosuvastatin 20 mg, 2185 individuals (27.6%) experienced a non-HDLC reduction ≥50% (dark green); 4887 individuals (61.8%) experienced a non-HDLC reduction >0 but <50% (light green); and 838 individuals (10.6%) experienced no reduction or an increase in non-HDLC compared with baseline (pink). In this analysis, incidence rates for the JUPITER primary endpoint were 11.1 per 1000 for the placebo group and 10.0, 6.0, and 5.2 per 1000 for those with no non-HDLC reduction, non-HDLC reductions <50%, and non-HDLC reductions ≥50%, respectively (Figure 2, right). When compared with placebo, the hazard ratios for these three groups according to on-treatment % reductions in non-HDLC were 0.99 (95%CI 0.60–1.66), 0.54 (95%CI 0.41–0.71), and 0.46 (95%CI 0.31–0.70) (P-trend <0.00001). After adjustment for covariates predictive of the change in lipid levels (including baseline non-HDLC), these multivariable adjusted hazard ratios were 1.04 (95%CI 0.62–1.74), 0.53 (95%CI 0.40–0.70), and 0.44 (95%CI 0.29–0.67) (P-trend <0.00001) (Table 1).
Figure 2.
Waterfall plot for individual trial participants allocated to rosuvastatin 20 mg for the per cent change in non-high-density lipoprotein cholesterol (left) and concordant incident event rates (per 1000 person years) for the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin primary endpoint (right). Data are shown for the placebo group (white bars) and for those allocated to rosuvastatin who had no reduction or an increase in non-high-density lipoprotein cholesterol (pink), a ≥0 but <50% reduction in non-high-density lipoprotein cholesterol (light green), and a ≥50% reduction in non-high-density lipoprotein cholesterol (dark green).
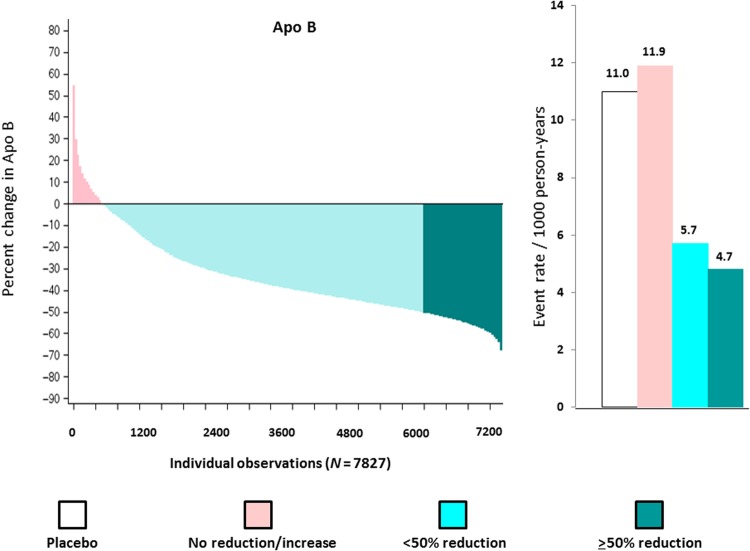
We repeated our analysis for apo B (median per cent reduction 40%) where wide variation was again seen in response to rosuvastatin. As shown in Figure 3 (left) among those allocated to rosuvastatin 20 mg, 1440 individuals (18.4%) experienced an apo B reduction ≥50% (dark green); 5807 individuals (74.2%) experienced an apo B reduction >0 but <50% (light green); and 580 individuals (7.4%) experienced no reduction or an increase in apo B compared with baseline (pink). For this analysis, incidence rates for the JUPITER primary endpoint were 11.0 per 1000 for the placebo group and 11.9, 5.7, and 4.7 per 1000 for those with no apo B reduction, apo B reductions <50%, and apo B reductions ≥50%, respectively (Figure 3, right). When compared with placebo, the hazard ratios for these three groups according to on-treatment per cent reductions in apo B were 1.14 (95% CI 0.66–1.97), 0.51 (95% CI 0.39–0.67), and 0.43 (95% CI 0.25–0.75) (P-trend <0.00001). After adjustment for covariates predictive of the change in lipid levels (including baseline apo B), these multivariable adjusted hazard ratios were 1.15 (95% CI 0.67–2.00), 0.50 (95% CI 0.38–0.65), and 0.41 (95% CI 0.24–0.71) (P-trend <0.00001) (Table 1).
Figure 3.
Waterfall plot for individual trial participants allocated to rosuvastatin 20 mg for the per cent change in apolipoprotein B (left) and concordant incident event rates (per 1000 person-years) for the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin primary endpoint (right). Data are shown for the placebo group (white bars) and for those allocated to rosuvastatin who had no reduction or an increase in apolipoprotein B (pink), a >0 but <50% reduction in apolipoprotein B (light green), and a ≥50% reduction in apolipoprotein B (dark green).
In sensitivity analyses using tertiles of % reduction rather than the ACC/AHA specified 50% reduction threshold, we observed similar findings. For example, incidence rates for the JUPITER primary endpoint were 11.1 per 1000 for the placebo group and 11.9, 7.7, 4.7, and 4.2 per 1000 for those with no apo B reduction, apo B reductions in the first tertile (0 to <34 mg/dL), apo B reductions in the second tertile (34–45 mg/dL), and apo B reductions in the third tertile (>45 mg/dL), respectively. When compared with placebo, the corresponding multivariable hazard ratios for these four groups according to on-treatment % reductions in apo B were 1.14 (95%CI 0.66–1.97), 0.71 (95%CI 0.50–0.99), 0.41 (95%CI 0.28–0.63), and 0.38 (95%CI 0.24–0.60) (P-trend across on-treatment apolipoprotein B groups <0.00001).
Finally, in analyses limited only to those on active rosuvastatin, the relationship between per cent cholesterol reduction and incident event rates remains significant with P-values of 0.01, 0.046, and 0.024 for per cent reductions in LDLC, non-HDLC, and apo B, respectively.
Discussion
We confirm here in a contemporary randomized trial of high-intensity statin therapy that the variability in % reduction for LDLC, non-HDLC, and apo B is wide for individual participants, with the magnitude of on-treatment % cholesterol reduction directly relating to the magnitude of risk reduction observed. In our study of rosuvastatin 20 mg daily, effects were robust to multivariable adjustment for those characteristics associated with greater lipid response to statin therapy and were minimally impacted in sensitivity analyses when tertile % reductions in on-treatment lipid levels were used instead of the pre-specified ≥50% threshold. In all of our analyses, we controlled for baseline lipid levels, and similar results were found in subgroup analyses limited to those who achieved on-treatment levels of LDLC between 50 and 75 mg/dL, and in analyses limited to those allocated to active statin therapy.
The wide variability in % cholesterol response to statin therapy described here is consistent with clinical experience and data recently presented by Boekholdt et al.,4 yet is not often recognized in clinical reports from statin trials nor in overviews of statin efficacy. The current contemporary data for a high-intensity statin regimen are also consistent with prior work indicating important behavioural, environmental, and genetic determinants of statin efficacy for individual patients.6–9
Our finding of sequentially greater clinical benefits with sequentially greater % reductions in cholesterol while on statin therapy parallels recent intravascular ultrasound data demonstrating greater progression of the % atheroma volume in statin hypo-responders when compared with statin responders.10 Our data are also consistent with outcomes from the IMPROVE-IT trial where greater event reductions were observed with greater LDLC reductions within the context of a randomized trial of ezetimibe added to statin therapy when compared with statin therapy alone.11
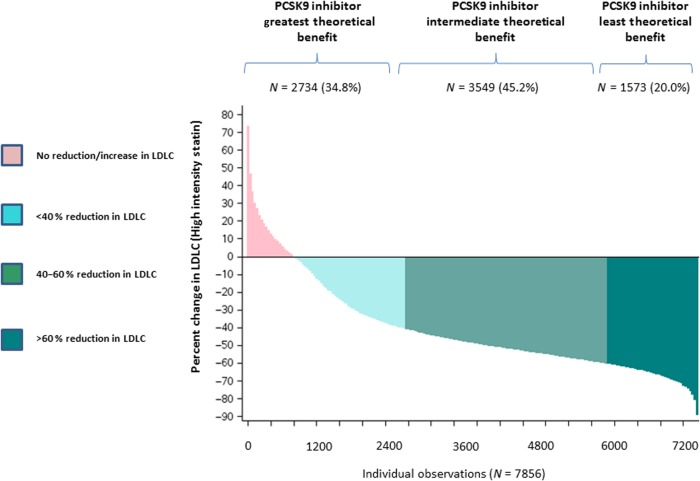
The concept of % reduction in cholesterol may have relevance for interpretation of ongoing outcome trials of PCSK9 inhibitors which can lower LDLC well beyond that achievable with statin therapy alone. Enrolment criteria in several ongoing PCSK9 inhibitor phase III development programmes typically include the attainment of LDLC levels of >70 mg/dL (11.8 mmol/L) or >100 mg/dL (2.6 mmol/L) following statin initiation. However, as shown in the current data, the % reductions in cholesterol achieved for individual patients on statin therapy are exceptionally wide even when being treated with a high-intensity regimen. This variability might impact on the effectiveness of PCSK9 inhibition; for example, as shown in Figure 4, PCSK9 inhibition might prove to have the greatest clinical benefit among those treated with a high-intensity statin who achieve only 20–30% initial reductions in LDLC. In contrast, among those treated with a high-intensity statin who have already achieved 70–80% reductions in LDLC, the ability to further reduce LDLC on a % basis is much smaller and hence the theoretical benefit of a PCSK9 inhibition in this setting might be more limited. It will thus be helpful if ongoing event reduction trials of PCSK9 inhibitors report results stratified by the % reduction in LDLC achieved by background statin therapy alone.
Figure 4.
Variability in low-density lipoprotein cholesterol response following high-intensity statin therapy and its theoretical implications for the allocation of PCSK9 inhibitors.
As in any study evaluating on-treatment thresholds in a trial of fixed dose therapy compared with placebo, our data are limited in that we cannot fully account for potential confounding factors that might influence drug response. In this regard, the fact that the recent IMPROVE-IT data demonstrate increased efficacy in a randomized trial of increasing LDLC reduction is reassuring.11 A further limitation of our analysis is that we used single measures of baseline and on-treatment level of LDLC, non-HDLC, and apolipoprotein B to define the % changes in these parameters. This limitation is, if anything, a bias toward the null and thus would lead to an underestimation of the importance of measuring on treatment % reduction in cholesterol levels. As recently demonstrated by the TNT investigators, visit-to-visit variability in on-treatment cholesterol levels are also an independent predictor of cardiovascular event rates, even after adjusting for on-treatment levels.12 Finally, this manuscript represents a secondary analysis of the JUPITER trial in which rosuvastatin 20 mg daily was given to individuals with low to normal levels of LDLC yet above average levels of hsCRP.
It is not our intent to suggest here that % reduction targets are better or worse than absolute on-treatment targets when seeking optimal care for individual patients. Rather, we seek to provide insights on statin variability (even when given at high-intensity doses) and to provide hard data from a contemporary statin trial with potential relevance for ongoing guideline discussions. We suspect that for specific patients, information on both absolute and % LDLC reduction are both important and note that this is the broad approach endorsed in recent European and Canadian guidelines.1,2
In sum, the current data confirm wide variation in the % reduction in cholesterol during high-intensity statin therapy as well as a direct relationship between the magnitude of this per cent reduction and the clinical benefit achieved.4 These data provide general support for the concepts of introducing % reduction in LDLC into broader clinical practice, along with fixed LDLC targets. Further consideration of % LDLC reduction as well as absolute LDLC reduction while on statin therapy might further provide a partial method to allocate PCSK9 inhibitors should these agents prove effective for cardiovascular event reduction.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
P.R., L.R. performed statistical analysis; P.R. handled funding and supervision. P.R., S.M. acquired the data. P.R. conceived and designed the research. P.R. drafted the manuscript. L.R., S.M. made critical revision of the manuscript for key intellectual content.
Funding
The JUPITER trial was supported by AstraZeneca.
Conflict of interest: P.M.R. has received investigator-initiated research grants from the National Heart Lung and blood Institute, the American Heart Association, the Leducq Foundation, AstraZeneca, Novartis, and Pfizer; served as a consultant to AstraZeneca, Lilly, and BostonHeart; and is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to AstraZeneca and Seimens.S.M. received research support from AstraZeneca, Atherotech Diagnostics, and NHLBI (HL1117861) and serves as a consultant to Quest diagnostics, Lilly, Pfizer, and Cerenis Therapeutics.
References
- 1.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvane M, Scholte op Remier WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 2.Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J Jr, Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151–167. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PWF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 4.Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amerenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM, Ridker PM, Grundy SM, Kastelein JJP. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events. A meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 6.Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F, Ridker PM. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ Cardiovasc Genet 2012;5:257–264. [DOI] [PubMed] [Google Scholar]
- 7.Thompson JF, Hyde CL, Wood LS, Paciga SA, Hinds DA, Cox DR, Hovingh GK, Kastelein JJ. Comprehensive whole-genome and candidate gene analysis for response to statin therapy in the Treating to New Targets (TNT) cohort. Circ Cardiovasc Genet 2009;2:173–181. [DOI] [PubMed] [Google Scholar]
- 8.Chasman DI, Posada D, Subrahmanyan L, Cook NR, Stanton VP Jr, Ridker PM. Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA 2004;291:2821–2827. [DOI] [PubMed] [Google Scholar]
- 9.Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield MJ, Devlin JJ, Nordio F, Hyde CL, Cannon CP, Sacks FM, Poulter NR, Sever PS, Ridker PM, Braunwald E, Melander O, Kathiresan S, Sabatine MS. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet 2015. doi 10.1016/S0140–6736(14)61730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kataoka Y, St. John J, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen S, Nicholls SJ. Atheroma progression in hyporesponders to statin therapy. Atheroscler Thromb Vasc Biol 2015. doi:10.1161/ATVBAHA.114.304477. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im KA, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. for the IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 12.Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH, on behalf of the TNT Steering Committee and Investigators. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes. Insights from the TNT trial. J Am Coll Cardiol 2015;65:1539–1548. [DOI] [PubMed] [Google Scholar]