Summary
Premature death among individuals with epilepsy is higher than in the general population, and sudden unexpected death is the most common cause of this mortality. A new multisite collaborative research consortium, the Center for sudden unexpected death in epilepsy (SUDEP) Research (CSR), has received major funding from the National Institutes of Health (NIH) to examine the possible biologic mechanisms underlying this potentially preventable comorbidity and develop predictive biomarkers for interventions that could lower SUDEP incidence. This inaugural report describes the structure of the CSR, its priorities for human and experimental research, and the strategic collaborations and advanced tools under development to reduce this catastrophic outcome of epilepsy. The CSR Partners Program will work closely with committed volunteer agencies, industry, and academic institutions to accelerate and communicate these advances to the professional and lay community.
Keywords: Cardiorespiratory, Autonomic dysfunction, Imaging, Genetics, Induced pluripotent stem cell, Neuropathology
Recent estimates indicate that sudden unexpected death in epilepsy (SUDEP) is responsible for approximately 7,000 deaths each year in the United States and Europe, and is the second most common cause, after stroke, of the number of adult life years lost—an estimated incidence of 1.16 deaths per 1,000 individuals with epilepsy.1 The cumulative risk of SUDEP is high, approaching 7% over 40 years, and 12% in individuals with epilepsy who are not taking antiepileptic medication and who are not in remission.2 The precise underlying causes are unknown, and no targeted prevention strategies currently exist, other than seizure reduction.3,4 In 2008, the American Epilepsy Society and the Epilepsy Foundation convened a task force, which led to a workshop sponsored by the National Institute of Neurological Disorders and Stroke (NINDS) at the NIH (National Institutes of Health) to assess current SUDEP knowledge and to make recommendations for future research,5 as summarized in the NINDS Epilepsy Research Benchmarks Area IIID3. At that time, very few postmortem SUDEP cases had been analyzed neuropathologically, and none genetically. Clinical assessment of living individuals with peri-ictal abnormalities that suggest increased SUDEP risk had not been studied for lethal outcomes. There were no validated experimental models of underlying SUDEP mechanisms. The Task Force recognized the limitations of single-center studies in accumulating a sufficient number of clinical cases for systematic investigation. It emphasized the need for hypothesis-driven, prospective, multicenter basic science and clinical research that integrated multimodal biologic parameters including cardiovascular, respiratory, autonomic, biochemical, and genetic factors. The development of tractable model systems to explore biomarkers for the inherited and acquired vulnerability to premature lethality was identified as a priority. This led to NINDS funding for two, 3-year P20 planning grants in 2011 to organize the infrastructure for a subsequent NIH Center Without Walls for SUDEP Research. In 2014, after a competitive review, the two planning consortia were successfully merged into the Center for SUDEP Research (CSR; http://www.sudepresearch.org), comprising 14 institutions collaborating in a broad spectrum of basic science and clinical approaches. In this report, we briefly describe the rationale and structure of the CSR, its scientific programs, research cores, and the CSR Partners Program for SUDEP outreach to the epilepsy community.
Overview
Pathophysiology, prediction, and prevention: the essential research mission of the CSR
The occurrence of SUDEP is usually seizure related.6 Poor seizure control, frequent generalized tonic–clonic seizures, and long-standing epilepsy are consistent risk factors. 4,7 Deaths are typically unwitnessed, nocturnal events associated with prone position,8 with circumstantial evidence of ictus.9 Deaths occurring in an epilepsy monitoring unit (EMU) reveal combined cardiorespiratory failure after generalized tonic–clonic seizures.10 Young people with epilepsy (20–40 years) are up to 24 times more likely to die suddenly than the general population,11 although SUDEP can occur at other ages.1 No antiepileptic medication has been consistently implicated in SUDEP.12,13 Because most individuals with epilepsy share similar population risk profiles yet do not die prematurely, a better definition of objective, individualized pathophysiologic risk indicators that can be routinely assessed following the diagnosis of epilepsy is urgently required. Genetic profiling may reveal molecular risk biomarkers. As additional deleterious mutations in genes linked to SUDEP are discovered and correlated with abnormal clinical measures, a checklist for assessing SUDEP risk can be developed and validated. Advancing the basis for personalized SUDEP risk prediction and ultimately its prevention, is a central scientific and clinical research goal of the CSR.
Clinical Investigation
Human peri-ictal cerebral, autonomic, and respiratory dysregulation studied in the EMU
Generalized tonic–clonic seizures are frequently followed by postictal generalized electroencephalography (EEG) suppression (PGES), an electrophysiologic pattern that correlates with peri-ictal sympathetic and parasympathetic dysregulation14 and seizure-related respiratory depression.15 Prolonged PGES is a quantifiable, age-driven16 candidate biomarker of individual SUDEP risk17 and has been reported in all observed EMU SUDEP cases.10 Interventions after generalized tonic–clonic seizures shorten PGES, potentially reducing SUDEP risk.18 Peri-ictal cardiac arrhythmias are common occurrences in SUDEP.10,19 The role of changes in heart rate variability (HRV) in seizure survivability is uncertain.20 Maintenance of blood pressure and heart rate mediated through the baroreflex may be altered in individuals with epilepsy during the interictal, nonseizure state, and also during blood pressure challenges in the immediate postictal state (Valsalva, tilt tests).21 During this vulnerable period, profound hypotension has been reported.22 Impaired baroreflex sensitivity, with subsequent compromised cerebral blood flow and an inability to recover from extreme postictal hypotension is a potential SUDEP mechanism. Peri-ictal respiratory apnea has been reported in near-SUDEP and recorded SUDEP cases. Severe, prolonged, end-tidal CO2 increases can appear during seizures with prolonged ictal apnea. Ictal hypoxemia was seen in 48.9% of children and 26.8% of adult seizures.23 Previously unrecognized patterns of tachypnea and profound cardiorespiratory dysfunction were followed by terminal apnea and cardiac arrest in 10 monitored cases of SUDEP.10 Brainstem dysfunction may contribute to fatal cardiorespiratory dysfunction, although this is a challenging region for human neurophysiologic study. Recent imaging evidence of structural abnormalities in the dorsal mesencephalon,24 as well as in cardiac and respiratory control structures in the forebrain25 suggest a role for premortem imaging in individuals with epilepsy.
A major focus of the CSR clinical research EMU network is to examine the complex interplay between seizure-induced suppression of cortical and brainstem function, disordered peri-ictal breathing, and autonomic dysfunction. The influence of seizure phenotype, prospectively studied with multimodal physiologic parameters including EEG, electrocardiography (ECG), oxygen saturation, CO2, breathing, and biochemical measures, will be correlated with advanced imaging to compare individuals with epilepsy who are at high and low risk for SUDEP. Exploratory studies to map critical seizure networks connecting forebrain and brainstem26 will better define critical SUDEP networks. Genotype– phenotype correlations in individuals with abnormal peri-ictal physiology will permit experimental validation of genetic contributions. This program carries substantial promise for premortem SUDEP risk identification.
Defining autonomic and imaging biomarkers of SUDEP
The objective of this project (principal investigators [PIs]: Lhatoo, Harper, and Diehl) is to identify specific seizure types and potential processes in brain structures, the failure of which may lead to autonomic or respiratory arrest and a fatal outcome. Measures that are not routinely monitored in EMUs, including blood pressure, overall and instantaneous HRV, baroreflex sensitivity, blood catecholamines, breathing patterns (rate, O2, CO2, apnea, and hypopnea), sweating during baseline, and peri-ictal events (including sleep and waking seizures) are under study.25 The analysis includes simple descriptive procedures, as well as time/frequency domain methods of cross-correlation, coherence, power spectral, and autoregressive modeling to determine interactions between autonomic dysregulation indices and EEG patterns such as PGES within this population. Imaging studies will determine the magnitude and laterality of gray and white matter abnormalities using high-resolution T1 volumetric and morphometric procedures and diffusion tensor imaging in autonomic and respiratory regulatory sites (e.g., right ventral medial frontal cortex, bilateral insulae, bilateral hippocampus, right ventrolateral medulla, caudal raphe, bilateral fastigial nuclei and cerebellar cortex, and dorsal and caudal thalamus), and relate that damage to EEG and autonomic patterns in subgroups of individuals monitored in the EMU with frequent (>1 month) refractory generalized tonic–clonic seizures (high SUDEP risk), compared to lower SUDEP risk patients (<1 month or no history of generalized tonic–clonic seizures). These identified risk biomarkers will be used to develop and validate a quantifiable SUDEP risk model for clinical use.
Building a multisite clinical data analysis infrastructure
The low annual incidence of SUDEP (~1%) requires multicenter participation to prospectively accrue sufficient SUDEP/near-SUDEP cases to power statistical study. Power analysis for autonomic studies suggests that at least 2,500 individuals are necessary for prospective analysis, placing considerable infrastructural demands on such projects. The clinical epilepsy phenotype; syndromic and genetic information; multimodal physiologic, biochemical, and respiratory measurements; and collection for standardization, integration, and presentation require significant bioinformatics expertise and infrastructural resources. The Informatics and Data Analytics Core (PI: Zhang) will develop the necessary digital infrastructure for the storage and analysis of complex multimodal, dynamic seizure variables (EEG, ECG, continuous blood pressure, O2, and CO2) with second to second measures. Data from individual cases recruited at multiple clinical sites within the EMU network are uploaded to a common digital analysis platform. A common analytics component will facilitate unified signal processing of acquired data and house a collaborative database for mining the large physiologic datasets acquired from individuals with epilepsy by participating investigators.27
The neuropathology of SUDEP
Advances in our understanding of SUDEP are critically dependent on comparisons of SUDEP postmortem brains with controls. This project (PIs: Thom and Devinsky) will recruit cases for study and gather evidence to test leading hypotheses regarding neurochemical dysfunction of selected neurotransmitters such as the serotonergic, purinergic, and adenosine systems in autonomic brainstem structures in cases of SUDEP. Advanced 9.4T magnetic resonance imaging (MRI) imaging, complemented by quantitative stereologic and immunohistochemical techniques are used to study neuronal and astrocytic densities in central autonomic network structures such as the insula, anterior cingulate cortex, and amygdala, along with cardiorespiratory and median raphe brainstem nuclei. This collaboration will examine a large collection of formalin-fixed SUDEP brains, along with prospectively collected frozen brain tissue from epilepsy surgery. Systematic neuropathologic analysis will provide a comparative reference map of histopathologic and neurochemical abnormalities that are altered in SUDEP.28
SUDEP brain morphometry, tractomics, and genomic cores
Two research cores were created as essential translational components of the Center. The Morphometrics Core (PI: Goldman), is responsible for imaging and neuropathologic analysis of brains from SUDEP cases recruited prospectively in the United States. These cases are entered into an efficient analysis pipeline that begins with high-resolution 7T MRI scanning and three-dimensional tractomic analysis of descending forebrain-brainstem pathways (Warfield, Harvard/Children’s Hospital) followed by neuropathologic tissue examination (Anderson, Harvard/Beth Israel Hospital). The data sets are then correlated with genotypic information. Detailed MRI volumetric analysis of brainstem is being standardized from imaging performed across the CSR multisite network, based on protocols developed in a recent MRI study of SUDEP cases that identified abnormalities in brainstem cardiorespiratory regions.24 This study focuses attention on an underexplored opportunity to identify early structural signs of SUDEP risk in living individuals with epilepsy.
The Molecular Diagnostic Core (PIs: Belmont and Goldman) plays an essential role in the CSR with the responsibility for genetic analysis of SUDEP cases and bioinformatics that can ultimately lead to risk prediction in individuals living with epilepsy. Along with monogenic causes of SUDEP, recent work reveals that inherited risk factors for epilepsy and SUDEP may be remarkably complex, both genetically and biologically.29,30 A whole-exome analysis of 18 SUDEP and probable SUDEP cases shows a significantly increased genome-wide polygenic burden per individual compared to epilepsy and nonepilepsy disease controls, suggesting a polygenic contribution to SUDEP risk.31 Gene variant profiling of a single SUDEP case demonstrates that premature lethality could likely be the result of mutations in not one, but three distinct genes, each conferring SUDEP risk.32 Various hypotheses regarding genetic contribution to personal SUDEP risk (rare gene sequence variants, copy number variants, and oligogenic patterns of common gene variants) are being tested using whole exome and whole genome approaches. Data will be analyzed using advanced bioinformatic filtering algorithms to extract and profile deleterious variants, which are deposited in a global database. Ultimately, genomic risk data will be linked to individuals studied at clinical sites with extensive phenotypical monitoring data, allowing those with physiologic abnormalities to be mined for high- and low-risk genetic profiles.
Experimental Approaches
Limbic control over brainstem respiratory stability
Delayed recovery of breathing following a seizure may be a major cause of SUDEP.33 Recent studies during presurgical evaluation of individuals with temporal lobe epilepsy show that apnea can coincide with propagation of seizures to the amygdala.26 Stimulation of the left or right amygdala with depth electrodes caused prolonged apnea (up to 47 s) and decreased O2 saturation (to as low as 87%). Remarkably, despite being awake and alert during amygdala stimulation, the individuals were unaware that they were not breathing. This project (PI: Richerson) centers on the experimental evaluation of the hypothesis that a combination of prolonged apnea and respiratory agnosia, combined with postictal depression of consciousness, may be important in causing some cases of SUDEP.
The exact pathways and mechanisms involved in propagation of seizures from the limbic forebrain to the brainstem respiratory network remain poorly understood. Neurons of the reticular activating system (RAS) are one possible connection, and their inhibition during the postictal state could contribute to respiratory depression and a decreased level of arousal. Serotonin neurons are a component of the RAS that stimulate respiratory activity34 and wakefulness35 in response to a rise in blood CO2. Experiments in mice are examining the role of serotonin neurons in SUDEP using long-term video/EEG/ECG monitoring. These studies show that the risk of seizures, postictal apnea, and postictal death are all increased in mice with genetic deletion of serotonin neurons.36 The action of serotonin in stimulating breathing, as well as forebrain-mediated wakefulness, is mediated in part through activation of 5-HT2a receptors.35 Thus, defects in the serotonin system could increase the risk of SUDEP, and pharmacologic agents that enhance serotonergic tone could lower it. Further definition of serotonin’s role in SUDEP using genetic mouse models may provide novel biomarkers of risk and also new avenues for treatment.
Cardiac genes, autonomic balance, and brainstem spreading depolarization
Two interrelated lines of translational research focus on identifying molecular mechanisms underlying abnormalities in cardiac rhythmicity, and brain-heart signaling as SUDEP risk mechanisms. The first of these projects (PIs: Parent and Isom) involves the examination of ion channel mutations derived from individuals with Dravet syndrome who carry a known elevated SUDEP risk. Rather than re-engineer these mutations into unrelated experimental model cells, the native genetic mutation is studied directly in neurons or cardiomyocytes derived from the individual. Using induced pluripotent stem cell technology, the effects of the mutation on excitability is examined in induced excitatory neurons, inhibitory neurons, autonomic neurons, and cardiac myocytes.37 The key advantage of this approach is that the mutation is evaluated in the same genetic context (i.e., in the presence of other genetic differences existing in that individual) and therefore reflects a far more meaningful functional assay of the genetic defect. This approach is uncovering crucial and unanticipated data on sodium currents in neurons38 and cardiac myocytes. Concurrent studies of a Dravet syndrome mouse model have also shown abnormally increased sodium current and hyperexcitability of cardiac myocytes in vitro, as well as ventricular arrhythmias in vivo in the setting of SUDEP.39 Currently other candidate mutations in cardiac ion channel genes are under analysis in model systems including cultured cells, and murine and transgenic rabbit models. For example, mouse models of epileptic encephalopathies caused by heterozygous putative gain-of-function mutations in Scn8a or homozygous loss-of-function mutations of Scn1b cause epilepsy and SUDEP.40,41 These studies are essential to move beyond single-cell firing patterns and evaluate the behavior of neural networks, cardiac rhythmicity, and autonomic reflexes.
A second project (PI: Noebels) continues the search for SUDEP phenotypes among monogenic mouse models with combined epilepsy and cardiac arrhythmia. These gene models were the first to demonstrate that SUDEP risk can be a product of a monogenic inherited error. Mutation of potassium ion channels, in particular those responsible for well-known long QT cardiac arrhythmias, are immediately translatable for predicting elevated sudden death risk in individuals with epilepsy.42–44 The list for human gene profiling of SUDEP cases currently exceeds 20 experimentally validated genes for SUDEP and this project aims to rapidly expand that number. This “reverse” genetic approach to SUDEP gene discovery performed in genetically engineered mouse mutants will accelerate progress in predicting this rare, but genetically very heterogeneous, human disorder. A parallel goal is to create mouse models of human SUDEP genes in collaboration with the Molecular Diagnostics Core by engineering candidate genes discovered in human SUDEP exomes. A third aim is to examine the cellular neurophysiology of these models for clues that may explain how the mutation confers biologic risk. One newly discovered mechanism underlying the failure of the brain to recover following a seizure is the phenomenon of “spreading depolarization.” When this all-or-none event is triggered in the brainstem, a wave of depolarization spreads slowly across medullary cardiorespiratory control centers, silencing the pacemaking neurons and preventing recovery of the heartbeat and normal respiration after a seizure. Mouse models of SUDEP-bearing mutations in ion channel genes (Kcna1a potassium channels), and Dravet syndrome (Scn1a sodium channels) show a dramatically lower threshold for this fatal event.45 Linking these genes to translational treatments designed to elevate the threshold for spreading depolarization may offer an important lifesaving intervention.
CSR Partners
The CSR senses the urgency for advancing SUDEP prevention research and has initiated an outreach program entitled CSR Partners to nonprofit, lay advocacy organizations, industry, and academic institutions. This program will foster communication and collaboration between CSR investigators and individuals with epilepsy and families who have lost a loved one to SUDEP. Such partnership is vital for the future of SUDEP research. In addition, CSR investigators are eager to inform the epilepsy community about ongoing research in the CSR, as well as research opportunities for individuals with epilepsy and their families. CSR Partners, therefore, has the important responsibility of providing updates on research activities within the CSR and promoting collaborative activities to the interested public.
CSR Pilot and Feasibility Program
The CSR’s Pilot Feasibility Program has provided grants for exploratory research (one clinical and one basic science) funded through the Administrative Core to two young investigators who will join the CSR and collaborate with CSR researchers to complete their projects. One institutional partner, Case Western Reserve University, has provided two additional pilot grants to local investigators. Expanding the exploratory project funding base for future multi-investigator collaborations while training new investigators in SUDEP research is an important shared goal of the CSR.
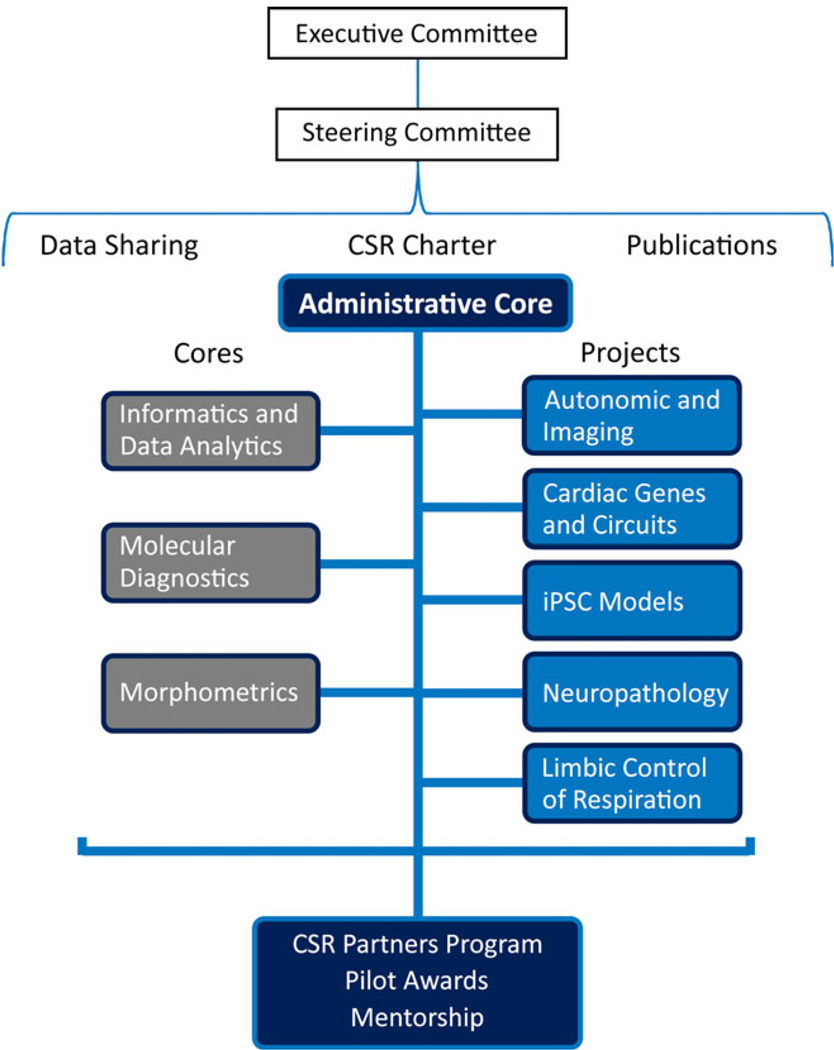
Figure 1.
Outline of NINDS Center for SUDEP Research.
Acknowledgments
The Center for SUDEP Research is supported by NINDS grants U01-NS090407, U01-NS090408, U01-NS090415, U01-NS090364, U01-NS090340, U01-NS090406, U01-NS090362, U01-NS090414, and U01-NS090405.
Biographies

Sam Lhatoo MD is a Principal Investigator of the Center for SUDEP Research.

Jeffrey Noebels MDPhD, is a Principal Investigator of the Center for SUDEP Research.
Footnotes
CSR Collaborators
Matt Anderson, Harvard Medical School (Morphometrics Core).
Lisa Bateman, Columbia University Medical College (Autonomic and Imaging Biomarkers of SUDEP).
John Belmont, Baylor College of Medicine (Molecular Diagnostics Core).
Maura Boldrini, New York University (Neuropathology of SUDEP).
Orrin Devinsky, New York University (Neuropathology of SUDEP).
Beate Diehl, University College London (Autonomic and Imaging Biomarkers of SUDEP).
Daniel Friedman, New York University (Neuropathology of SUDEP and Autonomic and Imaging Biomarkers of SUDEP).
Brandy Fureman, National Institute of Neurological Disorders and Stroke, National Institutes of Health (Administrative Program Director).
Brian Gehlbach, University of Iowa Carver College of Medicine (Autonomic and Imaging Biomarkers of SUDEP).
Alica Goldman, Baylor College of Medicine (Morphometrics Core & Molecular Diagnostics Core).
Ronald Harper, University of California, Los Angeles (Autonomic and Imaging Biomarkers of SUDEP).
Lori Isom, University of Michigan, (iPSC and Mouse Neurocardiac Models).
Samden Lhatoo, Case Western Reserve University (CSR Co-Director; Autonomic and Imaging Biomarkers of SUDEP and Clinical Administrative Core).
Kenneth Loparo, Case Western Reserve University (Informatics Core, signal analytics for Autonomic group).
Susanne Mueller, University of California, San Francisco (Morphometric Core).
Maromi Nei, Thomas Jefferson University Hospital (Autonomic and Imaging Biomarkers of SUDEP).
Jeffrey Noebels, PhD, Baylor College of Medicine (CSR Co-Director; Cardiac and Gene Circuits and Basic Science Administrative Core).
Doug Nordli, Ann & Robert H. Lurie Children’s Hospital of Chicago (Autonomic and Imaging Biomarkers of SUDEP, Respiratory and Arousal Mechanisms).
Jack Parent, University of Michigan (iPSC and Mouse Neurocardiac Models).
George Richerson, University of Iowa (Respiratory and Arousal Mechanisms).
Stephan Schuele, Feinberg School of Medicine at Northwestern University (Autonomic and Imaging Biomarkers of SUDEP).
Chad Shaw, Baylor College of Medicine (Molecular Diagnostics Core).
Sanjay Sisodiya, University College London (Neuropathology of SUDEP and Molecular Diagnostics Core).
Maria Thom, University College London (Neuropathology of SUDEP).
Simon Warfield, Harvard Medical School (Morphometrics Core).
Vicky Whittemore, National Institute of Neurological Disorders and Stroke, National Institutes of Health (Scientific Program Director).
Guo Qiang Zhang, Case Western Reserve University (Informatics and Data Analytics Core) (Fig. 1).
Administrators
Anita Zaremba, Case Western Reserve University.
Holly Robinson, Baylor College of Medicine.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publications and affirm that this report is consistent with those guidelines.
References
- 1.Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55:1479–1485. doi: 10.1111/epi.12666. [DOI] [PubMed] [Google Scholar]
- 2.Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. 2010;363:2522–2529. doi: 10.1056/NEJMoa0911610. [DOI] [PubMed] [Google Scholar]
- 3.Scorza CA, Cavalheiro EA, Scorza FA. SUDEP research: challenges for the future. Epilepsy Behav. 2013;28:134–135. doi: 10.1016/j.yebeh.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Surges R, Thijs RD, Tan HL, et al. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol. 2009;5:492–504. doi: 10.1038/nrneurol.2009.118. [DOI] [PubMed] [Google Scholar]
- 5.So EL, Bainbridge J, Buchhalter JR, et al. Report of the American Epilepsy Society and the Epilepsy Foundation joint task force on sudden unexplained death in epilepsy. Epilepsia. 2009;50:917–922. doi: 10.1111/j.1528-1167.2008.01906.x. [DOI] [PubMed] [Google Scholar]
- 6.Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia. 2011;52(Suppl 1):28–38. doi: 10.1111/j.1528-1167.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesdorffer DC, Tomson T, Benn E, et al. Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52:1150–1159. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- 8.Liebenthal JA, Wu S, Rose S, et al. Association of prone position with sudden unexpected death in epilepsy. Neurology. 2015;84:703–709. doi: 10.1212/WNL.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 9.Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology. 2005;64:1131–1133. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- 10.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 11.Ficker DM, So EL, Shen WK, et al. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology. 1998;51:1270–1274. doi: 10.1212/wnl.51.5.1270. [DOI] [PubMed] [Google Scholar]
- 12.Hesdorffer DC, Tomson T, Benn E, et al. Do antiepileptic drugs or generalized tonic-clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia. 2012;53:249–252. doi: 10.1111/j.1528-1167.2011.03354.x. [DOI] [PubMed] [Google Scholar]
- 13.Hesdorffer DC, Tomson T. Adjunctive antiepileptic drug therapy and prevention of SUDEP. Lancet Neurol. 2011;10:948–949. doi: 10.1016/S1474-4422(11)70194-6. [DOI] [PubMed] [Google Scholar]
- 14.Poh MZ, Loddenkemper T, Reinsberger C, et al. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology. 2012;78:1868–1876. doi: 10.1212/WNL.0b013e318258f7f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyal M, Hardin KA, Bateman LM. Postictal generalized EEG suppression is linked to seizure-associated respiratory dysfunction but not postictal apnea. Epilepsia. 2012;53:825–831. doi: 10.1111/j.1528-1167.2012.03443.x. [DOI] [PubMed] [Google Scholar]
- 16.Freitas J, Kaur G, Fernandez GB, et al. Age-specific periictal electroclinical features of generalized tonic-clonic seizures and potential risk of sudden unexpected death in epilepsy (SUDEP) Epilepsy Behav. 2013;29:289–294. doi: 10.1016/j.yebeh.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lhatoo SD, Faulkner HJ, Dembny K, et al. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68:787–796. doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- 18.Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia. 2013;54:377–382. doi: 10.1111/j.1528-1167.2012.03691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.der van Lende M, Surges R, Sander JW, et al. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry. 2015 doi: 10.1136/jnnp-2015-310559. pii: jnnp-2015-310559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeppesen J, Fuglsang-Frederiksen A, Brugada R, et al. Heart rate variability analysis indicates preictal parasympathetic overdrive preceding seizure-induced cardiac dysrhythmias leading to sudden unexpected death in a patient with epilepsy. Epilepsia. 2014;55:e67–e71. doi: 10.1111/epi.12614. [DOI] [PubMed] [Google Scholar]
- 21.Dutsch M, Hilz MJ, Devinsky O. Impaired baroreflex function in temporal lobe epilepsy. J Neurol. 2006;253:1300–1308. doi: 10.1007/s00415-006-0210-3. [DOI] [PubMed] [Google Scholar]
- 22.Bozorgi A, Chung S, Kaffashi F, et al. Significant postictal hypotension: expanding the spectrum of seizure-induced autonomic dysregulation. Epilepsia. 2013;54:e127–e130. doi: 10.1111/epi.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moseley BD, Nickels K, Britton J, et al. How common is ictal hypoxemia and bradycardia in children with partial complex and generalized convulsive seizures? Epilepsia. 2010;51:1219–1224. doi: 10.1111/j.1528-1167.2009.02490.x. [DOI] [PubMed] [Google Scholar]
- 24.Mueller SG, Bateman LM, Laxer KD. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. Neuroimage Clin. 2014;5:208–216. doi: 10.1016/j.nicl.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wandschneider B, Koepp M, Scott C, et al. Structural imaging biomarkers of sudden unexpected death in epilepsy. Brain. 2015 Aug 10; doi: 10.1093/brain/awv233. pii: awv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci. 2015;35:10281–10289. doi: 10.1523/JNEUROSCI.0888-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang GQ, Cui L, Lhatoo S, et al. MEDCIS: multi-modality epilepsy data capture and integration system. AMIA Annu Symp Proc. 2014;2014:1248–1257. [PMC free article] [PubMed] [Google Scholar]
- 28.Thom M, Michalak Z, Wright G, et al. Audit of practice in SUDEP post mortems and neuropathological findings. Neuropathol Appl Neurobiol. 2015 doi: 10.1111/nan.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klassen T, Davis C, Goldman A, et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noebels J. Pathway-driven discovery of epilepsy genes. Nat Neurosci. 2015;18:344–350. doi: 10.1038/nn.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leu C, Balestrini S, Maher B, et al. Genome-wide polygenic burden of rare deleterious variants in sudden unexpected death in epilepsy. Ebiomedicine. 2015 doi: 10.1016/j.ebiom.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klassen TL, Bomben VC, Patel A, et al. High-resolution molecular genomic autopsy reveals complex sudden unexpevcted death in epilepsy risk profile. Epilepsia. 2013;55:e6–e12. doi: 10.1111/epi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massey CA, Sowers LP, Dlouhy BJ, et al. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014;10:271–282. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brust RD, Corcoran AE, Richerson GB, et al. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep. 2014;9:2152–2165. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchanan GF, Smith HR, MacAskill A, et al. 5-HT2A receptor activation is necessary for CO2-induced arousal. J Neurophysiol. 2015;114:233–243. doi: 10.1152/jn.00213.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchanan GF, Murray NM, Hajek MA, et al. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592:4395–4410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parent JM, Anderson SA. Reprogramming patient-derived cells to study the epilepsies. Nat Neurosci. 2015;18:360–366. doi: 10.1038/nn.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Lopez-Santiago LF, Yuan Y, et al. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann Neurol. 2013;74:128–139. doi: 10.1002/ana.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auerbach DS, Jones J, Clawson BC, et al. Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS ONE. 2013;8:e77843. doi: 10.1371/journal.pone.0077843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagnon JL, Korn MJ, Parent R, et al. Convulsive seizures and SUDEP in a mouse model of SCN8A epileptic encephalopathy. Hum Mol Genet. 2015;24:506–515. doi: 10.1093/hmg/ddu470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin X, O’Malley H, Chen C, et al. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J Physiol. 2015;593:1389–1407. doi: 10.1113/jphysiol.2014.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman AM, Glasscock E, Yoo J, et al. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1:2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glasscock E, Yoo JW, Chen TT, et al. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–5175. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi Y, Wang J, Bomben VC, et al. Hyper-SUMOylation of the Kv7 potassium channel diminishes the M-current leading to seizures and sudden death. Neuron. 2014;83:1159–1171. doi: 10.1016/j.neuron.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med. 2015;7:282ra246. doi: 10.1126/scitranslmed.aaa4050. [DOI] [PMC free article] [PubMed] [Google Scholar]