Abstract
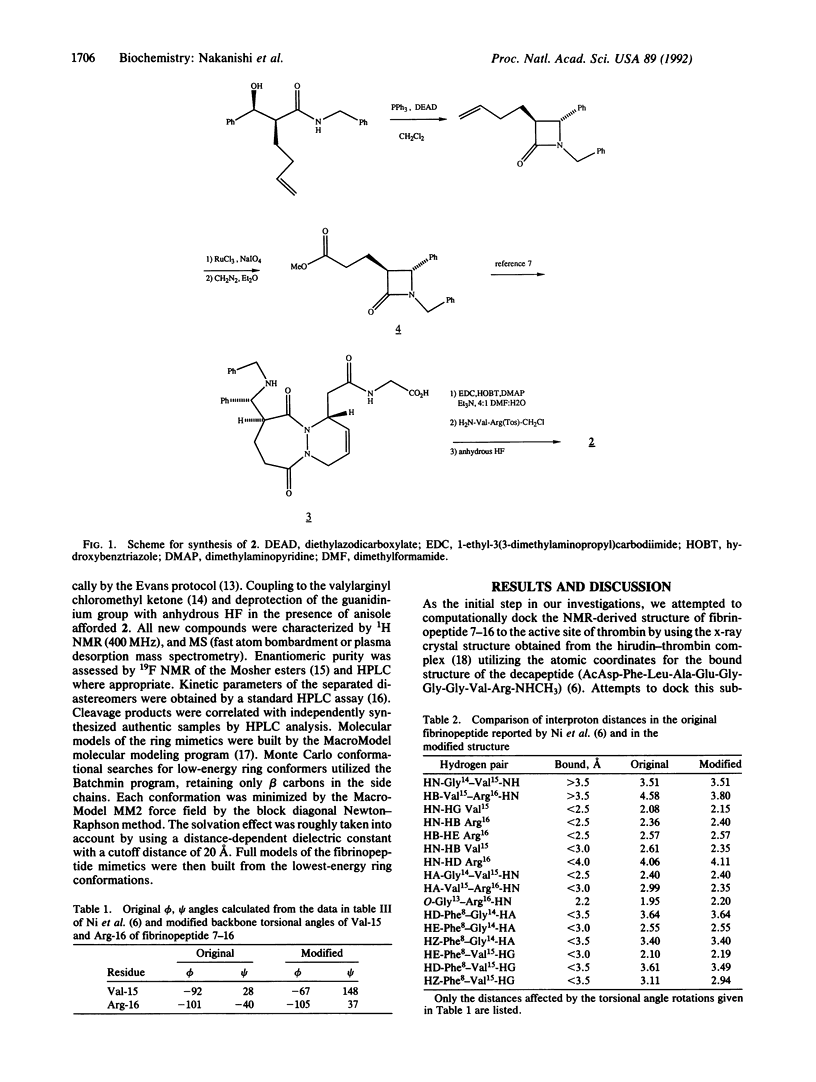
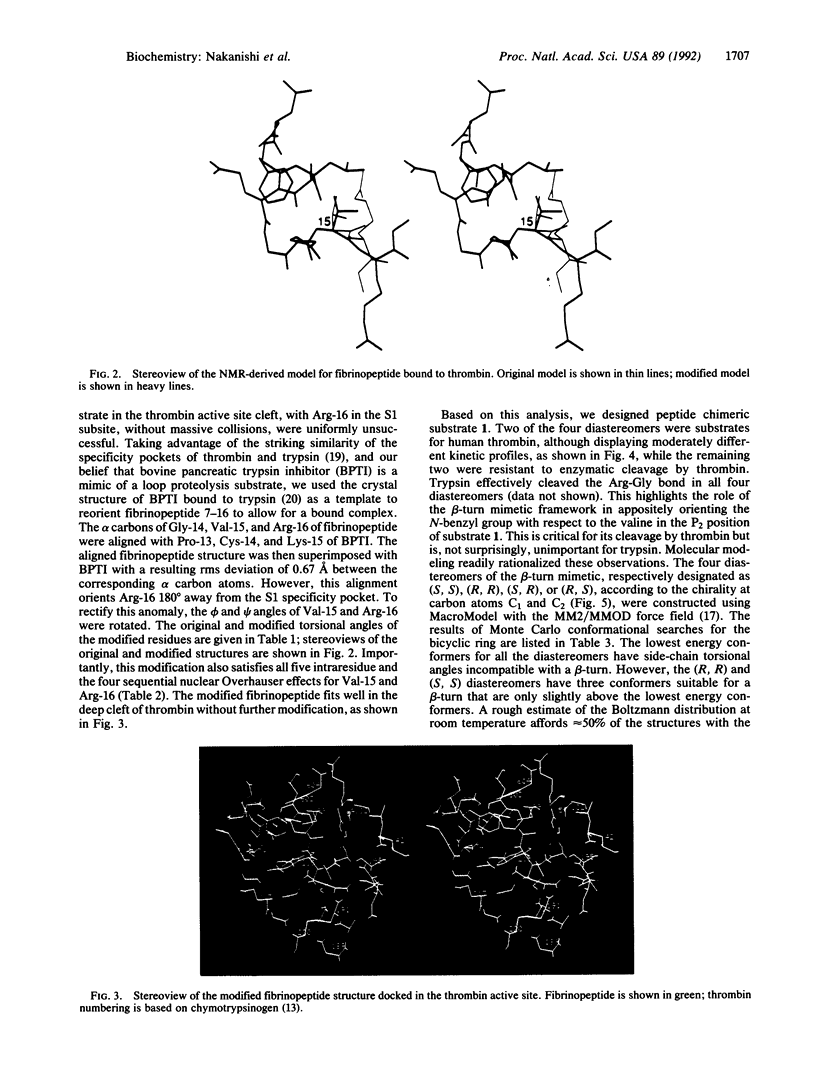
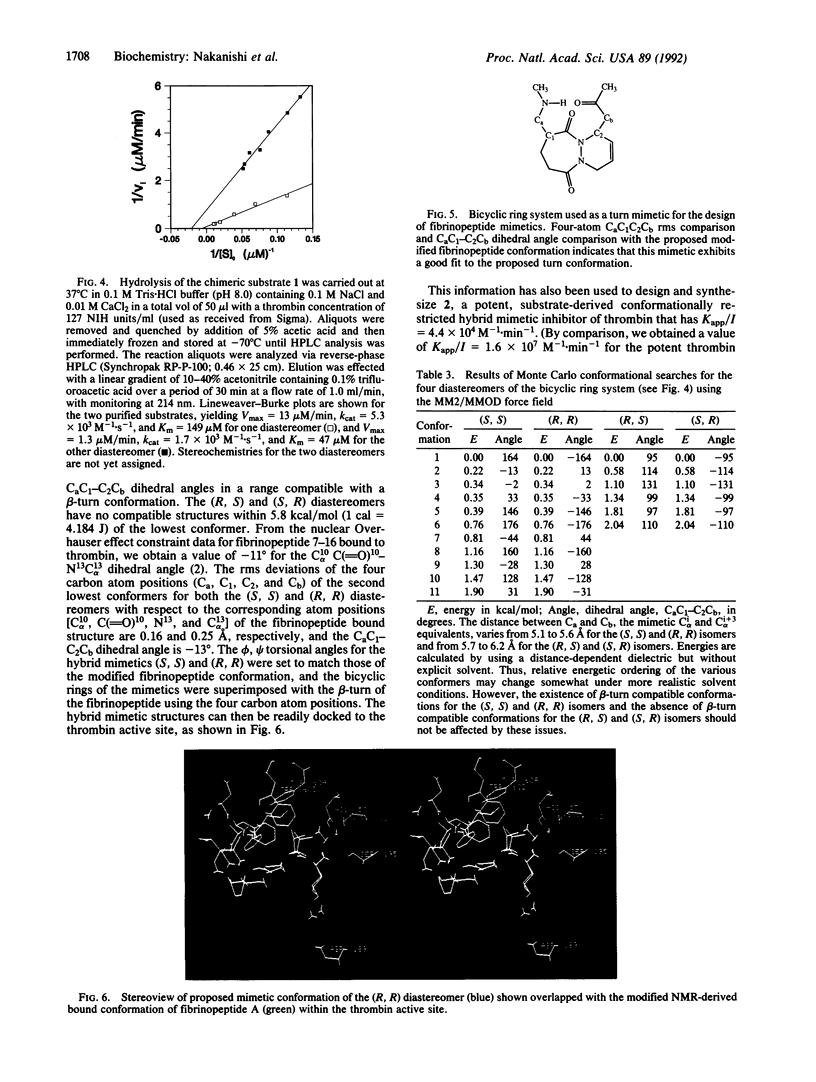
Recent work has suggested that the thrombin-bound conformation of fibrinopeptide A exhibits a strand-turn-strand motif, with a beta-turn centered at residues Glu-11 and Gly-12. Our molecular modeling analysis indicates that the published fibrinopeptide conformation cannot bind reasonably to thrombin but that reorientation of two residues by alignment with bovine pancreatic trypsin inhibitor provides a good fit within the deep thrombin cleft and satisfies all of the experimental nuclear Overhauser effect data. Based on this analysis, we have successfully designed and synthesized hybrid peptide mimetic substrates and inhibitors that mimic the proposed beta-turn structure. The results indicate that the turn conformation is an important aspect of thrombin specificity and that our turn mimetic design successfully mimics the thrombin-bound conformation of fibrinopeptide.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bek E., Berry R. Prohormonal cleavage sites are associated with omega loops. Biochemistry. 1990 Jan 9;29(1):178–183. doi: 10.1021/bi00453a024. [DOI] [PubMed] [Google Scholar]
- Bode W., Mayr I., Baumann U., Huber R., Stone S. R., Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Blow D. M., Fastrez J. Leaving group specificity in the chymotrypsin-catalyzed hydrolysis of peptides. A stereochemical interpretation. Biochemistry. 1973 May 22;12(11):2035–2041. doi: 10.1021/bi00735a002. [DOI] [PubMed] [Google Scholar]
- Henschen A., Lottspeich F., Kehl M., Southan C. Covalent structure of fibrinogen. Ann N Y Acad Sci. 1983 Jun 27;408:28–43. doi: 10.1111/j.1749-6632.1983.tb23232.x. [DOI] [PubMed] [Google Scholar]
- Kettner C., Shaw E. D-Phe-Pro-ArgCH2C1-A selective affinity label for thrombin. Thromb Res. 1979;14(6):969–973. doi: 10.1016/0049-3848(79)90014-8. [DOI] [PubMed] [Google Scholar]
- Marsh H. C., Jr, Meinwald Y. C., Thannhauser T. W., Scheraga H. A. Mechanism of action of thrombin on fibrinogen. Kinetic evidence for involvement of aspartic acid at position P10. Biochemistry. 1983 Aug 30;22(18):4170–4174. doi: 10.1021/bi00287a002. [DOI] [PubMed] [Google Scholar]
- Ni F., Meinwald Y. C., Vásquez M., Scheraga H. A. High-resolution NMR studies of fibrinogen-like peptides in solution: structure of a thrombin-bound peptide corresponding to residues 7-16 of the A alpha chain of human fibrinogen. Biochemistry. 1989 Apr 4;28(7):3094–3105. doi: 10.1021/bi00433a053. [DOI] [PubMed] [Google Scholar]
- Rholam M., Cohen P., Brakch N., Paolillo L., Scatturin A., Di Bello C. Evidence for beta-turn structure in model peptides reproducing pro-ocytocin/neurophysin proteolytic processing site. Biochem Biophys Res Commun. 1990 May 16;168(3):1066–1073. doi: 10.1016/0006-291x(90)91138-i. [DOI] [PubMed] [Google Scholar]
- Rholam M., Nicolas P., Cohen P. Precursors for peptide hormones share common secondary structures forming features at the proteolytic processing sites. FEBS Lett. 1986 Oct 20;207(1):1–6. doi: 10.1016/0014-5793(86)80002-3. [DOI] [PubMed] [Google Scholar]
- Rydel T. J., Ravichandran K. G., Tulinsky A., Bode W., Huber R., Roitsch C., Fenton J. W., 2nd The structure of a complex of recombinant hirudin and human alpha-thrombin. Science. 1990 Jul 20;249(4966):277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- Scheraga H. A. Chemical basis of thrombin interactions with fibrinogen. Ann N Y Acad Sci. 1986;485:124–133. doi: 10.1111/j.1749-6632.1986.tb34574.x. [DOI] [PubMed] [Google Scholar]





