Abstract
There are both theoretical and empirical underpinnings that provide evidence that the musculoskeletal system develops, functions, and ages as a whole. Thus, the risk of osteoporotic fracture can be viewed as a function of loading conditions and the ability of the bone to withstand the load. Both bone loss (osteoporosis) and muscle wasting (sarcopenia) are the two sides of the same coin, an involution of the musculoskeletal system.
Skeletal loads are dominated by muscle action; both bone and muscle share environmental, endocrine and paracrine influences. Muscle also has an endocrine function by producing bioactive molecules, which can contribute to homeostatic regulation of both bone and muscle. It also becomes clear that bone and muscle share genetic determinants; therefore the consideration of pleiotropy is an important aspect in the study of the genetics of osteoporosis and sarcopenia.
The aim of this review is to provide an additional evidence for existence of the tight genetic co-regulation of muscles and bones, starting early in development and still evident in aging. Recently, important papers were published, including those dealing with the cellular mechanisms and anatomic substrate of bone mechanosensitivity. Further evidence has emerged suggesting that the relationship between skeletal muscle and bone parameters extends beyond the general paradigm of bone responses to mechanical loading.
We provide insights into several pathways and single genes, which apparently have a biologically plausible pleiotropic effect on both bones and muscles; the list is continuing to grow. Understanding the crosstalk between muscles and bones will translate into a conceptual framework aimed at studying the pleiotropic genetic relationships in the etiology of complex musculoskeletal disease. We believe that further progress in understanding the common genetic etiology of osteoporosis and sarcopenia will provide valuable insight into important biological underpinnings for both musculoskeletal conditions. This may translate into new approaches to reduce the burden of both conditions, which are prevalent in the elderly population.
Keywords: Pleiotropy, Heritability, Genetic polymorphisms, Gene–environment interaction, Osteoporosis, Sarcopenia
Introduction
The risk of osteoporotic fracture can be viewed as a function of the ability of bone to withstand the applied loads. Skeletal loading conditions are largely dominated by muscle action, more so at younger ages. Muscles and bones develop, act, and age as a whole. Since bone and muscle cells share the same mesenchymal precursor, it is thus reasonable to hypothesize that adult bone strength and muscle mass share genetic determinants, and these determinants may also influence skeletal development as well as aging.
The physical forces produced during daily activities induce tissue-level strains, which are the physical cues directing skeletal growth [74]. Functional adaptation to physical forces ensures that structures like long bones are sufficiently stiff to support loading demands and sufficiently strong to resist fractures [74]. An interplay of multiple genetic factors and environmental demands is thus expected to shape bones in vivo. It is well accepted that sex (or gender) can be viewed as a form of environment. The distinct sexual dimorphism observed for muscles and bones underlies several possible etiologies of musculoskeletal degenerative disease. First, there may be differences in the response of the musculoskeletal systems of men and women to the environment. Alternatively, this sexual dimorphism may be a sex-hormone-related phenomenon or there may be a genetic explanation. It seems that different loading demands on the male skeleton combined with differences in the intrinsic milieu between the sexes make male skeleton more resistant to age-related losses. With advanced age, there is a decline of muscle input; also, bones might undergo a decline in their responsiveness to mechanical loads from exercise [127], which probably corresponds to the aging-related changes in cellular and hormonal mechanisms of muscle actions. The question here is, do the age-related declines in bone and muscle mass have a common etiology or do the two processes occur independently in parallel? Our underlying premise is that, for the most part, these and other related questions can be answered by considering genetic factors.
The aim of this review is therefore to summarize evidence from experimental and epidemiological studies for the biological, and especially the genetic, mechanisms underlying an interaction between bones and muscles in adults. We recently described this new conceptual framework in our perspective paper [82], in which we first provided an overview of the importance of muscle mass and physical exercise’s effects on the skeleton; however, we raised the possibility that both muscle and bone might be controlled by other factors such as genetic determinants; and further, we provided indications that, instead of merely a causal relationship, genetic influences are shared between muscles and bone strength. We then offered some insights on the possible nature of shared genetic factors. In the last year, several major developments occurred, such as solid evidence of the paracrine regulation of bones and muscles [118] and notably, genome-wide association studies (GWAS) for bone density [137] and lean mass. Paradigm-shifting manuscripts were published [128], including those dealing with the cellular mechanisms and anatomical substrate of bone mechanosensitivity [20]. Also, more evidence emerged suggesting that the relationship between muscle strength and bone parameters extends beyond the general paradigm of bone responses to mechanical loading.
We believe that further progress in understanding genetic etiologies of osteoporosis and sarcopenia lies in continuous uncovering of the molecular mechanisms, preferably those shared among the musculoskeletal traits. Guided by contemporary tools, this knowledge will ultimately lead to approaches aimed at reducing the burdens of both conditions via improved diagnosis and early-targeted treatment.
Relationship between bone and muscle throughout the life span
Allometry and pleiotropy during development and growth
The bone and muscle relationship starts early on, with patterning of the somites in embryonic life. For recent and comprehensive reviews of embryonic sources of muscle and bone development as well as progenitor cells, see Refs. [127] and [114]. In brief, muscle and bone develop from somites and share a common mesenchymal precursor. Somewhat surprisingly, muscle and bone develop independently and form attachments with one another secondarily, but in a highly regulated fashion. Bone morphogenetic proteins are important, since they are required not only for skeletal patterning during embryonic development, but also for bone response to mechanical stimulation and ensuing remodeling at specific anatomic sites in the skeleton [69].
Muscle precursors migrate to the extremity bud during limb morphogenesis in intra-uterine development. Muscle precursors are multipotent and able to form both adipocytes and osteogenic cells in proper conditions. The concept of a universal biomechanical unit [49,51] was explored by Matsuoka et al. [114] who, in particular, identified genes involved in the cellular modularity of muscle and bone of the shoulder girdle during the embryonic stages.
As the musculoskeletal apparatus grows, allometric relationships become more apparent and critical. Allometry is usually defined as the form/size/shape covariance or the relationship between the growth and size of one body part to the growth and size of the whole organism. In regard to the musculoskeletal system, morphological traits like joint shapes and sizes, long bone diaphyseal morphology, muscle–bone interfaces (entheses) and bone-muscle lever orientations, covary significantly with gross morphological parameters (such as length of body segments) due to the need to maintain an integrated, functioning system in spite of inter-individual variation in overall form and shape [30]. In theory, the pattern of (co)variation should evolve to match fitness demands (e.g., see works of Cheverud’s group [26]), and alleles whose pleiotropic effects contribute to the attainment of appropriate proportions by interdependent parts will generally be favored by natural selection [108]. Models of allometric scaling of bone variables have been proposed [25,119,120]. The theory of allometry is consistent with the possibility that genetic influences on bone and muscle are exerted in a pleiotropic manner. In addition to genetic regulation, other factors may exert effects on multiple traits during development, such as a hormonal signal (e.g., growth hormone or sex hormones) that regulates cellular activity [74].
Allometric shifts do not occur rapidly in an evolutionary and ontological sense; this is a long and intricate process. Although bone has often been considered as a dynamic responsive structure under a given loading condition, it cannot immediately attain an optimum state under a varying load, because of the slow speed of adaptation [11]. Recent empiric and theoretical work suggests that there is no single optimal bone architecture; instead many different architectural solutions produce adequate bone strength [123,165]. Some believe that, in evolutionary terms, most of bone-related traits were never selected – either for or against – and they are merely a mechanical consequence of a peculiar locomotion of humans [99].
Bones and muscles in adult life
In adult life, mineral mass and muscle mass content of the human body may also be functionally associated. For example, the area and radiographic density of the trunk musculature have been associated with both lower back pain and level of physical function in elderly individuals [66,67]. Bioimpedance (BIA) or DXA-measured lean (non-adipose soft tissue) mass also serves as indirect indicators of mechanical loading upon bone [132]. Higher lean mass is associated with greater muscle strength and better functioning [21,70,180]; leg lean muscle mass measured by DXA has been shown to be associated with mobility disability [178–180].
Studies of both humans (adults and children) and laboratory animals have documented a strong, positive correlation between muscle strength and bone mass [54]. Thus, muscle atrophy is concomitant with the observed bone loss [75]. There is a “mechanical” explanation for this phenomenon. Mechanical loads activate new bone formation on cortical and trabecular surfaces; strain can activate bone cells, which then respond with gene activation, increased metabolism, growth factor production and matrix synthesis [46,49]. Mechanical stimulation of bone cells may induce elevated levels of insulin-like growth factor I (IGF1), which stimulates the differentiation of osteoblasts into osteocytes [148]; osteocytes, in turn, are necessary to maintain bone mass in response to normal load [20].
In addition, the lumbar spine ex vivo, without assistance from muscles, buckles under compressive loads thousand times lower than those that in vivo spine withstands during daily tasks [29,116]. The ability of the in vivo spine to tolerate such high loads is mainly attributable to the dynamic stabilizing capacity of the trunk musculature [66,156]. In addition, the abdominal muscles by their attachment to the dorsolumbar fascia can not only flex and extend the lumbar spine, but also eliminate shear stress on the lumbar intervertebral segments. Similarly, it has been suggested that muscle-induced stiffness (of the abdominal muscles and iliopsoas) reduced the shear levels in the sacroiliac joint in preparation for load transfer from the spine to the legs [176]. Numerous muscles interact to create the mechanical environment of the bones, calling for an integrated approach to studying this complex structure–function relationship [108].
Environmental signals triggering bone response, conductors and recipients of those signals
When considering the genetic “determinants” of a functionally unified muscle–bone system [114], one would still expect there to be an interaction between genetic susceptibility and environmental factors [91]. Even if there is an obvious environmental trigger, there should be a molecular substrate in bone responsible for recording and responding to the action of muscles; this susceptibility to outside triggers should be genetically determined [30,183].
The mechanostat theory postulates that bone strength adapts primarily to muscle forces [49]. It has been a long-held belief that bone cross-sectional properties mostly reflect shape adaptations to only the most vigorous forms of loading rather than to typical habitual activities [127]. It has been also hypothesized that extremely small strains, if induced at a sufficiently high frequency, are strong determinants of bone intrinsic morphology [141]. Thus, evidence suggests that bone tissue depends as much on the persistent, low-magnitude strains that arise through dominant activities such as standing as it does on the relatively large, but rare strain events of intense (strenuous muscular) activity [2]. From this perspective, the bone loss that accompanies aging or period of prolonged bed rest, may result from both diminished numbers of low strain and high strain magnitude cycles, due to the muscle wasting (sarcopenia, or progressive atrophy of muscle mass) which parallels these conditions [36].
An exciting experiment by Xie et al. [190] emphasized effects of extremely low-magnitude, high-frequency mechanical stimuli on the quality of the adolescent musculoskeletal system in juvenile mice. Following 6 weeks of the whole-body vibration, their periosteal bone area, bone marrow area, cortical bone area, and the moments of inertia of the tibial metaphysis were all significantly greater than in controls (up to 29%, p<0.05); the mice that received the vibratory mechanical stimulus also had 14% greater trabecular bone volume (p<0.05) than controls. Very importantly, the soleus muscle also expressed gains, with total type I and type II fiber cross-sectional area as much as 29% greater in vibrated mice (p<0.05) [190]. This experiment thus confirmed that, first, the mechanosensitive elements of the musculoskeletal system are not necessarily dependent on strenuous, long-term activity to initiate response in young age, and secondly, that whole-body vibration provides a “pleiotropic” stimulus to both bone and muscle. It is important to identify a substrate for the responses of the various muscle receptors to vibration, which may include the receptors of stretch (muscle spindle primary ending), length (muscle spindle secondary endings), and tension (Golgi tendon organs) receptors [40].
In the absence of loading, bone is lost, while in the presence of loading, bone is either maintained or accrued. The skeleton is unique in its ability to adaptively remodel in response to its perception of mechanical loading or lack of loading or disuse [20] to prevent an underdesigned (or over-designed) structure [74]. How this external loading signal in bone is transmitted at the cellular level is not well established; the bone matrix is undergoing tissue deformation, but to what extent and how is not clear [20].
Osteocytes, the most abundant cells in bone, have been implicated as the link between external loading and the skeletal re-organization, since they are necessary to maintain bone mass in response to both normal load and extreme force (reviewed in [20]). In brief, osteocytes are embedded in mineralized matrix; they are distributed throughout the bone tissue volume, communicating with each other via gap junctions. They also communicate with osteoblasts on the bone surface through their dendritic processes within the tunnels called canaliculi, thereby forming a neuron-like network throughout the skeleton. Osteocytes have recently been shown to be the major bone cell population responsible for mechanotransduction [20]. It is still poorly understood how osteocytes translate the mechanical stimulus (tension or loading) information into the biochemical signals to direct bone formation by osteoblasts [104].
There are few studies that have examined whether discrete, localized loads, such as those created by muscle contractions on attachment sites, have local osteogenic effects. In one study, tensile forces induced the production of IGF1 and IGF1-receptors and proliferation of osteoblast-like cells at the site of load application [68]. The anatomical substrate for these actions of muscles on bones is not entirely clear. On one hand, tendons determine the translation of muscle force to the bone via the periosteum [32]. It is believed that muscle growth produces stretching of collagen fibers and periosteum at the muscle/bone interface, which then stimulates local periosteal growth [65]. On the other hand, specifically in entheses, there is a gradual transition between tissue types with distinctly different elastic moduli, which is thought to enhance the ability of tendons to dissipate force evenly during muscle contraction, thus resisting shear stresses at the bone surface [16,197]. We might hypothesize that recent molecular discoveries [118,193] as well as novel molecular pathways discovered by large-scale genetic analyses, will help to better identify the receptors and response elements.
Sex as environment for bone and muscle phenotypes: Role of sex hormones
Sexual dimorphism is marked in both skeletal development, adult maintenance, and aging and is not limited to skeletal dimensions, cortical thickness, and BMD. For example, in the Framingham cohorts, adult men have longer femora, with more obtuse neck shaft angles, longer and wider femoral necks, in addition to higher BMD [194]. The differences between the sexes in the structural components of bone strength seem to be partly due to their particular biomechanical responses, which might depend on differences between the hormonal mileau of men and women [80].
Hence, gender-specific predisposition to osteoporosis and fracture risk may lie in genes determining both the structural and mineral constituents of bone strength (and fragility). Despite pronounced sexual dimorphism in mass, structure and shape, there is no strictly sex-limited phenotype to be defined at the tissue level, i.e. distinctive features of the male and female skeleton result from quantitative and qualitative variations of bone modeling and remodeling, not from completely different mechanisms of regulation. Estrogen has also been suggested to sensitize the mechanosensory system to loading (reviewed in [153]). Bones of men respond to estrogen as well as to testosterone [90]. Replacement of testosterone in hypogonadal elderly men has successfully increased both muscle mass and strength [42]. The actions of androgens on muscles are well known in body-builders of each sex. Currently not much is known about the role endogenous estrogens, and administration of estrogen may play in the management of sarcopenia; it seems that the muscle effect of estrogens is not significant compared to that of androgens. Thus, estrogen replacement therapy (ERT) did not prevent loss of muscle composition and strength with aging [88,160]. On the other hand, the skeletal actions of androgens may result from direct activation of the androgen receptor (AR), or may alternatively depend on stimulation of the estrogen receptors (ESRs) following aromatization of androgens into estrogens that occurs in peripheral tissues, catalyzed by the enzyme, aromatase. Uniquely for women, though, the rise in estrogen levels at puberty leads to the deposition of additional cortical bone mass to satisfy the anticipated physiological needs of the subsequent reproductive period [94], such as in pregnancy and lactation [153].
Notably, differences in response to estrogen and testosterone have been shown for male and female chondrocytes, osteoblasts, myoblasts, and other cells [31,164]. Corsi et al. [31] recently found that, when stimulated with bone morphogenetic protein 4 (BMP4), skeletal muscle-derived stem cells (MDSCs) from male mice had a larger increase in osteogenic gene expression and a higher alkaline phosphatase activity than cells derived from female mice. These results suggest that male MDSCs have a significantly greater osteoprogenitor potential, which may serve a basis for enhanced bone regeneration, which is characteristic of males [31].
Aging of bone and muscle: Osteoporosis and sarcopenia
The process of involution of the musculoskeletal system seems to be a general phenomenon and an example of tissue atrophy with age [81]. Bones undergo a decline with age in their responsiveness to mechanical loads from exercise [127], a decline that parallels – and probably corresponds to – the decrease in muscle input that arises from energy deficiency and fiber loss. After the skeleton has reached maturity, bone remodeling is responsible for the complete replacement of old bone tissue with new tissue [112]. Bone’s ability to regenerate (remodel itself, even in absence of trauma) is characteristic of that tissue [20]. With advanced age and sex steroid deficiency, the placement and quality of new bone are altered, causing a derangement in the normal process of bone regeneration [112]. This alteration is caused by the change in areas of remodeling due to disease, accumulated micro-trauma, and by variations in muscle strength and direction of applied force. Catabolic illness, such as surgery, injury, palsy, and immobilization (due to bed rest [135] or space flight [22]), may contribute to rapid bone degeneration.
Similar to bone, muscle tissue deteriorates with the age. Age-associated loss of muscle fibers, fatty degeneration, and decreased number of functioning motor units bring about decline in muscle quality (i.e. force generated per unit of muscle mass) [100]. The weight of skeletal muscle comprises ~45% of body weight at age 21–30 but decreases to ~27% at age 70+years [161]. Sarcopenia (muscle wasting) is manifested by decreases in muscle strength and muscle mass with age, mainly as a result of the alterations in muscle morphology. This is due mostly to a decreased proportion and cross-sectional area of type II (fast-twitch) fibers; however, single fiber analysis suggests that the contractile proteins also become less effective with age [48].
A leading contemporary theory suggests that both decreasing muscle activity and muscle mass are the main causes of age-related bone loss [77]. Aging is typically accompanied by declines in physical activity; some relate it to the afore-mentioned energy deficiency in older persons. In addition, it is well known that adult bone is less sensitive to exercise-induced changes in peak muscle strain than younger, growing bone [166]. It is believed that exercise cannot add significant new bone mass in adults. Rather, the benefit of physical activity with regard to the adult skeleton lies mostly in conservation [47].
Genetics of bone and muscle mass: Pleiotropy?
Pleiotropy: what it means and what it does not
There are several potential mechanisms underlying associations between genetic polymorphisms and the musculoskeletal apparatus, including the mediation of environmental influences by genetic factors. The spectrum of pleiotropic effects of a gene on a morphological trait may include both “direct” and “indirect” effects (via some intermediate mechanism), with a possible continuum in-between [87]. There are at least two possible scenarios of gene actions (see scheme in Fig. 1), which include: (A) pleiotropic effects of gene polymorphisms on two traits, bone and muscle, (B) a conditional model (confounding), in which a gene is associated with one trait, and that trait in turn influences another trait. For example, considering bone and muscle size as traits of interest, genes might regulate how bone responds to actions of a large muscle (or, less plausible, how large a muscle should be to operate a larger bone). Testing Scenario (B) vs. Scenario (A) provides statistical evidence that the effect of the gene is “really” pleiotropic and not an indirect effect. In support of Scenario (A) (a “true” pleiotropy), one study reported that adjustment for lean mass increased the heritability estimate for femoral neck BMD in parent–adult child pairs [18].
Fig. 1.
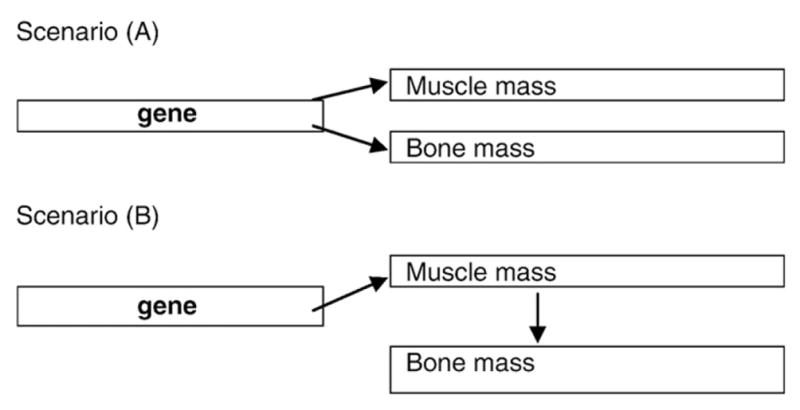
Diagram of possible action of a gene shared between muscle and bone.
It may be a single gene that can trigger changes in bone anatomy and as a result, affect muscle architecture. However, it is more plausible that alterations in certain systemic control factors active during development will have profound downstream consequences, e.g., small modulations of the growth hormone (GH) axis can generate fully coordinated morphological change [108]. There are a plethora of indications that GH and related factors (such as insulin-like growth factor I) may exert both direct anabolic effects on bones, as well as indirect effects, via influences on muscle. Furthermore, the rate and/ or duration of mitosis in the proliferative zones of femoral growth plates [186] or delayed mineralization of growth plate cartilage, may lead to elongation of the femoral neck with reduction of the neck shaft angle and height of the greater trochanter; these changes in bone geometry involve functional adaptations of soft tissues to maintain fitness in walking [108].
In summary, DNA variants having direct effects on bone may represent genes that alter the responsiveness of bone to its external loading demands. In contrast, SNPs that influence bone indirectly may represent genes that do not alter the responsiveness of bone but rather alter the stimulus imposed on the system [74] such as muscle strength. In any case, genetic strategies hold a promise to both propose (by statistical means) and validate (via introducing the perturbations) possible mechanisms.
Evidence for shared genetics between bone and muscle mass
Given that muscle cells and osteoblasts derive from a common mesenchymal precursor and that muscle and bone are directly connected to each other, beginning with the previously mentioned somite patterning, it is plausible that there would be potential genes determining characteristics of both traits. High genetic correlations ranging from 0.28 to 0.69, have been reported between femoral geometric parameters and total body lean mass in adults from Nebraska [158]. The bivariate genome linkage analysis in that same sample produced two chromosomal regions, 5q35 and 10q24, with pleiotropic effects on these phenotypes [34]. Our results in the Framingham Osteoporosis Study demonstrated similar bivariate genetic correlations between leg lean mass and cross-sectional femoral geometry; bivariate linkage analysis identified significant quantitative trait loci (QTL) shared by leg lean mass with shaft CSA on chr. 12p12–12p13 and with neck shaft angle, on 14q21–22 [85]. Most recently, in 102 monozygotic and 113 dizygotic older female twin pairs, muscle CSA of the lower leg, bending strength of the tibial shaft, and compressive strength of the distal tibia from pQCT scans, were shown to share genetic components [117].
Animal models confirm the above human observations: thus, inactivating mutations of the myostatin (growth differentiation factor 8, GDF8) gene induce a hypermuscular phenotype in mammals. pQCT data show that myostatin-knockout mice have significantly greater cortical bone mineral content at the L5 vertebra [60], as well as a larger size of the spinous processes and greater expansion of entheses on both the femur and humerus, than normal mice [59]. QTLs have been identified for traits related to both bony carcass and meat quality in Scottish sheep [79]; these traits were found to share regulatory genes also in beef cattle [147] and pigs [113]. Further, Structural Equation Modeling (SEM) was used by Lang et al. [96], to explore the extent to which select genetic loci manifest their pleiotropic effects through functional adaptations to biomechanic stimuli. QTL analysis was used to identify regions of chromosomes that simultaneously influenced skeletal mechanics (as tested to failure in three-point bending), muscle mass, and/or activity-related behaviors in young and aged mice of both sexes (B6XD2 intercross F2). Correlations among bone strength, muscle mass, and physical activity suggested that these traits may be modulated by common genetic and/or environmental mechanisms. The study pointed out QTLs at several mouse chromosomes that were responsible for mechanosensitivity. In a similarly interesting study, Kesavan et al. [89] applied bending loads to the tibiae of 10-week old female F2 mice derived from another intercross, B6XC3H. This expression study provided evidence for the presence of multiple genetic loci regulating bone anabolic response to loading in mice. Whether these genes have pleiotropic actions on muscles too, needs to be shown. Future studies should cross-validate these QTLs among different mouse strains and experiments and compare them with results from humans.
Implications for the genetic studies of osteoporosis: A need to consider pleiotropy
The most important clinical outcome of any anti-osteoporosis intervention is a reduction in the risk of fractures. Risk of fracture is also a function of muscle weakness, therefore a more comprehensive fracture risk assessment should account for the musculoskeletal unit as a whole [111]. It is also important to remember that the genetic contribution to a risk factor may differ from the genetic contribution to the ultimate disease phenotype. For example, DXA-derived aBMD, which has been commonly used as an endophenotype for fractures due to osteoporosis, was found to share only modest genetic variance with osteoporotic fractures [8,35,171,177]. Therefore, neither aBMD alone nor bone geometry, muscle mass or strength, can individually serve as perfect surrogates of the skeleton’s ability to withstand the forces that produce fracture.
How can a genetic study help to overcome these deficiencies? We have argued [82] that there are several benefits of exploring pleiotropy. First, the “post-genomic” era provides the opportunity to utilize a technically advanced hypothesis-free selection method to identify the major genetic variants (using GWAS, expression tests etc.). Second, the computational tools, exhaustive databases and advanced database querying methods support the conduct of multivariate analyses. Finally, newly proposed analytic approaches, such as “reverse phenotyping” [62,150], which uses genetic marker data to drive new phenotype definitions, would provide an opportunity to theoretically increase power by performing joint analyses of genetically correlated traits [7,63,185], as well as decrease the problem of multiple testing [126]. With the advent of genome-wide association scans [34], expression experiments [55], and bioinformatic tools [52], new candidate genes may emerge for further pursuit.
Candidate genetic mechanisms exerting effect on bones and muscles
Numerous candidate genes have been proposed as contributors to osteoporosis risk based on bone biology [133,136,170]. The candidate gene approach needs a strong prior hypothesis and has proven viable when there is a strong biological basis for considering a gene as a plausible candidate. Exploring the roles of candidate genes belonging to the signaling pathways/gene regulation network with biological relevance to both bones and muscles is also a promising strategy. The majority of candidate genes are traditionally suggested by basic studies in cell cultures and animal models.
We and others [82,181] postulated that possible pathways through which genes may influence bone mass and geometry should include genes for responsiveness of bone to applied mechanical loading (i.e., set points for mechanosensitivity, in a “mechanostat”-like mechanism [49,181]). To adapt bone structure to the strains derived from customary mechanical usage in each region of the skeleton, sex hormones or related factors could affect the threshold of the feedback system that controls bone remodeling [45,50,153]. However, there are examples of “serendipitous” discovery of potentially pleiotropic genes: thus, human chromosomal region 3p14–25 has been shown to be linked to BMD in multiple cohorts [71]. One gene in the region, the filamin B, is known for being involved in several rare human skeletal disorders, such as atelosteogenesis, spondylo-carpo-tarsal syndrome, and Larsen’s syndrome. Two recent independent studies explored FLNB as a positional candidate and confirmed its association with BMD in women, Caucasian [187] and Southern Chinese [103]. This gene is involved in actin binding and cytoskeleton functions and is active in skeletal muscle development.
In Table 1, we provide several pathways and single genes, which apparently have biologically plausible pleiotropic effects on both bones and muscles. Only those genes for which more than a single reference was available are listed in Table 1. Of note, this is not a comprehensive review of the literature nor a formal search of the relevant databases; these genes were identified by perusal of recent papers in PubMed for the above concepts.
Table 1.
Candidate genes and pathwaysa for pleiotropic action on bone and muscle.
| Gene (abbrev.) | Gene title | Action | Reference |
|---|---|---|---|
| Sex hormones | |||
| AR | Androgen receptor | Decreased AR activity results in a loss of bone mass | [17] |
| CAGn repeats associated with fat-free mass in men | [182] | ||
| ESR1 | Estrogen receptor 1 | In a meta-analysis, XbaI polymorphism associated with BMD and fracture risk in women | [72] |
| Esr1 knock-out mice unable to respond to physical exercise with a periosteal bone expansion compared to wildtype mice | [101] | ||
| PvuII polymorphism may modulate the effect of exercise on BMD | [159] | ||
| Note: no relationship between TA-repeat polymorphism and muscle mass and strength in young adult women | [58] | ||
| Growth hormone/insulin-like growth factors | |||
| IGF1 | Insulin-like growth factor I | CA-repeat promoter polymorphism has effects on femoral bone geometric parameters | [136] |
| CA-repeat polymorphism was associated with increased bone strength and muscle volume and strength | [95] | ||
| Alternative splicing was involved in the mechanotransduction of bone cells | [56,162] | ||
| Transforming growth factor-β superfamily | |||
| GDF8 | Myostatin | Myostatin-null mice had significantly greater cortical bone mineral content and larger entheses than normal mice | [59,60] |
| mRNA levels were reduced in response to heavy-resistance strength training in older adults | [138] | ||
| rs2293284 and rs7570532 were associated with hip peak BMD variation in Chinese women | [195] | ||
| Vitamin D signaling | |||
| VDR | Vitamin D receptor | In a meta-analysis, Cdx-2 polymorphism was associated with risk for vertebral fractures in women | [167] |
| BsmI polymorphism was associated with decreased vertebral area and femoral narrow neck width | [41] | ||
| BsmI polymorphism was associated with muscle strength | [57,188] | ||
| FokI polymorphism was associated with fat-free mass and sarcopenia in older men | [140] | ||
| Interactions between leisure physical activity and VDR BsmI genotype on the lumbar spine BMD in active post-menopausal women | [19] | ||
| Association between VDR polymorphisms and falls, balance and muscle strength | [14] | ||
| Inflammatory cytokines | |||
| IL6 | Interleukin 6 | −174 GC polymorphism was associated with increased risk of wrist fracture in post-menopausal women | [122] |
| −174 GC was associated with hip BMD in post-menopausal women | [44] | ||
| −174 GC was associated with fat-free mass in men but not women | [139] | ||
| Exercise increases IL-6 receptor production in human skeletal muscle | [86] | ||
| Other pathways | |||
| BMP2 | Bone morphogenetic protein-2 | Young males with the rs15705 C/C genotype were associated with an increased gain in skeletal muscle volume (p=0.0060) following resistance training | [37] |
| BMP2 was linked and associated with BMD at different skeletal sites | [157,192] | ||
| PPARγ | Peroxisome proliferator-activated receptor-gamma | Polymorphisms in the PPARγ were associated with aBMD in both mice and humans | [1] |
| Mutations in PPARγ result in increased fatty acid flux to the skeletal muscle | [145] | ||
| GCR | Glucocorticoid receptor | Contributed both to bone and lean mass in older persons, muscle strength in younger males | [173,174] |
| ER22/23EK polymorphism was associated with lower trochanteric BMD in elderly women | [175] | ||
| PTN | Pleiotrophin | Over-expression affects mouse long bone development, fracture healing and bone repair | [102] |
| Potential mediator of mechanotransduction signaling in regulating periosteal bone formation and resorption in mouse | [191] | ||
| Expression levels lowered in response to spaceflight | [121] | ||
| NOTCH1 | Notch homolog 1, translocation-associated | NOTCH1 inhibits bone resorption, both directly on osteoclast precursors and indirectly via osteoblast lineage cells | [12] |
| Significantly lower expression found in muscle biopsies from older men compared to muscle from younger men | [24] | ||
| RETN | Resistin | Serum levels showed a significant negative correlation with lumbar spine BMD in middle-aged men | [124] |
| Polymorphisms associated with muscle and bone phenotypes in men and women | [129] | ||
| SRY-17 | Transcription factor SRY (sex determining region Y)-box 17 | Involved in endochondral bone growth | [3] |
| Downregulated in older men’s muscles (as a part of the “sarcopenia signature”) | [55] | ||
| SOX6 | (sex determining region Y)-box 6 | Associated with BMD in GWAS studies | [137,105] |
| Expressed in a wide variety of tissues, most abundantly in skeletal muscle | [154] | ||
| NF-kB | Nuclear factor of kappaB | NF-kB proteins implicated in muscle wasting (short-term hindlimb unloading in rodents) | [76] |
| Activation of NF-kB can induce muscle atrophy in transgenic mice | [23] | ||
| LMNA | Lamin A/C | Mutations cause primary laminopathies, including skeletal muscular dystrophies | [73] |
| Lamin A/C knockdown had a negative impact on osteoblastogenesis and bone formation in vitro | [5] | ||
Pathways may overlap (the same gene may belong to more than one pathway).
“Classic” candidate genes: Sex hormones
Most hormones affect the remodeling of the skeleton via a direct osteogenic effect, although there may also be indirect effects through hormonal influence on muscle. Sex hormones are obvious candidates for regulation of both bones and muscles since there is pronounced sexual dimorphism in bone size, shape, mass and muscle mass/strength (detailed above). Sex steroids, androgens and estrogens, are believed to modulate the function of the muscle–bone unit. Their receptors are essential for normal skeletal growth and bone mineral acquisition [53] as well as being important regulators of recovery from disuse atrophy. Skeletal muscle myoblasts, myotubes, and mature fibers also express functional androgen and estrogen receptors [115]. Polymorphisms in the AR [64], two estrogen receptors, ESR1 and ESR2 [58,151,152], as well as in the aromatase gene CYP19 [53,84] have been extensively studied. Thus, in a recent GWAS of 19,195 adults from five Caucasian populations, SNPs in ESR1 were confirmed associated with FN and LS BMD [134].
Another candidate that has been studied with regard to bone and muscle effects is catechol-O-methyltransferase (COMT), an estrogen-degrading enzyme, involved also in the degradation of catecholamines. The COMT Val158Met polymorphism was associated with peak BMD in young men [106] and most recently, an interaction of COMT with physical activity on BMD was found in the same young men [107]. Girls with COMTLL compared to COMTHH genotype had more lean mass as measured by DXA, and an increased muscle area in the tibia as measured with pQCT [39].
Insulin-like growth factor I (IGF1)
Growth hormone (GH) and/or its related factors are active during musculoskeletal development and repair. Several reports [83,97] have documented a decrement in IGF1 plasma levels with age in humans. Growth hormone activates IGF1 gene transcription in vivo; IGF1 is an autocrine/paracrine growth factor, which may mediate the response of bone to mechanical loading via the IGF-1 receptor [78]. Mechanical stimulation of bone cells may induce elevated levels of IGF1, which in turn prompts the differentiation of osteocytes from osteoblasts and thus promotes bone formation [68]. IGF1 induces muscle hypertrophy by activating the IGF1 receptor, which then triggers multiple signaling pathways, including the PI3K and MAPK pathways. Inhibition of IGF-I production together with consistently high levels of inflammatory cytokines (such as IL-6) in old age is presumed to contribute to sarcopenia. The IGF2 gene also has been associated with total body fat-free mass in humans [149] and muscle growth in pigs [169]. The IGFs are thus believed to generate morphological change in bones in a fully coordinated manner with the skeletal muscle.
Vitamin D receptor (VDR)
The VDR gene has long been targeted as one of the genetic determinants influencing bone status because it is involved in bone homeostasis as a part of the vitamin D endocrine system. There are a few studies that also have examined the associations between VDR and muscle strength. Endo et al. [38] found that the muscle fibers of the VDR-null mice were smaller and had persistently elevated expression of early markers of myogenic differentiation, which are normally down-regulated in controls.
A number of single nucleotide polymorphisms within this wellknown and widely studied gene for osteoporosis have been studied, including the BsmI, ApaI and TaqI polymorphisms located in the 3′end of the gene [167] or Cdx-2 transcription factor-binding site which is located within the promoter region [9]. Table 1 provides indications of associations of VDR polymorphisms with bone geometry [41], fractures [167], muscle mass or strength [57,188], and most recently, falls and balance [14]. Interestingly, VDR showed no statistically significant association with BMD after adjustment for multiple testing in a recent meta-analysis of bone density traits from the GEFOS consortium [134].
Wnt/beta-catenin
Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5), a Wnt coreceptor, have been associated with BMD in men and women [43,170,172]. In mice, Akhter et al. [4] hypothesized that the denser and stiffer bones in G171V mice may represent greater sensitivity to normal mechanical stimuli resulting in an over-adaptation of the skeleton to weight-related forces. More recent studies confirmed this assertion [146]. In our study [92], genetic variation in polymorphisms of the LRP5 gene was found to modulate the relationship between physical activity and BMD in men, thus suggesting that LRP5 may play a role in the adaptation of bone to mechanical load in humans as well.
Both bone and muscle are signaled via the Wnt pathway [114]. Wnt signaling has been implicated in overload-induced skeletal muscle hypertrophy in mice [10]. Wnt/beta-catenin signaling regulates cell differentiation during embryonic and postnatal development; in particular, it inhibits adipogenic and enhances chondrogenic differentiation of pericytes [93]. Lanyon et al. [98] suggested that ESR1may play a role in shuttling β-catenin into the nucleus in response to mechanical strain in osteoblasts. This may in part explain how estrogen regulates bone mass [20]. β-catenin accumulates in the cytoplasm and then translocates into the nucleus to affect gene transcription by a mechanism that is not well understood (reviewed extensively in [20]).
Sclerostin, encoded by the SOST gene, was shown to bind to LRP5 and LRP6 and inhibit Wnt/β-catenin signaling in vitro. Sost(−/−) mice were resistant to mechanical unloading-induced bone loss [104]. These findings together suggest that Wnt signaling and specifically LRP5 and SOST may be involved in muscle/bone crosstalk.
Biological candidates: Response to load/mechanical stress
Bone is an exquisitely mechanosensitive organ, and bone homeostasis depends on ability of its cells to sense and respond to mechanical stimuli [110]. It is still poorly understood how, on sensing a mechanical stimulus, osteocytes translate the loading information into the biochemical signals to direct bone formation in osteoblasts [104]. Multiple chemicals transduce the mechanical signal. Osteocytes possess primary cilia that project from the cell surface and deflect during fluid flow. These primary cilia are required for osteogenic and bone resorptive responses to dynamic fluid flow [110]. Disruption of the ciliary protein polycystin 1, a mechanosensing protein, impairs the bone response to mechanical loading [110]. Also of interest is the observation that osteocytes express large amounts of Connexin 43, which is the component of gap junctions and a target of Wnt/β-catenin signaling [168]. Similarly, gap junctional communication is a feature of tendons, however, there is no data on whether tendons are directly communicate with osteocytes [15].
One of the “big” questions in mechanotransduction is the molecular nature of the receptor(s) for mechanical force (candidate mechanoreceptors). A number of important molecules were shown in vitro and in vivo to be modulated in osteocytes in response to increased mechanical stress, including ATP, nitric oxide (NO), prostaglandin E2 (PGE-2), IGF-1, glutamate transporter, c-fos, ion channel, and intracellular calcium, but few of these responses have been shown to be a prerequisite for loading adaptation [104]. Integrins have been implicated as mechanoreceptors in a wide range of cells including myocytes, fibroblasts, endothelial cells, chondrocytes, and bone cells (reviewed in [143]).
Integrins play an important, albeit not well defined, role in facilitating signaling by IGFs and by other growth factors. Signaling via integrin receptors is a prominent aspect of the afferent response of bones to loading; their expression is reduced during skeletal unloading. The interaction between certain integrins and the IGF receptor are of especial importance with respect to the ability of bone to respond to mechanical load: disruption of this interaction blocks IGF signaling and results in bone loss. The mechanical stimulation by osteocyte stretching, which prevents osteocyte apoptosis [130], is transmitted by integrins. Fluid flow has been shown to activate MAPK via β1 integrin [143].
Vasculature also seems to play a part in the muscle/bone interface. Since blood flow to the limb is proportional to lean (muscle) mass [61], one may expect that Haversian perfusion will also benefit from well-developed musculature. Muscle contractions might be responsible for basal transport of nutrients to the osteocytes [20], probably by intensifying local circulation. Nitric oxide is produced in response to mechanical loading; it seems to promote the osteogenic response to loading. Whether the nitric oxide derives primarily from bone or the vasculature in bone is not yet clear [143]. Bone NO derives primarily from the endothelial isoform of nitric oxide synthase (eNOS, coded by NOS3), with the highest eNOS expression occurring in osteocytes. The strained bone cell downregulates its expression of RANKL, and upregulates expression of eNOS [142]. A reduction in a number of NOS3-expressing osteocytes, coupled to an increase in their remoteness from canal surfaces (and from blood supply), may thus possibly contribute to the fragility of osteoporotic bone [109]. Of note, in two recent studies, NOS3 genetic variation did not appear to be a major contributor to adult bone density/ultrasound or geometry in older men and women [28,163].
Biological candidates: Muscle as endocrine organ
In some respects, muscle may serve as an endocrine organ, which can contribute to homeostatic regulation of both bone and muscle. Indeed, skeletal muscle produces bioactive molecules, including interleukin (IL)-6, IL-15, insulin-like growth factor-binding protein-5 (IGFBP5), and insulin-like growth factor-1 (IGF-1) [193]. Notable, the mRNA level of IGFBP5, which modulates the activity of IGF-1, is decreased in skeletal muscle during fasting [193]. Actions of interleukin 6 in bone and muscle loss also become apparent [155]. IL-6 is a cytokine that has been shown to be produced at high levels by human skeletal muscle (therefore called “myokine”) following endurance type exercise [128]. In vitro studies demonstrated that IL-6 inhibits the secretion of IGF-1, thus the negative effect of IL-6 on bone tissue might be mediated through IGF-1 [13].
A Japanese group [193] recently identified a novel skeletal muscle-derived secretory factor named musclin; its protein sequence is identical to that of osteocrin (Ostn) cloned from developing bones [118]. Ostn was also detected in tendons and ligaments, making it a viable candidate for both an anatomical and a physiological interface between muscles and bones. Ostn appears to modulate osteoblastic differentiation in the growth plate of chondrocytes. Thus, the Ostn-transgenic animals displayed elongated long bones [118]. It could also function as an autocrine and paracrine factor linked to glucose metabolism in skeletal muscle. Indeed, musclin expression is tightly regulated by nutritional status and its role could be linked to glucose metabolism/glycolytic activity of type IIB fibers [118]. Thus, musclin gene expression was markedly decreased by fasting, restored by refeeding, and upregulated by insulin [193]. There is a possibility of Ostn/Musclin acting as a “myokine” with actions on distal tissues such as liver and fat to regulate whole-body homeostasis [118].
Candidate genes: De-novo discovery
Genome-wide association studies
Unprecedented potential for the discovery of potentially pleiotropic associations is made possible by GWAS. For example, in GWAS for adult height, Sanna et al. [144] identified an osteoarthritis-associated locus GDF5-UQCC. The GDF5 gene codes for a cartilage-derived morphogenetic protein. The UQCC gene functions in concert with FGF2 and BMPs, therefore also can play role in bone metabolism. This finding is not surprising given that adult height is largely explained by the length of extremities and vertebral segments, which are a function of bone lengths [27]. Further GWAS by Weedon et al. [184] identified 20 loci that influence adult height; they confirmed GDF5 as one of the top genes and identified SOCS2; the latter is a negative regulator in the GH/IGF1 signaling pathway (also anabolic for both bone and muscle).
Further, in a recent study of 19,195 adults from five Caucasian populations [137], 20 genome-wide significant signals were found at several chromosomal locations. Especially notable are the following two newly identified genes, both significantly associated with femoral neck BMD: MEF2C (on 5q14) and SOX6 (11p15). In particular, MEF2C (myocyte enhancer factor 2), is a central regulator of diverse developmental programs; it is a ubiquitous transcription factor. The Mef2 genes are highly expressed in striated muscles [131]. Most recent study [33] demonstrated the expression of MEF2C in osteocytes, as well as regulation of DMP1 and SOST genes by MEF2C. The SRY (sex determining region Y)-box (SOX6) gene was identified via a SNP located 297 kb upstream from the gene. Also, the same gene was reported in a bivariate association with BMI-hip BMD in males [105]. SOX6 is a transcription factor of the SOX gene family, which is expressed in a variety of tissues, most abundantly in skeletal muscle. Sox6 knock-out mice present with mild skeletal abnormalities affecting size and mineralization of endochondral elements [154]. Other SOX-family genes regulate RUNX2-mediated differentiation of mesenchymal cells during endochondral ossification/skeletogenesis [196].
In summary, Table 1 provides indications that genes from multiple pathways, including inflammatory, growth hormone, and steroid metabolism, are candidates for pleiotroipic regulation of bone and muscle. The pathways, as genetic mechanisms governing complex metabolic processes, tend to be redundant, pleiotropic and polygenic, for safety reasons [153,189]. Other possible candidates such as leptin, transcription factor SRY-box 17, pleiotrophin, vascular endothelial growth factor, Notch signaling pathway, and glucocorticoid receptor, are also shown in Table 1; for these genes, less is known about their pleiotropic action on bone and muscle. This list needs to be added and refined by bioinformatic search of genetic association and expression databases, and a candidacy of any gene to be confirmed via functional studies. We did not review genes with overt pleiotropic functions observed in several monogenic syndromes involving bones, muscles, and other tissues.
Expression experiments
Physiology of the bone and muscle inter-relationship requires understanding of the gene expression patterns and regulatory networks involved in skeletal development, maintenance and remodeling, as well as muscle in exercise and unloading. Comprehensive analysis of gene expression became feasible recently with the advent of gene array technology [125]. Early reports evaluated gene expression mostly in osteoblastic cell lines undergoing osteogenic differentiation via stimulation by BMP2 or PTH. A recent study of preosteocytes and osteocytes [125] confimed many genes to be important for the osteoblast to osteocyte transition.
Analysis of microarray data from skeletal muscles of mice after a spaceflight found that 272 mRNAs were significantly altered by spaceflight, the majority of which displayed similar responses to hindlimb suspension [6]. Several mRNAs altered by spaceflight were associated with muscle growth. Thus, mRNA levels of the PPAR-γ coactivator-1α and the transcription factor PPAR-α were significantly decreased in spaceflight. Importantly, reloading (after landing) tended to counteract these expression changes [6]. In another experimental study, the expression patterns of two genes, Collagen X (ColX) and Indian hedgehog (Ihh), were shown to colocalize with biophysical stimuli induced by embryonic muscle contractions, identifying them as potentially being involved in the mechanoregulation of bone formation [123].
Most recently, another group [33] studied gene expression profiles from osteocytes purified from calvariae of 5–8 day-old mice. Among the 269 overexpressed osteocyte-specific genes ; there were many genes and transcription factors known to control muscle differentiation and contractility. The investigators proposed a regulatory network model, which included showed that many osteocyte-specific genes, including two well-known osteocyte markers DMP1 and SOST, had highly conserved clustering of cis-regulatory modules such as muscle-related Mef2c and Myogenin [33]. This thought-provoking finding thus supports the concept that a muscle-related gene network probably has a role in muscle contractility as well as dynamic movements of the osteocyte. Other sources of the heuristic discovery are emerging. Other genes with possible pleiotropic actions do exist and might be highlighted in the future. We may expect, for example, that GWAS of muscle characteristics might provide important discoveries (partly serendipitous), by pointing out genes thought to be candidates for height, BMI, and bone strength. The progress in these studies may help understand genetic underpinnings of broader musculoskeletal concepts, such as sarcopenia and frailty.
Summary and recommendations
In conclusion, bones and muscles develop and age together. The cellular mechanism for biological regulation of this phenotypic covariation needs to be better explored [74]; there are still many more questions than answers. It is not fully understood how bone senses mechanical loading, which cells are responsible for this ability, and whether bone loses its mechanosensitivity with aging. Additionally, there are other non-loading related mechanisms, such as the para- and endocrine crosstalk of muscle to bone, which should be studied to a greater extent. We postulate that to achieve success in the study of osteoporosis (or in genetics of sarcopenia) one needs to undertake a pleiotropic approach, to take into account the multivariate nature of complex diseases and to capitalize on statistical benefits of joint analyses [74,105]. A joint approach focusing on common genetic determination of both skeletal geometry and muscular mass will identify new signaling pathways, which in turn will pinpoint novel biological mechanisms. The study of model organisms as well as whole-genome expression and candidate gene association that simultaneously deals with two outcomes, muscular and skeletal, should prove helpful in this task.
Identification of a mechanism for mechanotransduction in bone or a hormonal factor affecting the system as a whole, could lead to therapeutic approaches for combating bone loss due to osteoporosis and disuse [110]. Identifying significant genetic variants underlying both bones and muscles, measured with state-of-the-art technology and replicated in large human cohorts and animal experiments, will provide valuable insight into important potential targets for risk stratification, as well as pharmacogenetic interventions aimed at increasing bone strength, muscle strength, or both.
There are new ways to evaluate how genetic variants affect multiple traits simultaneously. This knowledge “may provide a way to improve diagnosis by identifying at-risk individuals earlier in life, and to identify novel biological pathways that can be used to treat individuals earlier and more effectively” [74].
References
- 1.Ackert-Bicknell CL, Demissie S, Marinde Evsikova C, Hsu YH, DeMambro VE, Karasik D, et al. PPARG by dietary fat interaction influences bone mass in mice and humans. J Bone Miner Res. 2008;23:1398–408. doi: 10.1359/JBMR.080419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams DJ, Spirt AA, Brown TD, Fritton SP, Rubin CT, Brand RA. Testing the daily stress stimulus theory of bone adaptation with natural and experimentally controlled strain histories. J Biomech. 1997;30:671–8. doi: 10.1016/s0021-9290(97)00004-3. [DOI] [PubMed] [Google Scholar]
- 3.Agoston H, Khan S, James CG, Gillespie JR, Serra R, Stanton LA, et al. C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC Dev Biol. 2007;7:18. doi: 10.1186/1471-213X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, et al. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35:162–9. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Akter R, Rivas D, Geneau G, Drissi H, Duque G. Effect of lamin A/C knockdown on osteoblast differentiation and function. J Bone Miner Res. 2009;24:283–93. doi: 10.1359/jbmr.081010. [DOI] [PubMed] [Google Scholar]
- 6.Allen DL, Bandstra ER, Harrison BC, Thorng S, Stodieck LS, Kostenuik PJ, et al. Effects of spaceflight on murine skeletal muscle gene expression. J Appl Physiol. 2009;106:582–95. doi: 10.1152/japplphysiol.90780.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol. 1997;14:953–8. doi: 10.1002/(SICI)1098-2272(1997)14:6<953::AID-GEPI65>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Andrew T, Antioniades L, Scurrah KJ, Macgregor AJ, Spector TD. Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J Bone Miner Res. 2005;20:67–74. doi: 10.1359/JBMR.041015. [DOI] [PubMed] [Google Scholar]
- 9.Arai H, Miyamoto KI, Yoshida M, Yamamoto H, Taketani Y, Morita K, et al. The polymorphismin the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J Bone Miner Res. 2001;16:1256–64. doi: 10.1359/jbmr.2001.16.7.1256. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DD, Esser KA. Wnt/beta-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2005;289:C853–9. doi: 10.1152/ajpcell.00093.2005. [DOI] [PubMed] [Google Scholar]
- 11.Bagge M. A model of bone adaptation as an optimization process. J Biomech. 2000;33:1349–57. doi: 10.1016/s0021-9290(00)00124-x. [DOI] [PubMed] [Google Scholar]
- 12.Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283:6509–18. doi: 10.1074/jbc.M707000200. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284:E481–7. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 14.Barr R, Macdonald H, Stewart A, McGuigan F, Rogers A, Eastell R, et al. Association between vitamin D receptor gene polymorphisms, falls, balance and muscle power: results from two independent studies (APOSS and OPUS) Osteoporos Int. 2010;21(3):457–66. doi: 10.1007/s00198-009-1019-6. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin M, McGonagle D. Entheses: tendon and ligament attachment sites. Scand J Med Sci Sports. 2009;19:520–7. doi: 10.1111/j.1600-0838.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin M, Rufai A, Ralphs J. The mechanism of formation of bony spurs (enthesophytes) in the Achilles tendon. Arthritis Rheumatism. 2000;43:576–83. doi: 10.1002/1529-0131(200003)43:3<576::AID-ANR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl. 2001;22:718–31. [PubMed] [Google Scholar]
- 18.Blain H, Vuillemin A, Jeandel C, Jouanny P, Guillemin F, Le Bihan E. Lean mass plays a gender-specific role in familial resemblance for femoral neck bone mineral density in adult subjects. Osteoporos Int. 2006;17:897–907. doi: 10.1007/s00198-005-0062-1. [DOI] [PubMed] [Google Scholar]
- 19.Blanchet C, Giguere Y, Prud’homme D, Dumont M, Rousseau F, Dodin S. Association of physical activity and bone: influence of vitamin D receptor genotype. Med Sci Sports Exerc. 2002;34:24–31. doi: 10.1097/00005768-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–15. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49:1641–5. doi: 10.1046/j.1532-5415.2001.t01-1-49273.x. [DOI] [PubMed] [Google Scholar]
- 22.Buckey JC. Space Physiology. Oxford: University Press; 2006. [Google Scholar]
- 23.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Carey KA, Farnfield MM, Tarquinio SD, Cameron-Smith D. Impaired expression of Notch signaling genes in aged human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2007;62:9–17. doi: 10.1093/gerona/62.1.9. [DOI] [PubMed] [Google Scholar]
- 25.Carter DR, Orr TE. Skeletal development and bone functional adaptation. J Bone Miner Res. 1992;7(Suppl 2):S389–95. doi: 10.1002/jbmr.5650071405. [DOI] [PubMed] [Google Scholar]
- 26.Cheverud JM. Quantitative genetics and developmental constraints on evolution by selection. J Theor Biol. 1984;110:155–71. doi: 10.1016/s0022-5193(84)80050-8. [DOI] [PubMed] [Google Scholar]
- 27.Chinappen-Horsley U, Blake GM, Fogelman I, Kato B, Ahmadi KR, Spector TD. Quantitative trait loci for bone lengths on chromosome 5 using dual energy X-ray absorptiometry imaging in the Twins UK cohort. PLoS ONE. 2008;3:e1752. doi: 10.1371/journal.pone.0001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho K, Demissie S, Dupuis J, Cupples LA, Kathiresan S, Beck TJ, et al. Polymorphisms in the endothelial nitric oxide synthase gene and bone density/ultrasound and geometry in humans. Bone. 2008;42:53–60. doi: 10.1016/j.bone.2007.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech (Bristol, Avon) 1996;11:1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 30.Churchill SE. Particulate versus integrated evolution of the upper body in late pleistocene humans: a test of two models. Am J Phys Anthropol. 1996;100:559–83. doi: 10.1002/(SICI)1096-8644(199608)100:4<559::AID-AJPA9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Corsi KA, Pollett JB, Phillippi JA, Usas A, Li G, Huard J. Osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex. J Bone Miner Res. 2007;22:1592–602. doi: 10.1359/jbmr.070702. [DOI] [PubMed] [Google Scholar]
- 32.Daly RM, Bass SL. Lifetime sport and leisure activity participation is associated with greater bone size, quality and strength in older men. Osteoporos Int. 2006;17:1258–67. doi: 10.1007/s00198-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 33.Dean AK, Harris SE, Kalajzic I, Ruan J. A systems biology approach to the identification and analysis of transcriptional regulatory networks in osteocytes. BMC Bioinformatics. 2009;10(Suppl 9):S5. doi: 10.1186/1471-2105-10-S9-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng FY, Xiao P, Lei SF, Zhang L, Yang F, Tang ZH, et al. Bivariate whole genome linkage analysis for femoral neck geometric parameters and total body lean mass. J Bone Miner Res. 2007;22:808–16. doi: 10.1359/jbmr.070303. [DOI] [PubMed] [Google Scholar]
- 35.Deng HW, Mahaney MC, Williams JT, Li J, Conway T, Davies KM, et al. Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet Epidemiol. 2002;22:12–25. doi: 10.1002/gepi.1040. [DOI] [PubMed] [Google Scholar]
- 36.Desplanches D, Mayet MH, Ilyina-Kakueva EI, Sempore B, Flandrois R. Skeletal muscle adaptation in rats flown on Cosmos 1667. J Appl Physiol. 1990;68:48–52. doi: 10.1152/jappl.1990.68.1.48. [DOI] [PubMed] [Google Scholar]
- 37.Devaney JM, Tosi LL, Fritz DT, Gordish-Dressman HA, Jiang S, Orkunoglu-Suer FE, et al. Differences in fat and muscle mass associated with a functional human polymorphism in a post-transcriptional BMP2 gene regulatory element. J Cell Biochem. 2009;107:1073–82. doi: 10.1002/jcb.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–44. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson AL, Suuriniemi M, Mahonen A, Cheng S, Ohlsson C. The COMT val158met polymorphism is associated with early pubertal development, height and cortical bone mass in girls. Pediatr Res. 2005;58:71–7. doi: 10.1203/01.PDR.0000163383.49747.B5. [DOI] [PubMed] [Google Scholar]
- 40.Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve. 2007;36:21–9. doi: 10.1002/mus.20796. [DOI] [PubMed] [Google Scholar]
- 41.Fang Y, van Meurs JB, Rivadeneira F, van Schoor NM, van Leeuwen JP, Lips P, et al. Vitamin D receptor gene haplotype is associated with body height and bone size. J Clin Endocrinol Metab. 2007;92:1491–501. doi: 10.1210/jc.2006-1134. [DOI] [PubMed] [Google Scholar]
- 42.Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88:358–62. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, et al. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet. 2004;74:866–75. doi: 10.1086/420771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrari SL, Karasik D, Liu J, Karamohamed S, Herbert AG, Cupples LA, et al. Interactions of interleukin-6 promoter polymorphisms with dietary and lifestyle factors and their association with bone mass in men and women from the Framingham osteoporosis study. J Bone Miner Res. 2004;19:552–9. doi: 10.1359/JBMR.040103. [DOI] [PubMed] [Google Scholar]
- 45.Ferretti JL, Capozza RF, Cointry GR, Garcia SL, Plotkin H, Alvarez Filgueira ML, et al. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone. 1998;22:683–90. doi: 10.1016/s8756-3282(98)00046-5. [DOI] [PubMed] [Google Scholar]
- 46.Forwood MR. Mechanical effects on the skeleton: are there clinical implications? Osteoporos Int. 2001;12:77–83. doi: 10.1007/s001980170161. [DOI] [PubMed] [Google Scholar]
- 47.Forwood MR, Burr DB. Physical activity and bone mass: exercises in futility? Bone Miner. 1993;21:89–112. doi: 10.1016/s0169-6009(08)80012-8. [DOI] [PubMed] [Google Scholar]
- 48.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 49.Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 50.Frost HM. On the estrogen–bone relationship and postmenopausal bone loss: a new model. J Bone Miner Res. 1999;14:1473–7. doi: 10.1359/jbmr.1999.14.9.1473. [DOI] [PubMed] [Google Scholar]
- 51.Frost HM, Schonau E. The “muscle–bone unit” in children and adolescents: a 2000 overview. J Pediatr Endocrinol Metab. 2000;13:571–90. doi: 10.1515/jpem.2000.13.6.571. [DOI] [PubMed] [Google Scholar]
- 52.Gajendran VK, Lin JR, Fyhrie DP. An application of bioinformatics and text mining to the discovery of novel genes related to bone biology. Bone. 2007;40:1378–88. doi: 10.1016/j.bone.2006.12.067. [DOI] [PubMed] [Google Scholar]
- 53.Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A, et al. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:2803–10. doi: 10.1210/jc.2003-031342. [DOI] [PubMed] [Google Scholar]
- 54.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–74. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 55.Giresi PG, Stevenson EJ, Theilhaber J, Koncarevic A, Parkington J, Fielding RA, et al. Identification of a molecular signature of sarcopenia. Physiol Genomics. 2005;21:253–63. doi: 10.1152/physiolgenomics.00249.2004. [DOI] [PubMed] [Google Scholar]
- 56.Goldspink G, Yang SY. The splicing of the IGF-I gene to yield different muscle growth factors. Adv Genet. 2004;52:23–49. doi: 10.1016/S0065-2660(04)52002-3. [DOI] [PubMed] [Google Scholar]
- 57.Grundberg E, Brandstrom H, Ribom EL, Ljunggren O, Mallmin H, Kindmark A. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and bodyweight in Swedish women. Eur J Endocrinol. 2004;150:323–8. doi: 10.1530/eje.0.1500323. [DOI] [PubMed] [Google Scholar]
- 58.Grundberg E, Ribom EL, Brandstrom H, Ljunggren O, Mallmin H, Kindmark A. A TA-repeat polymorphism in the gene for the estrogen receptor alpha does not correlate with muscle strength or body composition in young adult Swedish women. Maturitas. 2005;50:153–60. doi: 10.1016/j.maturitas.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Hamrick MW. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:388–91. doi: 10.1002/ar.a.10044. [DOI] [PubMed] [Google Scholar]
- 60.Hamrick MW, Pennington C, Byron CD. Bone architecture and disc degeneration in the lumbar spine of mice lacking GDF-8 (myostatin) J Orthop Res. 2003;21:1025–32. doi: 10.1016/S0736-0266(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 61.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–83. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 62.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age gene/environment susceptibility—Reykjavik study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Havill LM, Rogers J, Cox LA, Mahaney MC. QTL with pleiotropic effects on serum levels of bone-specific alkaline phosphatase and osteocalcin maps to the baboon ortholog of human chromosome 6p23–21. 3. J Bone Miner Res. 2006;21:1888–96. doi: 10.1359/jbmr.060812. [DOI] [PubMed] [Google Scholar]
- 64.Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:271–7. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Herring S. In: Development of functional interactions between skeletal and muscular systems. Hall B, editor. BoneBoca Raton: CRC Press; 1994. pp. 165–91. [Google Scholar]
- 66.Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–7. doi: 10.1093/gerona/60.7.882. [DOI] [PubMed] [Google Scholar]
- 67.Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:1420–4. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- 68.Hirukawa K, Miyazawa K, Maeda H, Kameyama Y, Goto S, Togari A. Effect of tensile force on the expression of IGF-I and IGF-I receptor in the organ-cultured rat cranial suture. Arch Oral Biol. 2005;50:367–72. doi: 10.1016/j.archoralbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Ho AM, Marker PC, Peng H, Quintero AJ, Kingsley DM, Huard J. Dominant negative Bmp5 mutation reveals key role of BMPs in skeletal response to mechanical stimulation. BMC Dev Biol. 2008;8:35. doi: 10.1186/1471-213X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huygens W, Thomis MA, Peeters MW, Vlietinck RF, Beunen GP. Determinants and upper-limit heritabilities of skeletal muscle mass and strength. Can J Appl Physiol. 2004;29:186–200. doi: 10.1139/h04-014. [DOI] [PubMed] [Google Scholar]
- 71.Ioannidis JP, Ng MY, Sham PC, Zintzaras E, Lewis CM, Deng HW, et al. Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J Bone Miner Res. 2007;22:173–83. doi: 10.1359/jbmr.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ioannidis JP, Stavrou I, Trikalinos TA, Zois C, Brandi ML, Gennari L, et al. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density and fracture risk in women: a meta-analysis. J Bone Miner Res. 2002;17:2048–60. doi: 10.1359/jbmr.2002.17.11.2048. [DOI] [PubMed] [Google Scholar]
- 73.Jacob KN, Garg A. Laminopathies: multisystem dystrophy syndromes. Mol Genet Metab. 2006;87:289–302. doi: 10.1016/j.ymgme.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 74.Jepsen KJ. Systems analysis of bone. WIREs Syst Biol Med. 2009;14:73–88. doi: 10.1002/wsbm.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Judex S, Garman R, Squire M, Busa B, Donahue LR, Rubin C. Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res. 2004;19:607–13. doi: 10.1359/JBMR.040110. [DOI] [PubMed] [Google Scholar]
- 76.Kandarian S. The molecular basis of skeletal muscle atrophy—parallels with osteoporotic signaling. J Musculoskelet Neuronal Interact. 2008;8:340–1. [PubMed] [Google Scholar]
- 77.Kaptoge S, Dalzell N, Jakes RW, Wareham N, Day NE, Khaw KT, et al. Hip section modulus, a measure of bending resistance, is more strongly related to reported physical activity than BMD. Osteoporos Int. 2003;14:941–9. doi: 10.1007/s00198-003-1484-2. [DOI] [PubMed] [Google Scholar]
- 78.Kapur S, Mohan S, Baylink DJ, Lau KH. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J Biol Chem. 2005;280:20163–70. doi: 10.1074/jbc.M501460200. [DOI] [PubMed] [Google Scholar]
- 79.Karamichou E, Richardson RI, Nute GR, Gibson KP, Bishop SC. Genetic analyses and quantitative trait loci detection, using a partial genome scan, for intramuscular fatty acid composition in Scottish Blackface sheep. J Anim Sci. 2006;84:3228–38. doi: 10.2527/jas.2006-204. [DOI] [PubMed] [Google Scholar]
- 80.Karasik D, Ferrari SL. Contribution of gender-specific genetic factors to osteoporosis risk. Ann Hum Genet. 2008;72:696–714. doi: 10.1111/j.1469-1809.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 81.Karasik D, Hannan MT, Cupples LA, Felson DT, Kiel DP. Genetic contribution to biological aging: the Framingham study. J Gerontol A Biol Sci Med Sci. 2004;59:218–26. doi: 10.1093/gerona/59.3.b218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karasik D, Kiel DP. Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner Res. 2008;23:788–802. doi: 10.1359/jbmr.080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karasik D, Rosen CJ, Hannan MT, Broe KE, Dawson-Hughes B, Gagnon DR, et al. Insulin-like growth factor binding proteins 4 and 5 and bone mineral density in elderly men and women. Calcif Tissue Int. 2002;71:323–8. doi: 10.1007/s00223-002-1002-0. [DOI] [PubMed] [Google Scholar]
- 84.Karasik D, Shearman A, Cupples L, Demissie S, Gruenthal K, Housman D, et al. Association of aromatase gene polymorphisms with bone mineral density in men and women: the Framingham Offspring Study. J Bone Miner Res. 2003;18(S2):S325–S325. [Google Scholar]
- 85.Karasik D, Zhou Y, Cupples LA, Hannan MT, Kiel DP, Demissie S. Bivariate genome-wide linkage analysis of femoral bone traits and leg lean mass: Framingham study. J Bone Miner Res. 2009;24:710–8. doi: 10.1359/JBMR.081222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keller P, Penkowa M, Keller C, Steensberg A, Fischer CP, Giralt M, et al. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. Faseb J. 2005;19:1181–3. doi: 10.1096/fj.04-3278fje. [DOI] [PubMed] [Google Scholar]
- 87.Kelly SA, Czech PP, Wight JT, Blank KM, Garland T., Jr Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J Morphol. 2006;267:360–74. doi: 10.1002/jmor.10407. [DOI] [PubMed] [Google Scholar]
- 88.Kenny AM, Dawson L, Kleppinger A, Iannuzzi-Sucich M, Judge JO. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long-term users of estrogen-replacement therapy. J Gerontol A Biol Sci Med Sci. 2003;58:M436–40. doi: 10.1093/gerona/58.5.m436. [DOI] [PubMed] [Google Scholar]
- 89.Kesavan C, Baylink DJ, Kapoor S, Mohan S. Novel loci regulating bone anabolic response to loading: expression QTL analysis in C57BL/6JXC3H/HeJ mice cross. Bone. 2007;41:223–30. doi: 10.1016/j.bone.2007.04.185. [DOI] [PubMed] [Google Scholar]
- 90.Khosla S. Oestrogen, bones and men: when testosterone just isn’t enough. Clin Endocrinol (Oxf) 2002;56:291–3. doi: 10.1046/j.1365-2265.2002.01428.x. [DOI] [PubMed] [Google Scholar]
- 91.Khoury MJ, McCabe LL, McCabe ER. Population screening in the age of genomic medicine. N Engl J Med. 2003;348:50–8. doi: 10.1056/NEJMra013182. [DOI] [PubMed] [Google Scholar]
- 92.Kiel DP, Ferrari SL, Cupples LA, Karasik D, Manen D, Imamovic A, et al. Genetic variation at the low-density lipoprotein receptor-related protein 5 (LRP5) locus modulates Wnt signaling and the relationship of physical activity with bone mineral density in men. Bone. 2007;40:587–96. doi: 10.1016/j.bone.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101:581–9. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 94.Kontulainen SA, Macdonald HM, McKay HA. Change in cortical bone density and its distribution differs between boys and girls during puberty. J Clin Endocrinol Metab. 2006;91:2555–61. doi: 10.1210/jc.2006-0136. [DOI] [PubMed] [Google Scholar]
- 95.Kostek MC, Delmonico MJ, Reichel JB, Roth SM, Douglass L, Ferrell RE, et al. Muscle strength response to strength training is influenced by insulin-like growth factor 1 genotype in older adults. J Appl Physiol. 2005;98:2147–54. doi: 10.1152/japplphysiol.00817.2004. [DOI] [PubMed] [Google Scholar]
- 96.Lang DH, Conroy DE, Lionikas A, Mack HA, Larsson L, Vogler GP, et al. Bone, muscle and physical activity: structural equation modeling of relationships and genetic influence with age. J Bone Miner Res. 2009;24(9):1608–17. doi: 10.1359/JBMR.090418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, et al. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–62. doi: 10.1210/jcem.83.12.5308. [DOI] [PubMed] [Google Scholar]
- 98.Lanyon L, Armstrong V, Saxon L, Sunters A, Sugiyama T, Zaman G, et al. Estrogen receptors critically regulate bones’ adaptive responses to loading. Clin Rev Bone Miner Metab. 2008;5:234–48. [Google Scholar]
- 99.Latimer B. The perils of being bipedal. Ann Biomed Eng. 2005;33:3–6. doi: 10.1007/s10439-005-8957-8. [DOI] [PubMed] [Google Scholar]
- 100.Lauretani F, Bandinelli S, Bartali B, Iorio AD, Giacomini V, Corsi AM, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27:1145–54. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 102.Li G, Bunn JR, Mushipe MT, He Q, Chen X. Effects of pleiotrophin (PTN) over-expression on mouse long bone development, fracture healing and bone repair. Calcif Tissue Int. 2005;76:299–306. doi: 10.1007/s00223-004-0145-6. [DOI] [PubMed] [Google Scholar]
- 103.Li GHY, Kung AWC, Huang QY. Common variants in FLNB/CRTAP, not ARHGEF3 at 3p, are associated with osteoporosis in southern Chinese women. Osteoporosis International. 2010 doi: 10.1007/s00198-009-1043-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–61. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 105.Liu YZ, Pei YF, Liu JF, Yang F, Guo Y, Zhang L, et al. Powerful bivariate genomewide association analyses suggest the SOX6 gene influencing both obesity and osteoporosis phenotypes in males. PLoS ONE. 2009;4:e6827. doi: 10.1371/journal.pone.0006827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lorentzon M, Eriksson AL, Mellstrom D, Ohlsson C. The COMT val158met polymorphism is associated with peak BMD in men. J Bone Miner Res. 2004;19:2005–11. doi: 10.1359/JBMR.040909. [DOI] [PubMed] [Google Scholar]
- 107.Lorentzon M, Eriksson AL, Nilsson S, Mellstrom D, Ohlsson C. Association between physical activity and BMD in young men is modulated by catechol-O-methyltransferase (COMT) genotype: the GOOD study. J BoneMiner Res. 2007;22:1165–72. doi: 10.1359/jbmr.070416. [DOI] [PubMed] [Google Scholar]
- 108.Lovejoy CO, Meindl RS, Ohman JC, Heiple KG, White TD. The Maka femur and its bearing on the antiquity of human walking: applying contemporary concepts of morphogenesis to the human fossil record. Am J Phys Anthropol. 2002;119:97–133. doi: 10.1002/ajpa.10111. [DOI] [PubMed] [Google Scholar]
- 109.Loveridge N, Fletcher S, Power J, Caballero-Alias AM, Das-Gupta V, Rushton N, et al. Patterns of osteocytic endothelial nitric oxide synthase expression in the femoral neck cortex: differences between cases of intracapsular hip fracture and controls. Bone. 2002;30:866–71. doi: 10.1016/s8756-3282(02)00732-9. [DOI] [PubMed] [Google Scholar]
- 110.Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci USA. 2007;104:13325–30. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mannen Cawthon P, Fullman RL, Marshall L, Mackey DC, Fink HA, Cauley JA, et al. Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008;23:1037–44. doi: 10.1359/JBMR.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–37. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 113.Mao H, Guo Y, Yang G, Yang B, Ren J, Liu S, et al. A genome-wide scan for quantitative trait loci affecting limb bone lengths and areal bone mineral density of the distal femur in a White Duroc x Erhualian F2 population. BMC Genet. 2008;9:63. doi: 10.1186/1471-2156-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–55. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol. 2006;100:2012–23. doi: 10.1152/japplphysiol.01583.2005. [DOI] [PubMed] [Google Scholar]
- 116.McGill SM, Norman RW. Partitioning of the L4–L5 dynamic moment into disc, ligamentous, and muscular components during lifting. Spine. 1986;11:666–78. doi: 10.1097/00007632-198609000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Mikkola TM, Sipila S, Rantanen T, Sievanen H, Suominen H, Tiainen K, et al. Muscle cross-sectional area and structural bone strength share genetic and environmental effects in older women. J Bone Miner Res. 2009;24:338–45. doi: 10.1359/jbmr.081008. [DOI] [PubMed] [Google Scholar]
- 118.Moffatt P, Thomas GP. Osteocrin—beyond just another bone protein? Cell Mol Life Sci. 2009;66:1135–9. doi: 10.1007/s00018-009-8716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nevill AM, Holder RL, Maffulli N, Cheng JC, Leung SS, Lee WT, et al. Adjusting bone mass for differences in projected bone area and other confounding variables: an allometric perspective. J Bone Miner Res. 2002;17:703–8. doi: 10.1359/jbmr.2002.17.4.703. [DOI] [PubMed] [Google Scholar]
- 120.Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual X-ray absorptiometry. Bone. 2003;32:62–8. doi: 10.1016/s8756-3282(02)00927-4. [DOI] [PubMed] [Google Scholar]
- 121.Nikawa T, Ishidoh K, Hirasaka K, Ishihara I, Ikemoto M, Kano M, et al. Skeletal muscle gene expression in space-flown rats. Faseb J. 2004;18:522–4. doi: 10.1096/fj.03-0419fje. [DOI] [PubMed] [Google Scholar]
- 122.Nordstrom A, Gerdhem P, Brandstrom H, Stiger F, Lerner UH, Lorentzon M, et al. Interleukin-6 promoter polymorphism is associated with bone quality assessed by calcaneus ultrasound and previous fractures in a cohort of 75-year-old women. Osteoporos Int. 2004;15:820–6. doi: 10.1007/s00198-004-1610-9. [DOI] [PubMed] [Google Scholar]
- 123.Nowlan NC, Prendergast PJ. Evolution of mechanoregulation of bone growth will lead to non-optimal bone phenotypes. J Theor Biol. 2005;235:408–18. doi: 10.1016/j.jtbi.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 124.Oh KW, Lee WY, Rhee EJ, Baek KH, Yoon KH, Kang MI, et al. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol (Oxf) 2005;63:131–8. doi: 10.1111/j.1365-2265.2005.02312.x. [DOI] [PubMed] [Google Scholar]
- 125.Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–92. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pan WH, Lynn KS, Chen CH, Wu YL, Lin CY, Chang HY. Using endophenotypes for pathway clusters to map complex disease genes. Genet Epidemiol. 2006;30:143–54. doi: 10.1002/gepi.20136. [DOI] [PubMed] [Google Scholar]
- 127.Pearson OM, Lieberman DE. The aging of Wolff’s “law”: ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol Suppl. 2004;39:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 128.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 129.Pistilli EE, Gordish-Dressman H, Seip RL, Devaney JM, Thompson PD, Price TB, et al. Resistin polymorphisms are associated with muscle, bone, and fat phenotypes in white men and women. Obesity (Silver Spring) 2007;15:392–402. doi: 10.1038/oby.2007.543. [DOI] [PubMed] [Google Scholar]
- 130.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–43. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 131.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134:4131–40. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 132.Prendergast P, van der Helm F, Duda G. Analysis of muscle and joint loads. In: Mow V, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. 3. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 29–89. [Google Scholar]
- 133.Qureshi A, McGuigan F, Seymour D, Hutchison J, Reid D, Ralston S. Association between COLIA1 Sp1 alleles and femoral neck geometry. Calcif Tissue Int. 2001;69:67–72. doi: 10.1007/s002230010037. [DOI] [PubMed] [Google Scholar]
- 134.Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151:528–37. doi: 10.7326/0003-4819-151-8-200910200-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, et al. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;36:1019–29. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 136.Rivadeneira F, Houwing-Duistermaat JJ, Beck TJ, Janssen JA, Hofman A, Pols HA, et al. The influence of an insulin-like growth factor I gene promoter polymorphism on hip bone geometry and the risk of nonvertebral fracture in the elderly: the Rotterdam study. J Bone Miner Res. 2004;19:1280–90. doi: 10.1359/JBMR.040405. [DOI] [PubMed] [Google Scholar]
- 137.Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41:1199–206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA. Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 2003;228:706–9. doi: 10.1177/153537020322800609. [DOI] [PubMed] [Google Scholar]
- 139.Roth SM, Schrager MA, Lee MR, Metter EJ, Hurley BF, Ferrell RE. Interleukin-6 (IL6) genotype is associated with fat-free mass in men but not women. J Gerontol A Biol Sci Med Sci. 2003;58:B1085–8. doi: 10.1093/gerona/58.12.b1085. [DOI] [PubMed] [Google Scholar]
- 140.Roth SM, Zmuda JM, Cauley JA, Shea PR, Ferrell RE. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci. 2004;59:10–5. doi: 10.1093/gerona/59.1.b10. [DOI] [PubMed] [Google Scholar]
- 141.Rubin CT, Sommerfeldt DW, Judex S, Qin Y. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov Today. 2001;6:848–58. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]
- 142.Rubin J, Murphy TC, Zhu L, Roy E, Nanes MS, Fan X. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J Biol Chem. 2003;278:34018–25. doi: 10.1074/jbc.M302822200. [DOI] [PubMed] [Google Scholar]
- 143.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sanna S, Jackson AU, Nagaraja R, Willer CJ, Chen WM, Bonnycastle LL, et al. Common variants in the GDF5-UQCC region are associated with variation in human height. Nat Genet. 2008;40:198–203. doi: 10.1038/ng.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910–7. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 146.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, et al. The Wnt coreceptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 147.Schenkel FS, Miller SP, Ye X, Moore SS, Nkrumah JD, Li C, et al. Association of single nucleotide polymorphisms in the leptin gene with carcass and meat quality traits of beef cattle. J Anim Sci. 2005;83:2009–20. doi: 10.2527/2005.8392009x. [DOI] [PubMed] [Google Scholar]
- 148.Schmid K, McSharry W, Pameijer C, Binette JP. Chemical and physiochemical studies on the mineral deposits of the human atherosclerotic aorta. Atherosclerosis. 1980;37:199–210. doi: 10.1016/0021-9150(80)90005-2. [DOI] [PubMed] [Google Scholar]
- 149.Schrager MA, Roth SM, Ferrell RE, Metter EJ, Russek-Cohen E, Lynch NA, et al. Insulin-like growth factor-2 genotype, fat-free mass, and muscle performance across the adult life span. J Appl Physiol. 2004;97:2176–83. doi: 10.1152/japplphysiol.00985.2003. [DOI] [PubMed] [Google Scholar]
- 150.Schulze TG, McMahon FJ. Defining the phenotype in human genetic studies: forward genetics and reverse phenotyping. Hum Hered. 2004;58:131–8. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]
- 151.Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, et al. Association between estrogen receptor alpha gene variation and cardiovascular disease. Jama. 2003;290:2263–70. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- 152.Shearman AM, Karasik D, Gruenthal KM, Demissie S, Cupples LA, Housman DE, et al. Estrogen receptor Beta polymorphisms are associated with bone mass in women and men: the Framingham study. J Bone Miner Res. 2004;19:773–81. doi: 10.1359/JBMR.0301258. [DOI] [PubMed] [Google Scholar]
- 153.Sievänen H. Hormonal influences on the muscle–bone feedback system: a perspective. J Musculoskelet Neuronal Interact. 2005;5:255–61. [PubMed] [Google Scholar]
- 154.Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, et al. The transcription factors L-Sox5 and Sox6 are essential for cartilage. Formation. 2001;1:277–90. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 155.Solomon AM, Bouloux PM. Modifying muscle mass—the endocrine perspective. J Endocrinol. 2006;191:349–60. doi: 10.1677/joe.1.06837. [DOI] [PubMed] [Google Scholar]
- 156.Stokes IA, Gardner-Morse M. Quantitative anatomy of the lumbar musculature. J Biomech. 1999;32:311–6. doi: 10.1016/s0021-9290(98)00164-x. [DOI] [PubMed] [Google Scholar]
- 157.Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, et al. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 2003;1:E69. doi: 10.1371/journal.pbio.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun X, Lei SF, Deng FY, Wu S, Papacian C, Hamilton J, et al. Genetic and environmental correlations between bone geometric parameters and body compositions. Calcif Tissue Int. 2006;79:43–9. doi: 10.1007/s00223-006-0041-3. [DOI] [PubMed] [Google Scholar]
- 159.Suuriniemi M, Mahonen A, Kovanen V, Alen M, Lyytikainen A, Wang Q, et al. Association between exercise and pubertal BMD is modulated by estrogen receptor alpha genotype. J Bone Miner Res. 2004;19:1758–65. doi: 10.1359/JBMR.040918. [DOI] [PubMed] [Google Scholar]
- 160.Taaffe DR, Newman AB, Haggerty CL, Colbert LH, de Rekeneire N, Visser M, et al. Estrogen replacement, muscle composition, and physical function: the Health ABC Study. Med Sci Sports Exerc. 2005;37:1741–7. doi: 10.1249/01.mss.0000181678.28092.31. [DOI] [PubMed] [Google Scholar]
- 161.Tan RS. Aging Men’s Health: a Case-Based Approach. New York: Thieme; 2005. [Google Scholar]
- 162.Tang LL, Wang YL, Sun CX. The stress reaction and its molecular events: splicing variants. Biochem Biophys Res Commun. 2004;320:287–91. doi: 10.1016/j.bbrc.2004.05.167. [DOI] [PubMed] [Google Scholar]
- 163.Taylor BC, Schreiner PJ, Zmuda JM, Li J, Moffett SP, Beck TJ, et al. Association of endothelial nitric oxide synthase genotypes with bone mineral density, bone loss, hip structure, and risk of fracture in older women: the SOF study. Bone. 2006;39:174–80. doi: 10.1016/j.bone.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 164.Tosi LL, Boyan BD, Boskey AL. Does sex matter in musculoskeletal health? The influence of sex and gender on musculoskeletal health. J Bone Joint Surg Am. 2005;87:1631–47. doi: 10.2106/JBJS.E.00218. [DOI] [PubMed] [Google Scholar]
- 165.Turner CH. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos Int. 2002;13:97–104. doi: 10.1007/s001980200000. [DOI] [PubMed] [Google Scholar]
- 166.Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res. 1995;10:1544–9. doi: 10.1002/jbmr.5650101016. [DOI] [PubMed] [Google Scholar]
- 167.Uitterlinden AG, Ralston SH, Brandi ML, Carey AH, Grinberg D, Langdahl BL, et al. The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med. 2006;145:255–64. doi: 10.7326/0003-4819-145-4-200608150-00005. [DOI] [PubMed] [Google Scholar]
- 168.van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, et al. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci. 1998;111(Pt 12):1741–9. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- 169.Van Laere AS, Nguyen M, Braunschweig M, Nezer C, Collette C, Moreau L, et al. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature. 2003;425:832–6. doi: 10.1038/nature02064. [DOI] [PubMed] [Google Scholar]
- 170.van Meurs JB, Rivadeneira F, Jhamai M, Hugens W, Hofman A, van Leeuwen JP, et al. Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res. 2006;21:141–50. doi: 10.1359/JBMR.050904. [DOI] [PubMed] [Google Scholar]
- 171.van Meurs JB, Schuit SC, Weel AE, van der Klift M, Bergink AP, Arp PP, et al. Association of 5′ estrogen receptor alpha gene polymorphisms with bone mineral density, vertebral bone area and fracture risk. Hum Mol Genet. 2003;12:1745–54. doi: 10.1093/hmg/ddg176. [DOI] [PubMed] [Google Scholar]
- 172.van Meurs JB, Trikalinos TA, Ralston SH, Balcells S, Brandi ML, Brixen K, et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. Jama. 2008;299:1277–90. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W, et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf) 2003;59:585–92. doi: 10.1046/j.1365-2265.2003.01888.x. [DOI] [PubMed] [Google Scholar]
- 174.van Rossum EF, Voorhoeve PG, te Velde SJ, Koper JW, Delemarre-van de Waal HA, Kemper HC, Lamberts SW. The ER22/23EK polymorphism in the glucocorticoid receptor gene is associated with a beneficial body composition and muscle strength in young adults. J Clin Endocrinol Metab. 2004;89:4004–9. doi: 10.1210/jc.2003-031422. [DOI] [PubMed] [Google Scholar]
- 175.van Schoor NM, Dennison E, Lips P, Uitterlinden AG, Cooper C. Serum fasting cortisol in relation to bone, and the role of genetic variations in the glucocorticoid receptor. Clin Endocrinol (Oxf) 2007;67:871–8. doi: 10.1111/j.1365-2265.2007.02978.x. [DOI] [PubMed] [Google Scholar]
- 176.van Wingerden JP, Vleeming A, Buyruk HM, Raissadat K. Stabilization of the sacroiliac joint in vivo: verification of muscular contribution to force closure of the pelvis. Eur Spine J. 2004;13:199–205. doi: 10.1007/s00586-003-0575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Villadsen MM, Bunger MH, Carstens M, Stenkjaer L, Langdahl BL. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism is associated with osteoporotic vertebral fractures, but is a weak predictor of BMD. Osteoporos Int. 2005;16:411–6. doi: 10.1007/s00198-004-1704-4. [DOI] [PubMed] [Google Scholar]
- 178.Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–6. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 179.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–21. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 180.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 181.Volkman SK, Galecki AT, Burke DT, Paczas MR, Moalli MR, Miller RA, et al. Quantitative trait loci for femoral size and shape in a genetically heterogeneous mouse population. J Bone Miner Res. 2003;18:1497–505. doi: 10.1359/jbmr.2003.18.8.1497. [DOI] [PubMed] [Google Scholar]
- 182.Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, et al. Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J Appl Physiol. 2005;98:132–7. doi: 10.1152/japplphysiol.00537.2004. [DOI] [PubMed] [Google Scholar]
- 183.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 184.Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40:575–83. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Williams JT, Van Eerdewegh P, Almasy L, Blangero J. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. I. Likelihood formulation and simulation results. Am J Hum Genet. 1999;65:1134–47. doi: 10.1086/302570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wilsman NJ, Farnum CE, Leiferman EM, Fry M, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res. 1996;14:927–36. doi: 10.1002/jor.1100140613. [DOI] [PubMed] [Google Scholar]
- 187.Wilson SG, Jones MR, Mullin BH, Dick IM, Richards JB, Pastinen TM, et al. Common sequence variation in FLNB regulates bone structure in women in the general population and FLNB mRNA expression in osteoblasts in vitro. J Bone Miner Res. 2009;24:1989–97. doi: 10.1359/jbmr.090530. [DOI] [PubMed] [Google Scholar]
- 188.Windelinckx A, De Mars G, Beunen G, Aerssens J, Delecluse C, Lefevre J, et al. Polymorphisms in the vitamin D receptor gene are associated with muscle strength in men and women. Osteoporos Int. 2007;18:1235–42. doi: 10.1007/s00198-007-0374-4. [DOI] [PubMed] [Google Scholar]
- 189.Wolf JB, Pomp D, Eisen EJ, Cheverud JM, Leamy LJ. The contribution of epistatic pleiotropy to the genetic architecture of covariation among polygenic traits in mice. Evol Dev. 2006;8:468–76. doi: 10.1111/j.1525-142X.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- 190.Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104:1056–62. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]
- 191.Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, et al. Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem. 2005;96:1049–60. doi: 10.1002/jcb.20606. [DOI] [PubMed] [Google Scholar]
- 192.Xiong DH, Shen H, Zhao LJ, Xiao P, Yang TL, Guo Y, et al. Robust and comprehensive analysis of 20 osteoporosis candidate genes by very high-density single-nucleotide polymorphism screen among 405 white nuclear families identified significant association and gene–gene interaction. J Bone Miner Res. 2006;21:1678–95. doi: 10.1359/JBMR.060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yasui A, Nishizawa H, Okuno Y, Morita K, Kobayashi H, Kawai K, et al. Foxo1 represses expression of musclin, a skeletal muscle-derived secretory factor. Biochem Biophys Res Commun. 2007;364:358–65. doi: 10.1016/j.bbrc.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 194.Yates LB, Karasik D, Beck TJ, Cupples LA, Kiel DP. Hip structural geometry in old and old–old age: similarities and differences between men and women. Bone. 2007;41:722–32. doi: 10.1016/j.bone.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang ZL, He JW, Qin YJ, Hu YQ, Li M, Zhang H, et al. Association between myostatin gene polymorphisms and peak BMD variation in Chinese nuclear families. Osteoporos Int. 2008;19:39–47. doi: 10.1007/s00198-007-0435-8. [DOI] [PubMed] [Google Scholar]
- 196.Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, et al. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci U S A. 2006;103:19004–9. doi: 10.1073/pnas.0605170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zumwalt A. The effect of endurance exercise on the morphology of muscle attachment sites. J Exp Biol. 2006;209:444–54. doi: 10.1242/jeb.02028. [DOI] [PubMed] [Google Scholar]


