Abstract
Epilepsy is a common central nervous system (CNS) disease characterized by recurrent transient neurological events occurring due to abnormally excessive or synchronous neuronal activity in the brain. The CNS is affected by systemic acid–base disorders, and epileptic seizures are sensitive indicators of underlying imbalances in cellular pH regulation. Na+/H+ exchangers (NHEs) are a family of membrane transporter proteins actively involved in regulating intracellular and organellar pH by extruding H+ in exchange for Na+ influx. Altering NHE function significantly influences neuronal excitability and plays a role in epilepsy. This review gives an overview of pH regulatory mechanisms in the brain with a special focus on the NHE family and the relationship between epilepsy and dysfunction of NHE isoforms. We first discuss how cells translocate acids and bases across the membrane and establish pH homeostasis as a result of the concerted effort of enzymes and ion transporters. We focus on the specific roles of the NHE family by detailing how the loss of NHE1 in two NHE mutant mice results in enhanced neuronal excitability in these animals. Furthermore, we highlight new findings on the link between mutations of NHE6 and NHE9 and developmental brain disorders including epilepsy, autism, and attention deficit hyperactivity disorder (ADHD). These studies demonstrate the importance of NHE proteins in maintaining H+ homeostasis and their intricate roles in the regulation of neuronal function. A better understanding of the mechanisms underlying NHE1, 6, and 9 dysfunctions in epilepsy formation may advance the development of new epilepsy treatment strategies.
Keywords: ADHD, Autism, Excitability, NHE1, NHE6, NHE9
1. Introduction
Epilepsy is a common central nervous system disease in which a sudden, abnormal discharge of brain neurons results in seizure activity, which can appear as a wide range of neurological events including impaired awareness, sensations of déjà vu or generalized tonic-clonic jerking. This disease can result in life-long disabilities and serious adverse effects on the physical, mental, and socioeconomic status of patients and society (Kerr, 2012). The prevalence of epilepsy is 0.6% of the population, resulting in an estimated $9.6 billion in medical expenses in the United States alone (Yoon et al., 2009). Approximately 60% of cases are idiopathic while the remaining cases are secondary to some form of brain insult, including stroke, tumors, infections, traumatic brain injuries, or underlying genetic conditions (Forsgren et al., 2005; WHO, 2015).
Epilepsy is a heterogeneous collection of seizure disorders that result from different underlying defects in neuronal membrane excitability and synaptic function (Staley, 2015). These phenotypic variances can be influenced by dysregulation of ion channels (Armijo et al., 2005), neurotransmitter systems (Masino et al., 2014; Rowley et al., 2012), neuropeptides (Kovac and Walker, 2013), hormones (Tauboll et al., 2015), ion transporters (Cox et al., 1997; Kahle et al., 2008), calcium-activated kinases (Butler et al., 1995), and/or vesicle release proteins (Rosahl et al., 1995). Genetic testing of epilepsy patients can help determine which brain functions have been compromised, allowing a more targeted treatment plan to be prescribed (El Achkar et al., 2015; Olson et al., 2014).
The currently available epilepsy treatments target a wide range of molecular substrates, reflective of the heterogeneous origins of epileptic seizures. Initial treatments for emergent seizures are typically benzodiazepines, such as diazepam and lorazepam, which enhance the effects of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) (Rogalski and Rogalski, 2015). Once a specific diagnosis of the cause of the seizure is determined, or if seizures become refractive to benzodiazepines, additional treatments targeting the GABAergic system may be applied, including GABA agonists, structural analogs, reuptake inhibitors, and inhibitors of GABA degrading enzymes (reviewed in Greenfield, 2013). Other classes of antiepileptic drugs (AEDs), such as channel blockers for Na+ (Catterall, 2014), glutamate (Gupta et al., 2014; Steinhoff, 2015), and Ca2+ (Goren and Onat, 2007), can be added to treatment regimens to successfully manage seizures. Levetiracetam is being increasingly used due to its low probability of pharmaco resistance and limited side effects. This drug minimizes seizures by several molecular mechanisms revolving around Ca2+ regulation, including decreasing [Ca2+]i and preventing Ca2+ from binding to synaptic vesicle protein 2, thus impairing neurotransmitter release (Deshpande and Delorenzo, 2014). Overall, up to two-thirds of newly diagnosed patients who receive appropriate drug treatments become seizure-free, highlighting the importance of therapeutic interventions for the recovery of a normal quality of life (Kwan and Brodie, 2000). However, approximately 30% of patients do not respond to any available treatments, which illustrates an urgent need for novel therapeutic interventions focused on new molecular targets.
2. Systemic acid–base disorders and seizures
The CNS is a tightly regulated milieu that can be perturbed by alteration in peripheral conditions, such as pH-altering systemic acid–base disorders. A wide variety of neuronal signaling mechanisms are pH-dependent, including membrane voltage – and ligand – gated ion channels, transmitter uptake through transporters, intracellular signal transduction, and intercellular communication via gap junctions (Takahashi and Copenhagen, 1996). Accordingly, the alteration of pH can lead to a great diversity of changes in nerve cell function, including synchronization of discharges, resulting in epilepsy (Obara et al., 2008). Many mice that exhibit spontaneous seizure activity and aberrant gates were found to have mutations in pH-sensitive voltage-gated Ca2+ channel subunits, giving rise to the “Stargazer”, “Ducky”, “Lethargic”, “Leaner” and “Tottering” mutant mouse lines used in epilepsy research today (Grone and Baraban, 2015; Letts, 2005; Tombaugh and Somjen, 1997). However, one of the strongest intrinsic mechanisms for the modulation of neuronal excitability in the brain is pH regulation. Shifts toward an extracellular alkaline pH generally enhance neuronal excitability, while acidic extracellular environments encourage neuronal depression (Ruusuvuori and Kaila, 2014). These pH shifts can vary in scale, but large, life-threatening shifts can result from systemic acid–base dysregulation, such as during renal or hepatic failure, which are often accompanied by epileptic seizures (Lacerda et al., 2006). Transient pH shifts routinely occur in the microdomains surrounding bursting neurons, creating a pH differential between the intracellular and extracellular compartments (de Curtis et al., 1998; Dulla et al., 2009; Makani and Chesler, 2010; Ruusuvuori and Kaila, 2014). These pH differentials play important roles in the modulation of neuronal excitability, which will now be discussed in more detail.
3. The influence of pH on neuronal excitability
In general, neuronal activity is associated with localized fluctuations between acidic and alkaline pHo, which act as feedback mechanisms to regulate firing activity by modulating neuronal excitability. These shifts are usually the result of movement of acid–base equivalents across the cell membrane such that a shift toward an alkaline pHo is accompanied by an intracellular acidification and vice versa (Sinning and Hubner, 2013). These H+ shifts affect neuronal excitability through a variety of mechanisms, including modification of receptor and ion channel properties, enzymatic activity, and protein bonds. Although our knowledge is far from complete, experiments over the past decades have illuminated some mechanistic aspects of how pH alters neuronal excitability.
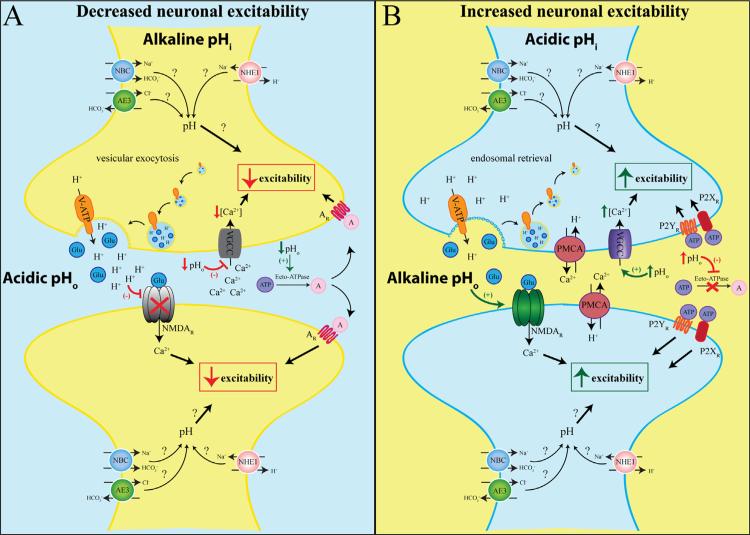
During an action potential, H+ ions that were co-packaged with neurotransmitters by the H+-VATPase are released and induce a rapid but brief (~10 ms) acidification of the synaptic cleft. Transient incorporation of the V-ATPase into the cell membrane during vesicle fusion further acidifies the extracellular space (Zhang et al., 2010). This H+ accumulation can then alter the function of other synaptically localized proteins. For example, presynaptic voltage-gated calcium channels (VGCCs) mediate the Ca2+ influx upon neuronal depolarization that induces vesicular release of neurotransmitters. Several studies have shown that these channels are pH sensitive, reducing their conductance and shifting their opening to voltage to more positive values when pHo is acidic (Chen et al., 1996; Krafte and Kass, 1988). Thus, extracellular H+ dampen presynaptic Ca2+ accumulation thereby acting as a negative feedback mechanism when successful vesicular release has occurred (DeVries, 2001) and Fig. 1. This brief synaptic acidification also affects the post-synapse function. NMDA receptors are highly sensitive to pH (Traynelis and Cull-Candy, 1990). Acidic pHo converts NMDA receptors to an inactivated form with decreased receptor opening frequency while alkaline environments enhance receptor function (Tang et al., 1990; Traynelis and Cull-Candy, 1990). This feature may serve to limit excessive activation during normal brain activity (Fig. 1), but may also underlie the excessive NMDA receptor activation seen in seizure models resulting from the alkaline pHo (Velisek et al., 1994).
Fig. 1.
The influence of pH on neuronal excitability. When extracellular pH (pHo) is acidic and intracellular pH (pHi) is alkaline, neuronal excitability is decreased. Upon neuronal stimulation, vesicular release of neurotransmitters, such as glutamate (Glu, blue), is accompanied by the release of co-packaged H+. Transient incorporation of the V-ATPase into the plasma membrane induces further expulsion of H+ from the cytosol into the synaptic cleft and causes acidic pHo. The extracellular H+ blocks the voltage-gated Ca2+ channel (VGCC) and reduces presynaptic Ca2+ accumulation, thus inhibiting additional neurotransmitter release and neuronal excitability. Furthermore, acidic pHo also reduces the opening frequency of postsynaptic NMDA receptors (NMDAR), thus decreasing postsynaptic excitability. Extracellular ATP (purple) is broken down into adenosine (pink) driven by ecto-ATPase which is stimulated by low pHo. Adenosine can bind and activate adenosine receptors (AR) and further decrease neuronal excitability. Neuronal excitability is increased when pHi is acidic and pHo is alkaline. The V-ATPase is retrieved from the membrane into early recycling endosomes after neuronal firing, reducing H+ extrusion and elevating pHo. In response to accumulated intracellular Ca2+, the pre- and postsynapse plasma membrane Ca2+/H+ ATPases (PMCA) expel Ca2+ and import H+, further elevating pHo. The alkaline pHo enhances NMDAR activity, facilitating postsynaptic depolarization. Furthermore, alkaline pHo inhibits ecto-ATPases and prevents ATP degradation. The increased availability of ATP activates P2X and P2Y receptors, initiating cascades that could enhance neuronal excitability. Many other pH regulatory players discussed in Section 4 (NHEs, AEs, NBCs) may also contribute to neuronal excitability.
ATP and adenosine can also regulate neuronal excitability in a pH-dependent manner. ATP binds to the purinergic P2X and P2Y receptors which often result in increased neuronal excitability (Abbracchio et al., 2009). In the extracellular space, ATP is rapidly broken down into adenosine and activates adenosine receptors, which largely inhibits excitatory neurotransmission (Wei et al., 2011). Extracellular acidification as a result of increased CO2 levels has been observed to increase adenosine availability, thereby reducing excitatory synaptic transmission (Gourine et al., 2005). Conversely, extracellular alkalization inhibits the enzyme ecto-ATPase, which converts ATP into adenosine, resulting in decreased availability of adenosine. This results in reduced activation of adenosine receptors while abundant ATP activates P2X and P2Y receptors culminating in enhanced synaptic transmission (Dulla et al., 2005) (Fig. 1). Intracellular acidification caused by epileptic-like bursting, but not normal brain activity, has also been shown to increase adenosine release (Dulla et al., 2005, 2009). This may be a protective mechanism to help reduce neuronal excitability and halt seizure activity during pathological conditions.
The brief period of acidification after vesicular release is reversed through the actions of another set of pH-sensitive proteins (Fig. 1). Once the presynaptic terminal has been flooded with Ca2+ to induce vesicular release, presynaptic plasma membrane Ca2+/H+ ATPases (PMCAs) reestablish resting Ca2+ levels by importing H+ and extruding Ca2+, thus beginning the intracellular re-acidification (Thomas, 2009). Large Ca2+ influxes are also seen in the postsynaptic spine after NMDA receptor activation. PMCAs on the postsynapse induce a highly localized extracellular alkalinization in the vicinity of NMDA receptors, enhancing depolarization (Chen and Chesler, 2015). Extracellular acidification by membrane inserted V-ATPase is reversed as early recycling endosomes retrieve the V-ATPases from plasma membrane, reducing the number of H+ being extruding thus allowing a return to an alkaline extracellular pH (Zhang et al., 2010). Furthermore, membrane bound and extracellular carbonic anhydrases, particularly CA4 and CA14, help to buffer the alkaline shift. However, this process is relative slow compared to the speed of synaptic transmission and the relative contributions of regional isoforms require further clarification (Shah et al., 2005; Tong et al., 2006).
Many other proteins discussed as pH regulators in Section 4 likely have roles in the cyclical pH fluctuations at the synapses, however, the direct relation between most of these proteins and neuronal excitability has not been clearly deduced. A summary of known and potential mechanisms for pH regulation at the synapse are presented in Fig. 1.
4. Acid–base regulatory mechanisms
pH regulation is a concerted balancing effort by the lungs, kidneys, and various ion transporter systems including sodium-bicarbonate transporters, chloride-bicarbonate exchangers, and sodium-proton exchangers. These transporters are expressed in different tissue types with varying subcellular localizations to regulate pH in specific microdomains. The dysregulation of these pH regulatory systems is associated with numerous pathologies which have been thoroughly reviewed previously (Supuran, 2008). Notably, pH regulation plays a prominent role in the development of seizure disorders and intellectual disabilities. Broad overviews of pH regulatory mechanisms are available elsewhere (Casey et al., 2010), so we will focus on the regulation of pH in the brain.
Cellular pH is perpetually fluctuating as a result of cellular activity, therefore, pH regulation is a continuously ongoing process. Because cells maintain a negative resting membrane potential, protons tend to be driven inside the cell from the extracellular space. Furthermore, acidic by-products are generated within the cell by metabolic reactions, such as ATP from glycolysis. Together, these factors result in a slightly acidic intracellular space (pHi ~7.1–7.2). In order to maintain optimal pHi and prevent toxic levels of acidity, H+ ions need to be extruded into the extracellular space, which is kept at pHo ~ 7.3– 7.4. This balance is imperative to the proper regulation of protein structures, protein interactions, enzymatic activity, and transmembrane ion gradients which allow the cell to function optimally (Whitten et al., 2005).
The brain presents with an additional layer of pH regulatory complexity owing to its frequent electrical activity. Rapid pH fluctuations occur in response to neuronal activity and alter neuronal function in accordance with pH feedback mechanisms (Chesler, 2003; Kaila and Ransom, 1998; Ruffin et al., 2014), as discusses in Section 3. Thus, pH fluctuations need to be tightly regulated in order to maintain signaling fidelity and prevent epileptic activity. Numerous mechanisms exist within cells to import or extrude acid–base equivalents. Many of these have been evolutionarily conserved, highlighting their critical role in cell viability (Brett et al., 2005a; Parker and Boron, 2013). We will now provide a short overview of key pH regulatory mechanisms present in the brain.
4.1. Sodium-bicarbonate transporters
Sodium coupled-bicarbonate transporters (NBCs) are part of the Slc4 gene family. Five mammalian NBCs move Na+ and HCO3– unidirectionally through electroneutral (NBCn1, NDCBE, and NBCn2) or electrogenic (NBCe1 and NBCe2) isoforms with varying stoichiometries (Parker and Boron, 2013). Expression of many Slc4 family members in mammalian tissues has been thoroughly characterized previously (Damkier et al., 2007; Majumdar and Bevensee, 2010) and these findings will only be briefly summarized here. All five NBCs are expressed in the brain. NBCe1 (Slc4a4) is abundantly expressed in both neurons and glia, with highest expression in the hippocampus, cerebellum, piriform cortex, and olfactory bulb. Three NBCe1 splice variants are known, although only NBCe1-B and NBCe1-C appear in the brain (Majumdar et al., 2008). NBCe2 is expressed in the apical membrane of choroid plexus endothelial cells, and at least at the mRNA level in the hippocampus, cerebrum, and cerebellum. NBCn1 (Slc4a7) is expressed in the cortex, cerebellum, choroid plexus, and several regions within the hippocampus, including neurons in the pyramidal layer and dentate gyrus as well as in postsynaptic densities. NDCBE1 (Slc4a8) transports Cl– in addition to Na+ and HCO3– and is expressed in hippocampal pyramidal neurons and in Purkinje cell and dendrites within the molecular layer of the cerebellum. NBCn2 (Slc4a10), also known as NCBE, appears in the choroid plexus, hippocampus, cortex, and to a lesser degree, the cerebellum (Majumdar and Bevensee, 2010).
NBCs generally act as acid extruders, although alterations in membrane potential and ion gradients can evoke acid loading activity (Majumdar and Bevensee, 2010). Extrusion of acid equivalents serves to reduce the firing threshold of neurons by alkalizing the intracellular compartment. Additionally, activity of electrogenic NBCs in astrocytes can modulate the pH of the synaptic microenvironment, adding an additional layer of control over neuronal excitability. Neuronal action potentials lead to an accumulation of extracellular K+, which in turn depolarizes perisynaptic astrocytes to activate astrocytic NBC activity, termed depolarization-induced alkalization (DIA). The influx of Na+ and HCO3– into astrocytes acidifies the synaptic environment, thus hindering neuronal excitability mediated by pH-sensitive channels (reviewed in Chesler, 2003).
To further study the roles of NBCs in pH regulation, transgenic knockout (KO) mice have been developed. Hippocampal neurons from Slc4a10/NBCn2 KO mice did not differ from wildtypes in baseline pH levels, however, KO pyramidal neurons took longer to recover from experimentally induced intracellular acidification, demonstrating a clear role of NBCn2 in pHi regulation (Jacobs et al., 2008). Accordingly, these mice showed increased resistance to seizure induction as the proconvulsant pentylenetetrazole (PTZ) took nearly twice as long to induce seizures in NBCn2 KO mice. Furthermore, while half of the wildtype mice died as a result of PTZ or pilocarpine-induced seizures, all NBCn2 KO mice successfully recovered (Jacobs et al., 2008). NBCe1 null mice have also been generated, although they die before weaning due to complications from metabolic acidosis, thus precluding any detailed study of neurological function and seizure susceptibility (Gawenis et al., 2007). However, a role for NBCe1 in seizures can be inferred from other animal studies. Gerbils separated into “seizure-resistant” and “seizure sensitive” groups based on their response to seizure inducing stimuli showed no difference in NBCe1 expression prior to seizure induction. However, beginning 30 minutes after seizure initiation, seizure sensitive gerbils exhibited a progressive increase in NBCe1 expression in the hippocampal CA1-3 and dentate gyrus which returned to baseline 6 hour postictal (Kang et al., 2002). This increased NBC expression after seizure induction may serve to acidify the extracellular space to reduce excitability and inhibit further seizure propagation.
It has been shown that alterations in human NBC function are associated with a range of intellectual disabilities. Reduced membrane expression and decreased NBC transporter activity have been characterized as a result of SLC4A4 mutations initially identified in humans. Some of these patients display signs of mental retardation, developmental disorders, and frequent migraines, while others are free of neurological symptoms (Deda et al., 2001; Liu et al., 2012; Majumdar and Bevensee, 2010; Suzuki et al., 2010). Comparative genomic hybridization identified a spontaneous deletion of exon 1 in SLC4A10/NBCn2 in monozygotic twins with autism (Sebat et al., 2007). The varied symptomology resulting from NBC mutations points to a complex role of the NBC family in brain development and function with possible functional compensation by other NBC isoforms or other acid–base transporters, such as NHE1 (Damkier et al., 2009).
4.2. Chloride-bicarbonate exchangers
In addition to the sodium-dependent bicarbonate transporters, the Slc4 gene superfamily also contains 4 Na+-independent, Cl–-dependent HCO3– anion exchangers (AE1-AE4). AEs are transmembrane proteins which generally act as acid loaders, extruding HCO3– in exchange for Cl– influx. AE4 (Slc4a9) is not yet fully characterized. Initial cloning of the gene demonstrated Cl– transport function of the protein, although phylogenetically this isoform is much more closely related to the Na+-coupled HCO3– transporters (Alka and Casey, 2014; Ko et al., 2002). Recent evidence suggests that AE4 functions as a Na+-dependent Cl–/HCO3– transporter in salivary glands, making AE4 distinct in its transport profile (Pena-Munzenmayer et al., 2015). AE3 is the primary Cl–/HCO3– exchanger in the brain, although AE2 is abundant in the choroid plexus (Hentschke et al., 2006; Romero et al., 2013). A brain-enriched transcriptional variant of AE3, known as AE3fl (full-length) or bAE3, is longer than the cardiac-enriched variant (cAE3) and contains residues targeted by PKC, allowing for more fine-tuned modulation of channel behavior (Alvarez et al., 2001). The cytoplasmic N-terminals of AE2 and AE3, contain pH-sensing domains to more tightly regulate their activity and maintain physiological pH (Zhang et al., 1996).
Mutations in AE3 have been associated with increased susceptibility to seizures. The Ala867Asp variant of the SLC4A3 gene was identified in a large number of patients with idiopathic generalized epilepsy (Sander et al., 2002). HEK cells expressing this mutant form of AE3 showed a ~50% reduction in exchange activity, although how this results in increased excitability in neurons remains unclear (Vilas et al., 2009). AE3 KO mice do not exhibit spontaneous seizures, but do have a lower seizure induction threshold than wildtype mice (Hentschke et al., 2006). Furthermore, AE3 KO mice are more likely to die after seizure induction. Although it is clear AE3 plays an important role in pH regulation further work is needed to clarify its contributions to epileptic activity under different conditions.
4.3. Carbonic anhydrases
CAs catalyze the hydration of CO2 into H+ and bicarbonate (HCO3–), mediating one of the most important pH buffering mechanisms in the brain. Thirteen enzymatically active mammalian carbonic anhydrase (CA) isoforms are expressed as soluble, secreted, mitochondrial, or membrane-associated zinc metalloenzymes (Ruusuvuori and Kaila, 2014; Supuran, 2008). Three additional isoforms (XIII, X, and XIV) lack the zinc-binding active site, and are therefore catalytically inactive (Aggarwal et al., 2013). CAs are expressed in both neurons and glia, although most of their pH buffering properties are associated with their function in astrocytes, due in large part to the involvement of astrocytes in energy metabolism (Deitmer, 2001; Deitmer and Rose, 1996). The presence of CAs in multiple cell types indicates the requirement not only of coordination between various transporter systems but also between cell types to maintain proper pH regulation.
CA function in the brain is not limited to their enzymatic activity. CAII, which is abundantly expressed in astrocytes, can directly bind to monocarboxylate transporter 1 (MCT1) and regulate glial pHi by enhancing lactate/H+ cotransport, independent of its catalytic function (Agnati et al., 1995; Stridh et al., 2012). Indeed, CAII has been shown to enhance the function of other pH regulatory ion channels including Na+/H+ exchangers, Na+/HCO3– transporters, Cl–/HCO3– transporters, as well as other MCT family members either through direct binding or catalytic activity (Klier et al., 2014; Li et al., 2002; McMurtrie et al., 2004; Schueler et al., 2011). Mice with mutations in CAVIII, a catalytically inactive CA, show robust neurological alterations including functional defects of excitatory synapses in the cerebellum and an ataxic wobbly gate (Hirasawa et al., 2007).
Several experimental models and transgenic mice have identified roles for CAs in seizure pathology. CA II null mice are more resistant to flurothyl-induced seizures than their wildtype litter mates (Velisek et al., 1993). Kainic acid-induced seizures increased CAII expression in the hippocampal CA1 region and CAXII expression in layer 1 of the cortex, suggesting these isoforms may also be pro-epileptogenic (Halmi et al., 2006). Similarly, while wild type animals uniformly experience electrographic seizures in response to hyperthermia, CAVII KO mice show less severe or frequently absent febrile seizures under the same conditions (Ruusuvuori et al., 2013). CAVII KO mice exhibited comparable levels of increased body temperatures and respiratory alkalosis as compared to the wildtype animals, indicating seizure prevention may not be directly mediated through pH regulation. Rather, CAVII likely facilitates seizure induction through the generation of HCO3– and efflux of HCO3– via GABAA receptors, which drives excitatory GABAergic depolarization (Ruusuvuori et al., 2013).
CA activity is strongly linked to seizure activity through its regulation of GABAergic transmission. Activated GABAA receptors (GABAARs) bring Cl– into the cell and HCO3– out of the cell, effectively acting as acid loaders. GABA released by interneurons can bind to postsynaptic receptors on principle neurons to elicit Cl– influx, thus hyperpolarizing the neurons and preventing action potentials. However, repeated activation of GABAARs results in neuronal depolarization, enhancing seizure activity. This is because repeated GABAA stimulation leads to a massive Cl− influx, which needs to be removed from the cell through the neuronal outward K+/Cl– cotransporter KCC2. The subsequent rise in [K+]o in combination with the locally increased pHo from HCO3– extrusion from the GABAAR stimulates neuronal depolarization, leading to bursting activity (Viitanen et al., 2010). This phenomenon is contingent upon the activity of CAs as HCO3– generation determines the GABAAR reversal potential and is required to fuel GABAAR receptor activity which drive neuronal depolarization (Ruusuvuori et al., 2004). Accordingly, inhibition of CA activity with antiepileptic drug acetazolomide in slice cultures prevents epileptiform discharges by reducing the availability of HCO3– (Hamidi and Avoli, 2015). Extensive details of these paradoxical actions of GABA and seizure activity are available in other excellent reviews (Avoli and de Curtis, 2011; Blaesse et al., 2009).
Clearly, GABA has an exceedingly complex role in epileptiform brain activity, preventing seizures in some instances while enhancing discharge synchronicity to induce and maintain seizures in others (Avoli and de Curtis, 2011). Similarly, the role of GABA receptors in pH regulation is also multifaceted. Ionotropic GABAA receptor activation itself can acidify the intracellular compartment through its extrusion of HCO3– (Kaila et al., 1992; Kaila and Voipio, 1987; Luckermann et al., 1997). Activation of metabotropic GABAB receptors has been shown to increase expression levels of acid–base transporters including NHE1 and NBCe1 (Kang et al., 2003). NHE1 is a crucial acid extruder with prominent roles in epilepsy and developmental disorders and will thus be discussed in detail in Section 9.
4.4. Sodium-proton exchangers
NHEs catalyze the electroneutral exchange of one extracellular Na+ ion for one intracellular H+ that leads to a higher intracellular pH (pHi) and lower extracellular pH (pHo) (Jean et al., 1985). Mice lacking NHE isoform 1 (NHE1) experience seizures beginning at 2–3 weeks of age and usually die prior to weaning (Bell et al., 1999; Cox et al., 1997). Interestingly, NHE1 null mice show an increased expression of voltage-sensitive Na+ channels, indicating a complex pathway for the role for NHEs on the regulation of neuronal excitability and seizure induction (Gu et al., 2001; Xia et al., 2003). In addition, recent studies reveal a link between dysfunction of NHE isoform 6 (NHE6) in cellular organelles and several developmental brain disorders, with epilepsy as a common feature (Kondapalli et al., 2014). In subsequent sections of this review, we will summarize the functions of the NHE family and specifically discuss findings of NHE1, NHE6, and NHE9 in the pathogenesis of epilepsy.
4.5. Respiratory regulation
While ion transporters and enzymes work to regulate pH in niche environments, system-level pH is largely regulated by respiration. As blood passes through the lungs, large quantities of CO2 are removed and released through expiration thus controlling the partial pressure of CO2 (pCO2). Systemic pH and CO2 levels are monitored by chemosensitive neurons within in the brain which can then adjust respiratory rate to regulate CO2 efflux and ensure pH homeostasis (Putnam et al., 2004). The efflux of CO2 results in an overall increase in pH and neuronal excitability. Conversely, a reduced breathing rate will decrease pH, leading to an acidic environment and decreased excitability. Indeed, respiratory alkalosis is frequently present in children with febrile seizures (Schuchmann et al., 2006, 2011).
Regulation of breathing rate is frequently used in the study of seizure disorders. Asking patients to hyperventilate is an effective and non-invasive method to create an alkaline pH and precipitate seizure activity in experimental settings (Guaranha et al., 2005). Conversely, actively inducing an acidic extracellular environment has been proposed as a potential treatment for seizure disorders. This can be achieved by asking patients to hold their breath or through inhalation of CO2. Self-acidification by the inhalation of 5% CO2 was shown to reduce seizure duration in both animal seizure models and human epilepsy patients (Tolner et al., 2011; Yang et al., 2014). Interestingly, neonatal asphyxia is associated with seizure development during the hypoxia recovery period. This is attributed to an overshoot in the pH balancing mechanisms in which H+ are rapidly extruded across the blood–brain barrier by sodium-proton exchangers, thus inducing a sustained alkaline pH exclusively in the brain. Administration of an NHE blocker in a rodent model of neonatal asphyxia ameliorated seizures by balancing pH during the recovery period (Helmy et al., 2012). Several other drugs targeting pH regulatory mechanisms have been developed to prevent and treat seizures which will be discussed below.
5. NHE family and specific functions
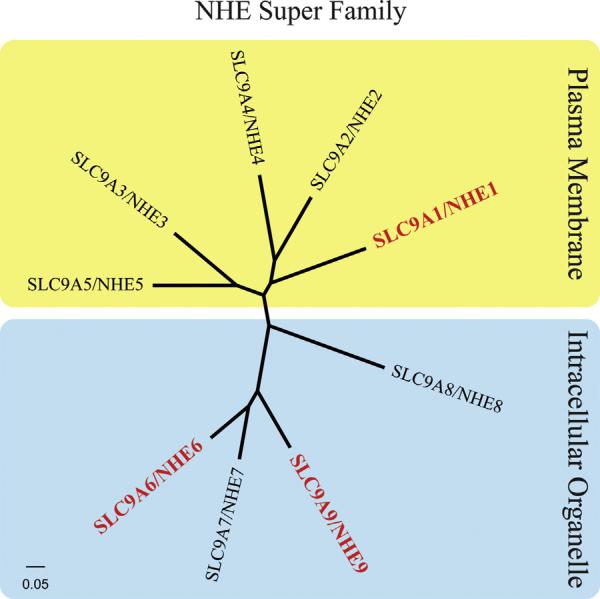
NHEs are ubiquitously expressed in prokaryotes and lower and higher eukaryotes (Girardi and Di Sole, 2012). The NHE family consists of 9 members (NHE1-9) and a cluster of distantly NHE-related genes, NHA1 and NHA2 (Orlowski and Grinstein, 2007; Kondapalli et al., 2014). NHEs have a broad tissue distribution in mammals, with NHE1 being the most widely expressed isoform (Xu et al., 2015). NHE family members have similar membrane topologies. Each has 12 N-terminal membrane-spanning domains involved in ion binding and Na+ and H+ cation translocation and a large C-terminal cytoplasmic domain which serves as a scaffold for intracellular signaling (Shi et al., 2013; Slepkov et al., 2007). The members of the mammalian NHE family can be broadly classified into plasma membrane clusters vs. intracellular organellar clusters (Fig. 2), depending on their cellular localization as described below.
Fig. 2.
NHE super family. The plasma membrane and intracellular organelle clusters of NHE isoforms are illustrated: NHE members 1–5 are located in the plasma membrane (yellow) and the NHE members 6–9 are located in the intracellular organelles (blue). Red font denotes the isoforms which have been discussed in detail in this review. Canonical sequence alignment was performed using ClustalX and the tree was constructed using FigTree (Thompson et al., 1997).
5.1. Localization of plasma membrane NHE family members
The NHE family members NHE1–NHE5 are primarily localized to the plasma membranes of diverse cell types, although NHE3 and NHE5 can also enter endosomal pools (Bobulescu et al., 2005; Orlowski and Grinstein, 2007). Among the plasma membrane cluster isoforms, NHE1 is distributed throughout the brain and the peripheral nervous system where it undertakes basic housekeeping functions, including regulating cytoplasmic pH and maintaining cell volume (Fuster and Alexander, 2014). NHE1 activity is a primary cellular alkalinizing mechanism, resulting from extrusion of H+ derived from metabolism or acidification (Fuster and Alexander, 2014). NHE1 also contributes to the Na+ influx into the cell, which is coupled to Cl– and H2O uptake. NHE1-mediated water influx restores cell volume following cell shrinkage, a process referred to as regulatory volume increase (Rotin and Grinstein, 1989).
Other plasma membrane isoforms, such as NHE2, NHE3 and NHE4, are primarily localized to the epithelia of peripheral tissues, but are also present in certain areas of the brain where they fulfill more specialized roles (Ma and Haddad, 1997). Table 1 has summarized all NHE isoforms, their function, their expression profile, and related pathophysiology in the CNS.
Table 1.
Na+/H+ family members.
| Protein name | Gene name | Localization | Tissue distribution | Mutation associated neurological disease phenotype | References |
|---|---|---|---|---|---|
| NHE1 | SLC9A1 | Plasma membrane | Ubiquitous | Epilepsy, ataxia, growth retardation | Bell et al. (1999) |
| NHE2 | SLC9A2 | Plasma membrane | Brain, gut, skeletal muscle, kidney, uterus, testis, heart, lung and stomach | No neurological phenotype reported | Fuster and Alexander (2014) |
| NHE3 | SLC9A3 | Plasma membrane | Gut, kidney, lung, heart, brain, stomach, gall bladder and epididymis Ventrolateral brainstem |
No neurological phenotype reported | Fuster and Alexander (2014), Orlowski and Grinstein (2004), Studer et al. (2014) |
| NHE4 | SLC9A4 | Plasma membrane | Gut, kidney, brain, stomach, skeletal muscle and uterus Hippocampus | No neurological phenotype reported | Bookstein et al. (1996), Pizzonia et al. (1998) |
| NHE5 | SLC9A5 | Plasma membrane | Brain, spleen, testis, and skeletal muscle | No neurological phenotype reported | Attaphitaya et al. (1999), Baird et al. (1999), Klanke et al. (1995) |
| NHE6 | SLC9A6 | Intracellular organelle | Ubiquitous | X-linked Angelman-like condition, Christianson syndrome, corticobasal degeneration with tau deposition, epilepsy, autistic behavior intellectual disability, ataxia | Christianson et al. (1999), Garbern et al. (2010), Gilfillan et al. (2008) |
| NHE7 | SLC9A7 | Intracellular organelle | Ubiquitous | No neurological phenotype reported | Fuster and Alexander (2014) |
| NHE8 | SLC9A8 | Intracellular organelle | Ubiquitous | No neurological phenotype reported | Fuster and Alexander (2014) |
| NHE9 | SLC9A9 | Intracellular organelle | Ubiquitous | Autism, epilepsy, addiction, and attention-deficit/hyperactivity disorder (ADHD) | Fisher et al. (2002), Morrow et al. (2008), Pescosolido et al. (2014), Schwede et al. (2014), Vink et al. (2009) |
5.2. Intracellular organelle NHE family members
The members of the second NHE group, NHE6–NHE9, are expressed in organelle membranes (Pescosolido et al., 2014). Within the NHE superfamily (Table 1), NHE6 and NHE9 are currently the only known organellar isoforms that cause neurological disorder in humans when mutated (Fuster and Alexander, 2014). Therefore, the following discussion focuses on these two intracellular organelle isoforms.
5.2.1. NHE6
The NHE6 protein product is encoded by SLC9A6, a portion of the SLC9 gene family located on chromosome band Xq26.3 (Orlowski and Grinstein, 2004). NHE6 primarily resides within early and recycling endosomes of the endocytic pathway, but is also a minor contributor to plasma membrane structure (Nakamura et al., 2005). The endosomal pathway has various functions critical to maintaining proper cellular activity. Components of the endocytic pathway experience a steady intracellular pH decrease as they progress from endosome to lysosome (from pH 6.3 in early endosomes to pH 5.5 in late endosomes to pH 4.6 in lysosomes) (Mellman, 1992). NHE6 works in concert with the vacuolar-type H+ ATPase (V-ATPase) to regulate organellar pH. By pumping H+ into the lumen of endosomes, the V-ATPase steadily generates a relatively acidic luminal pH, which can then be de-acidified by the outward leakage of H+ through NHE6 (Kondapalli et al., 2014; Nakamura et al., 2005). The precise stoichiometry of this exchange and the relative contributions of each protein to endosomal pH have not been quantified. Unlike the plasma membrane NHEs, organellar NHEs are able to pair H+ extrusion with the import of either Na+ or K+ (Ohgaki et al., 2011). In this manner, the NHE6 antiporter is able to regulate both pH and monovalent ion content of early and recycling endosomal compartments and can likely influence vesicular volume (Brett et al., 2002; Kondapalli et al., 2014).
As a primary constituent of early and recycling endosomes, NHE6-mediated H+ leakage is believed to play an integral role in maintaining the differential pH levels observed throughout the endocytic pathway (Nakamura et al., 2005). The NHE6-dependent regulation of acidification of intracellular compartments is essential for intracellular trafficking (Brett et al., 2005b), enzymatic protein processing (Prasad and Rao, 2015), establishment of cell polarity (Ohgaki et al., 2010), and clathrin-dependent endocytosis of molecules, including transferrin (Xinhan et al., 2011). Regulation of both the pH and monovalent ion content are crucial for the proper packaging of the appropriate neurotransmitters into vesicles (Futai et al., 2000). For example, membrane potential regulates the packaging of glutamate (Moriyama et al., 1990) whereas pH value dictates the transport of monoamines and GABA (Schuldiner et al., 1995). It has been proposed that the presence of NHE6 along the endocytic pathway serves to reverse the luminal pH reduction induced by the V-ATPase and consequently allow the V-ATPase to increase the membrane potential required for the repeated influx of neurotransmitters into vesicles (Hnasko and Edwards, 2012). However, this is a speculative role for NHE6, and its direct role in neurotransmitter packaging has yet to be established.
Under normal conditions, the slightly acidic internal environment of early endosomes promotes the dissociation of receptor–ligand complexes, subsequently facilitating receptor recycling (Mellman, 1992). Synaptic function requires proper targeting and recycling of transporters and receptors from the cell surface, thus, without proper receptor return, synaptic transmission and synaptic plasticity are compromised (Choquet and Triller, 2013; van der Sluijs and Hoogenraad, 2011). NHE6 distribution between the membrane and endosomes is regulated by NHE6s C-terminal interaction with receptor for activated C-kinase (RACK1). Disruption of this interaction alkalizes endosomal pH, reduces NHE6 expression at the plasma membrane, and disturbs transferrin uptake (Ohgaki et al., 2008). Thus, binding proteins that modulate NHE6 function also have important roles in the regulation of surface protein endocytosis and trafficking.
Loss-of-function mutations of NHE6 proteins have been shown to result in the hyperacidification of endosomal vesicles (Ohgaki et al., 2010; Xinhan et al., 2011). Without the ability to alkalize the lumen of intracellular compartments through the NHE6-mediated leaky H+ pathway, H+ is continuously transported into endosomes via the V-ATPase, resulting in overacidification of the endosome interior. Because synaptic transmission is dependent on tightly regulated receptor recycling and neurotransmitter packaging, loss of NHE6 may perturb this important process. For example, hyperacidification of vesicles due to NHE6 deletion has been shown to increase the rate of endosomal TrkB receptor degradation, causing a reduced efficacy of BDNF neurotrophic signaling.
Disrupted NHE6 function elicits a broad range of cellular complications arising from impaired regulation of vesicular pH and localization. Ultimately, these functional complications may lead to circuit defects that underlie the neurodevelopmental disorders associated with NHE6 mutation. Indeed, NHE6 mutant mice exhibit reduced levels of neuronal arborization, functional connectivity, and mature synapses, all of which are characteristic of intellectual disabilities (Ouyang et al., 2013; Verpelli et al., 2014). These neurological deficits will be explored in more detail in Section 6.
5.2.2. NHE9
As part of the NHE super family, NHE9 is encoded by SLC9A9 and participates in the regulation of organellar pH and volume via cation/proton antiport (Kondapalli et al., 2013). According to primary protein structural analysis, NHE9 has high homology with NHE6 with 55% similarity among the amino acid sequences (Nakamura et al., 2005). NHE9 appears to perform similar functions to NHE6 by balancing the activity of the V-ATPase, but within different components of the endocytic pathway. NHE9 is primarily localized to late recycling endosomes, but does show a small degree of colocalization with NHE6 in early endosomes and at the plasma membrane (Nakamura et al., 2005).
As with NHE6, overexpression of NHE9 results in vesicular alkalization while deletion of NHE9, in conjunction with NHE6, enhances endosomal acidification (Kondapalli et al., 2013; Nakamura et al., 2005; Roxrud et al., 2009). Gene dosing experiments in astrocytes indicate that NHE9 levels regulate surface expression of the potent glutamate transporter GLAST and thus affect glutamate clearance from the synaptic cleft (Kondapalli et al., 2013). Autism-associated NHE9 protein variants were less efficient at clearing extrasynaptic glutamate, which may underlie autistic patients’ intellectual disabilities and predisposition to epilepsy (Kondapalli et al., 2013).
NHE9 also interacts with various other molecules and receptors to promote signaling pathways between local networks of neurons within the hippocampus and cortex (Kondapalli et al., 2014; Zhang-James et al., 2011). For example, similar to NHE6, NHE9 has a binding region for RACK1 (Ohgaki et al., 2008). RACK1 knockdown results in hyperacidification of endosomal lumens but has no effect on the total amount of NHE9 protein. This leads to the hypothesis that RACK1 may play an important role in sequestering NHE9 proteins to the membranes of proper intracellular compartments in order to elicit essential regulatory activities for the endocytic pathway (Ohgaki et al., 2008). The roles of NHE9 mutation in epilepsy and other brain disorder will be discussed in Sections 7 and 8.
6. NHE1 and epilepsy
NHE1 is expressed in all mammalian cell types and is a primary regulator of intracellular pH (pHi) homeostasis and cell volume (Orlowski and Grinstein, 1997). The major function of NHE1 is to counteract excessive intracellular acidification and maintain the acid–base balance in the cytoplasm (Orlowski and Grinstein, 1997). In hippocampal and cortical neurons, NHE1 is mainly activated by a decrease in intracellular pH (Raley-Susman et al., 1991). When intracellular acidosis occurs, NHE1 activation is indispensable for the extrusion of H+ to restore physiological pHi. Additionally, the import of Na+ by NHE1 alters the intracellular milieu, triggering the activation of several other transporters. Increased [Na+]i stimulates the activity of the Na+/K+ ATPase, which extrudes 3 Na+ and imports 2 K+ at the cost of 1 molecule of ATP per exchange. A high [Na+]i will also stimulate the Na+/Ca2+, thus increasing the intracellular Ca2+, leading to a variety of downstream molecular changes (Masereel et al., 2003). In this manner NHE1 can alter cellular function both by the regulation of pH and stimulation of intracellular signaling cascades.
Mouse models provide complementary tools for identifying disease-associated genes and defining the mechanisms underlying human epilepsy. The specific molecular pathways detailing how mutations in NHE1 can lead to epilepsy remain to be fully elucidated, although abundant evidence suggests it is an important contributor. Despite its ubiquitous expression, loss of NHE1 function produces a predominantly neurological phenotype (Lukashova et al., 2013). Many studies indicate that an NHE1 null mutation (knockout, KO) leads to spontaneous slow wave epilepsy (swe) in mice (Cox et al., 1997). Loss of NHE1 increases excitability of the CNS, which contributes to the epilepsy phenotype. Bell et al. generated a traditional NHE1 KO mouse line (Bell et al., 1999). Both the NHE1 KO mutant and the swe mutant mice show an ataxic gait first evident at 2 weeks of age. There is a high excitability in neonates, followed by a brief period of total behavioral arrest. Just prior to weaning, mutants exhibit an increased rate of mortality and with a postmortem appearance suggestive of death by convulsive seizure (Bell et al., 1999). Next, we will discuss the specific individual characteristics of each mouse model.
6.1. NHE1 KO mice
Severe neurological events that appear to be seizures (which usually result in death) are one of the neurological abnormalities characterizing NHE1 KO mice (Bell et al., 1999). NHE1 KO mice exhibit a decreased rate of postnatal growth and high mortality with only ~10% of mice surviving 5 weeks after birth (Bell et al., 1999). Cortical astrocytes isolated from NHE1 KO mice show decreased steady state pHi and attenuated pHi recovery from acidification, even in the presence of HCO3– (Kintner et al., 2004). Electrophysiological recordings in hippocampal neurons from NHE1 KO mice revealed and increased excitability and higher Na+ channel subtype I current density (Gu et al., 2001). This increased current density was the result of increased Na+ channel subtype I expression in the hippocampus and Na+ channel subtype II expression in the cortex (Xia et al., 2003). Therefore, the expression and activity of membrane transport proteins in the brain are changed in NHE1 KO mice, which can result in increased neuronal excitability or pHi changes.
6.2. SWE mice
In 1997, Cox et al. characterized slow-wave epilepsy (swe) mice. These mice exhibit CNS-restricted features including locomotor ataxia and a novel seizure phenotype similar to common human epilepsies. Electrocorticographic (ECoG) recordings show that the homozygous swe mutants show frequent bursts of 3/sec generalized spike-wave activity and behavioral arrest. These seizures are found in younger but not older mice, are influenced by genetic background, and are accompanied by infrequent tonic-clonic seizures. This phenomenon is often observed in patients with childhood (25–46%) or juvenile onset (74–81%) generalized absence (petit mal) epilepsy. Interestingly, with high resolution genetic mapping and analysis of candidate genes, the defective gene in swe mice has been identified as the ubiquitously expressed SLC9A1 gene, coding for the protein NHE1 (Cox et al., 1997). Thus, NHE1 activity defects may leave certain neurons less able to regulate transient changes in pH and result in a reduced threshold for the initiation or propagation of synchronous epileptic discharges.
It is also possible that the crucial mechanisms of neuroexcitability in swe mice are related not only to dysfunctions of neurons, but also dysregulated function in the surrounding astrocytes (Cox et al., 1997). Glia regulate pHo to maintain ion homeostasis through a specific electrogenic Na+/HCO3– membrane cotransporter and NHE1 (Deitmer and Rose, 1996). Loss of NHE1 activity could reduce the effectiveness of glia in pHo homeostasis and enhance the neuroexcitability by mimicking chronic extracellular alkalosis. A similar extracellular alkalosis following hyperventilation (reducing pCO2 and increasing pHo) is often used to reliably activate absence seizures in children with petit mal epilepsy (Adams and Lueders, 1981).
Lastly, the NHE1 protein is expressed in different immune cells including monocytes, neutrophils, and macrophages/microglia. NHE1 activity is crucial for immune cell function by extruding H+ and regulating pHi during respiratory bursting (Shi et al., 2013). In the CNS, NHE1 function is required for full microglial (macrophage) activation and is involved in microglial phagocytosis (Shi et al., 2013). Microglia-neuron interactions play a crucial role in epilepsy by altering neural network excitability, which was associated with increased glutamatergic transmission through the potentiation of NMDA receptor mediated activity (Ferrini and De Koninck, 2013). Therefore, changes of NHE1 function in microglia may also affect hyper-excitability.
7. NHE6 and X-linked intellectual disabilities
Epilepsy and intellectual disability disorders exhibit a high level of comorbidity, suggesting similarities in their underlying molecular etiologies (Brooks-Kayal, 2010; Robertson et al., 2015). NHE6 is one of the most recurrently mutated loci in patients with X-chromosome linked intellectual disability (XLID) (Tarpey et al., 2009). SLC9A6 mutations have been reported to result in three phenotypes: an X-linked Angelman-like condition (Gilfillan et al., 2008), Christianson syndrome (Christianson et al., 1999), and corticobasal degeneration with tau deposition (Garbern et al., 2010). Although accompanied by varying signs and symptoms, these three phenotypic categories share similarities in that they are each characterized by the presentation of epilepsy, autistic behavior, intellectual disability (ID), and ataxia (Stromme et al., 2011). Details of the specific mutations and their resulting phenotypes have been thoroughly reviewed by Kondapalli et al. (2014).
An NHE6 knockout mouse has been generated and characterized by Stromme et al. (2011). NHE6 knockout mice exhibit an accumulation of GM2 ganglioside and cholesterol within the late endosomes and lysosomes of neurons in specific sub regions of the hippocampus, amygdala, and cerebral cortex. The enzyme responsible for the degradation of GM2 gangliosides, lysosomal β-hexosaminidase, was undetectable in knockout mouse neurons in the regions of GM2 accumulation. Furthermore, prominent Purkinje cell pathology was evident in the cerebellum of NHE6 knockouts. Despite prevalent cellular pathology, the behavioral phenotype consists only of mild motor impairments and motor hyperactivity without presentation of spontaneous seizures (Stromme et al., 2011). While this mouse begins to model the ataxia observed in humans with NHE6 mutations, the mild phenotype suggests possible compensation by other NHE family members. Interestingly, similar GM2 accumulations and cerebellar degeneration are seen in lysosomal disorders, suggesting that NHE6 abnormalities may also contribute to other types of diseases (Walkley, 2004). Impaired endosomal function, including improper protein processing and receptor trafficking, likely underlie the cellular and behavioral pathologies, as was detailed in Section 5.2.1.
7.1. X-linked Angelman-like syndrome
The X-linked Angelman-like condition is the most common of the three intellectual disability phenotypes seen clinically and is attributed to SLC9A6 mutations (Stromme et al., 2011). Patients with this condition exhibit similar symptoms to those with true Angelman syndrome, including epilepsy, ataxia, happy demeanor, drooling, and limited or absent speech, but lack UBE3 gene pathology that is characteristic of Angelman syndrome (Takahashi et al., 2011). Patients with Angelman-like syndrome can also be distinguished from Angelman patients based on their low body weight, cerebellar atrophy, and a distinct EEG pattern consisting of a 10–14 Hz background frequency. However, it should be noted that conditions termed as “Angelman-like syndromes” are not exclusively characterized by SLC9A6 mutations. Genetic screening of patients with these symptoms have revealed instances where neither UBE3 nor SLC9A6 mutations were present, highlighting the complex etiology of intellectual disability (Fichou et al., 2009).
7.2. Christianson syndrome
Christianson syndrome (CS) is a syndromic form of X-linked mental retardation (XLMR) first reported by Christianson et al. (1999). Christianson syndrome usually presents in infancy and is characterized by severe developmental delay, seizures, absent speech, ataxia, and acquired microcephaly (Pescosolido et al., 2014; Riess et al., 2013; Schroer et al., 2010; Tzschach et al., 2011). Magnetic resonance spectroscopy has shown increased levels of glutamate/glutamine in patient brains, which likely contributes to seizure kindling and the observed atrophy of the hippocampus and cerebellum (Schroer et al., 2010).
CS was initially attributed to a 2 base pair frameshift mutation in the SLC9A6 gene in the South African family in which it was first identified (Gilfillan et al., 2008). Since then, several other protein truncating or splicing SLC9A6 mutations have been identified in patients diagnosed with CS from independent lineages (Mignot et al., 2013; Pescosolido et al., 2014; Riess et al., 2013; Tzschach et al., 2011). Tzschach et al. (2011) report a 2 year-old boy with a 315 kb deletion within Xq26.3, the region associated with the NHE6 gene, SLC9A6. The patient exhibited epilepsy, severe intellectual disability, ataxia, absent speech, and gastroespohogeal reflux, consistent with the phenotype of Christianson Syndrome. Mignot et al. (2013) report a 22 year-old male with an unique SLC9A6 point mutation who also presented with retinitis pigmentosum, suggesting retinal degeneration may also be a symptom of CS. The heterogeneity of SLC6A9 mutations that have been identified in CS illustrate the importance for proper NHE6 structure and function in normal brain development.
7.3. Corticobasal degeneration with tau deposition
Corticobasal degeneration with tau deposition is another phenotypic result attributed to NHE6 mutation first reported by Garbern et al. (2010). They reported a 9 base pair deletion in exon 8 of SLC9A6 that resulted in the loss of three highly conserved amino acids within the transmembrane domain. These patients exhibit profound mental retardation but less pronounced microencepahly and facial dysmorphia than other NHE6 mutation associated diseases. Uniquely, these patients have tangled tau depositions in glia and neurons throughout the brain (Garbern et al., 2010). Tau deposition is a primary characteristic of several neurodegenerative diseases, including Alzheimer's disease and Pick's disease (Murray et al., 2014). NHE6 expression is decreased in the brains of Alzheimer patients and was shown to be important for the proper endosomal processing of amyloid precursor protein (Prasad and Rao, 2015). The tau deposition observed in individuals with NHE6 mutations can provide additional insights into the etiology of neurodegenerative diseases affecting both sexes.
8. NHE9 and intellectual disability
SLC9A9 mutations that result in mutated NHE9 protein are implicated in individuals presenting with autism, epilepsy, addiction, and attention-deficit/hyperactivity disorder (ADHD) (Fisher et al., 2002; Morrow et al., 2008; Pescosolido et al., 2014; Schwede et al., 2014; Vink et al., 2009). Autism is accompanied by 10–30% comorbidity with epilepsy and up to 70% comorbidity with intellectual disability (Gabis et al., 2005; Yeargin-Allsopp et al., 2003). Kondapalli et al. (2013) reported three autism-linked substitutions that occur within the NHE9 coding region. Patients with L236S and S438P mutations in the SLC9A9 transmembrane domain presented with comorbid epilepsy, while the patient with a V176I mutation in the more variable peripheral region of the protein structure did not (Kondapalli et al., 2013). Morrow et al. (2008) reported a nonsense mutation at Arg423 in two male siblings and their mother which resulted in a premature stop codon within the transmembrane region of SLC9A9. The male siblings were diagnosed with autism, one with diagnosed comorbid epilepsy while the other had experienced seizures in childhood but was no longer epileptic. The mother reported experiencing only childhood language delay. Interestingly, this mutation is structurally similar to the NHE1 mutation seen in swe mice. Swe mice have a premature stop codon insertion at Lys44 of SLC9A1, which is also within the transmembrane domain. These findings highlight the importance of the transmembrane domain to proper NHE function but also that additional factors play a role in whether individuals with the same SLC9A9 mutations will exhibit the same phenotype.
Attention-deficit hyperactivity disorder (ADHD) is characterized by impulsivity, inability to focus, and hyperactivity. It is not classified as an X-linked intellectual disability, but it does affect more males than females, with ratios ranging from 1:3 to 1:16, depending on country (Novik et al., 2006). Several genome wide association studies have suggested correlations between SLC9A9 and ADHD (Brookes et al., 2006; Lasky-Su et al., 2008; Markunas et al., 2010). NHE9 interacts with regulatory proteins at the intracellular C-terminal juxtamembrane domain (Zhang-James et al., 2011). Specifically, NHE9 has been shown to interact with calmodulin (CaM), calcineurin homologous protein (CHP), and RACK 1, all of which foster synaptic transmission and plasticity (Zhang-James et al., 2011). CaM, CHP, and RACK1 are regulated by intracellular Ca2+ signaling cascades and provide the ability to phosphorylate and dephosphorylate proteins (Bertrand et al., 1994; Lin and Barber, 1996; Ohgaki et al., 2008; Zhang-James et al., 2011). Mutations in SLC9A9 may interfere with interactions between NHE9 and these signaling molecules and thus disrupt normal synaptic transmission and neuronal activity, leading to conditions including epilepsy, autism, and ADHD.
9. Epilepsy and NHE6/NHE9 mutations
The SLC9A9 gene that encodes for NHE9 has been suggested to play a role in the development of various disorders including ADHD, autism, inattentiveness, and hyperactivity (Zhang-James et al., 2012). Although missense substitutions in the SLC9A9 gene have not been functionally evaluated, they are hypothesized to exhibit a genetic linkage to autism (Kondapalli et al., 2013). Aside from the well-known phenotypic characteristics of autism spectrum disorder (ASD), including impaired language and social communication, ASD has also been reported to show significant comorbidity with both epilepsy and intellectual disability (ID). Epilepsy is more frequent among ADHD patients than among the general public. Children with comorbid ADHD/epilepsy have poorer attention and memory functions than children with only one pathology (Lee et al., 2015). Interestingly, both ADHD and ID are present in individuals exhibiting NHE6-associated neurodevelopmental and degenerative disorders (Kondapalli et al., 2013; Zhang-James et al., 2011). The co-expression of these traits suggests similar underlying genetic defects, potentially caused in part by NHE6 mutations.
One hypothesis for the link between epilepsy, ID and ASD in patients that exhibit mutations in both NHE6 and NHE9 revolves around abnormal accumulation of glutamate in patients with NHE mutations (Gilfillan et al., 2008; Kondapalli et al., 2013; Schroer et al., 2010). The majority of extra-synaptic glutamate is cleared by the glutamate transports GLAST and GLT-1 in astrocytes, which also express NHE1, NHE9, and likely NHE6 (Anderson and Swanson, 2000; Cahoy et al., 2008; Cengiz et al., 2014; Kondapalli et al., 2013). The transport of both glutamine and glutamate is sensitive to pH changes, thus NHE proteins, like NHE6 and NHE9, can play a role in controlling the transportation of these compounds (Gilfillan et al., 2008; Swanson et al., 1995). Mutations disrupting NHE6 and NHE9 functions might lead to glutamate toxicity and result in the phenotypic conditions exhibited in epilepsy, ID, and autism (Kondapalli et al., 2013).
10. Pharmacological inhibitors
Anti-epileptic drugs (AEDs) are used to control the patients’ symptoms and improve their quality of life. Based on their mechanisms of action, the currently available AEDs can be roughly grouped into three categories consisting of drugs that induce ion channel regulation, increase inhibitory neurotransmission, or decrease excitatory transmission (Kwan et al., 2001). Drugs that modulate voltage-gated ion channels, such as Na+, K+, and Ca2+ channels, can alter the intrinsic excitability of the cell to control firing rate. Enhancing inhibitory neurotransmission by targeting the GABAergic system has led to many new antiepileptic treatments that decrease the brain's overall excitability. Finally, drugs that attenuate excitatory neurotransmission by reducing glutamate release can also reduce seizures. These categories are not mutually exclusive as a single drug may exert effects on multiple systems in order to balance neuronal excitability and inhibition. In addition, neurotransmitter transporters indirectly modulate neurotransmission via the regulation of pH. In the following section we will discuss some AEDs which improve seizures through the regulation pH.
10.1. Elevation of GABA-mediated inhibition
Animal experiments and clinical trials have proven that GABA plays an important role in the causative mechanisms and subsequent treatment of epilepsy (Kwan et al., 2001). GABA is released at up to 40% of all synapses and functions as the primary inhibitory neurotransmitter to maintain inhibitory tone that counterbalances neuronal excitation in the mammalian CNS (Olsen and Avoli, 1997). GABA exerts its effects by binding to either metabotropic or ionotropic GABA receptors. Activation of ionotropic GABAA ligand-gated ion channels controls chloride entry into the cell. Metabotropic GABAB receptors are G protein-linked receptors which increase K+ conductance and decreases Ca2+ access (Treiman, 2001). Excess GABA is depleted from the synaptic cleft through GABA transporters (GATs) present on perisynaptic astrocyte processes and axon terminals (Conti et al., 2004). The four identified GATs (GAT-1, GAT-2, GAT-3, and BGT-1) exhibit different expression patterns and sensitivities (Borden et al., 1992; Conti et al., 2004). The variety of GABA receptors and transporters provide a broad range of targets for antiepileptic therapies to regulate neuronal excitability.
The initial line of treatment for patients presenting with emergent status epilepticus of unknown origin is administration of benzodiazepines. Intravenous lorazepam is preferred, although non-intravenous diazepam or midazolam are suitable alternatives (Abend et al., 2014; Rogalski and Rogalski, 2015). These anti-epileptic drugs (AEDs) enhance GABA-mediated neuronal inhibition by acting as GABAAR positive allosteric modulators. Benzodiazepines bind between the α/γ subunits and induce a conformational change in the receptor leading to enhanced affinity for the natural ligand, GABA (Sigel and Steinmann, 2012). This increases the channel's opening frequency thereby enhancing Cl– entry and attenuating neuronal depolarization (Twyman et al., 1989).
Barbiturates, another class of AEDs, are also allosteric GABAAR modulators that increase the mean open time of the channel (MacDonald et al., 1989). At sufficiently high concentrations, barbiturates, such as phenobarbital, can activate GABAAR by themselves (Rho et al., 1996). However, they do not specifically bind GABARs and have several other targets in the brain and periphery. Their ability to induce more dramatic Cl– currents than benzodiazapines and to increase propensity for side effects makes the barbiturates a second-line choice for epilepsy treatment (Loscher and Rogawski, 2012). Of note, the closely related GABAA-ρ receptor, formerly known as GABAc receptor, is insensitive to both barbiturates and benzodiazepines and has a less clear role in epilepsy (Johnston, 1996; Watanabe et al., 2010).
The second line of effective anticonvulsants depends on increasing the amount of synaptically available GABA. One way to achieve this is by inhibiting the degradation of GABA. GABA-transaminase (GABA-T) is the enzyme which is responsible for the catabolism of GABA (Jung et al., 1977). The AED viagabatrin is an inhibitor of GABA-T that works by irreversibly binding to the active site of the enzyme. It blocks the degradation of GABA leading to an increased concentration of synaptic GABA and brain-wide inhibitory tone, thereby reducing seizure activity (Schechter et al., 1977; Treiman, 2001).
Another approach to increasing the availability of GABA is to block its removal from the synaptic cleft. The AED tiagabine is a prototypic GABA uptake blocker. Tiagabine specifically blocks GAT-1, which is expressed in both synaptic terminals and perisynaptic astrocyte processes, primarily in the cerebral cortex and hippocampus (Madsen et al., 2010; Meldrum and Chapman, 1999). Tiagabine can temporarily prolong the existence of GABA in the synaptic cleft as well as influence the enhance of synaptic GABA concentration (Kwan et al., 2001). This drug also has a demonstrated effect on the intracellular pH of CA3 neurons. Udo Bonnet et al. demonstrate that 10 or 50 μM tiagabine can reversibly decrease the steady-state pHi to similar degrees, indicating even low concentrations of this drug can elicit pH regulation (Bonnet et al., 2010). This slight drop in pHi is likely a contributing mechanism to tiagabine's anti-epileptic properties (Bonnet et al., 2000).
A third widely used AED, valproate, also increases the concentration of GABA in the brain. However, the exact mechanism(s) of action remain to be conclusively determined, but may involve the inhibition of GABA-T, blocking GABA reuptake, or increasing GABA release (Bonnet et al., 2002; Leach et al., 1996; Rowley et al., 1995). Several other enzymes and signaling pathways have been suggested targets of valproate, which may explain its efficacy in treating a broad range of conditions, including cancer and neurodegenerative diseases (Terbach and Williams, 2009). An additional therapeutic pathway for valproate may be its ability to influence pHi. Hippocampal CA3 neurons treated with valproate undergo rapid intracellular acidification, which has been suggested to reduce membrane excitability and limit seizure induction (Bonnet et al., 2002). This effect was independent of valproate's influence on GABAergic transmission, highlighting valproate's broad range of therapeutic means and the importance of pHi in controlling neuronal activity. It is of note that intracellular acidification has also been seen with other anti-epileptics, including acetazolamide and sulthiamine, indicating that exceptions exist to the general schema presented in Fig. 1 (Leniger et al., 2002).
10.2. CA inhibitors
It is well known that CAs play a crucial role in the transport of CO2 in a variety of tissues and regulate pH in many organs (for more details see Section 4.3). CA inhibitors have multiple actions that underlie their efficacy in the prevention of illnesses, like epilepsy. CA inhibitors have been widely explored as antiglaucoma, anticancer, anticonvulsant, antiobesity, antifungal, and antibacterial agents (Supuran, 2013).
Widely used CAs for the management of seizures include acetazolamide, zonisamide, and topiramate (Thiry et al., 2008). The newest generation of anticonvulsants garner positive results either as monotherapies or adjunctive therapy in adult and pediatric patients (Treiman, 2001). For each of these drugs, CA inhibition is only one of several purported paths whereby they can reduce seizures. Acetazoloamide has been reported to be one of the more potent CA inhibitors (Dodgson et al., 2000). Acetazolamide's anti-seizure properties are correlated with its brain-level inhibition of CA and depend upon the drug's free SO2NH2 group (Millichap et al., 1955). Administration of acetazolamide induces increased extracellular CO2 levels which, like holding one's breath, acidify the extracellular space and reduce seizure induction (Heuser et al., 1975). In addition to CA inhibition, acetazolamide also increases GABA levels, offering another route for seizure control.
Topiramate is able to modulate neuronal pH through CA activity, but also by modulating the efficacy of the Na-HCO3– anion exchanger (Leniger et al., 2004). Additional therapeutic effects of topiramate come from its ability to block voltage- gated Ca+ and Na+ channels, allosterically modulate kainate/AMPA receptors, or enhancement of GABAergic transmission (Angehagen et al., 2004; DeLorenzo et al., 2000; Zhang et al., 2000). However, not all CA inhibitors exert demonstrable effects on pH. Zonisamide alters CA activity, although its application did not impact neuronal pH (Bonnet et al., 2010). These varied modes of action make the range of available CA inhibiters more efficacious in the treatment of different subtypes of epilepsy with different features.
10.3. NHE inhibitors
NHE isoforms are highly abundant in various brain regions and subcellular compartments. NHEs regulate brain pH changes which consequentially modulate neuronal activity and the function of cell machinery, therefore NHEs are extremely important for epileptic activity (for more details see Section 4). Pharmacological inhibition of NHEs has a demonstrated ability to reduce excitatory synaptic transmission and exert anticonvulsant actions. The diuretic drug amiloride and its analogs were the first NHE inhibitors to be developed (Masereel et al., 2003; Orlowski and Grinstein, 2004). NHE1 and NHE2 are the most sensitive to amiloride-mediated inhibition while NHE3, NHE4, and NHE7 are amiloride-resistant isoforms (Ali et al., 2008; Masereel et al., 2003). Additionally, amiloride inhibits voltage-gated Na+ channels and the Na+/Ca2+ exchangers (Schellenberg et al., 1983; Velly et al., 1988). Amiloride analogs, EIPA (ethylisopropyl), DMA (dimethyl amiloride), MIBA (methylisobutyl amiloride) and HMA (5-(N,N-hexamethylene amiloride)), have been synthesized and are more effective than amiloride with less inhibitory potency on Na+ channels and the Na+/Ca2+ exchanger (Masereel et al., 2003).
In vivo data have demonstrated the anticonvulsant efficacy of amiloride in the different animal models of seizure and epilepsy (Ali et al., 2008). Amiloride-treated mice show increased resistance to both electrical and chemical induced seizures (Ali et al., 2004). Additionally, amiloride improved short term memory function as assessed by spontaneous alternation as well as normalizing affective behavior in the forced swim test, all without compromising motor function (Ali et al., 2004). Although the exact mechanisms of these benefits were not clarified, they likely involve the role of NHE1 in pH regulation. Traditional AEDs, like benzodiazepines, have side effects including drowsiness, impaired motor function, and decreased cognitive abilities (Stewart, 2005). The improved neurological function and stable motor control on animals treated with amiloride suggests that NHE inhibitors may be a better tolerated treatment option than those currently available. Thus, NHE inhibitors, in addition to their function as anticonvulsants, have additional behavior stabilizing benefits with limited motor side effects, making them a promising class of novel AEDs.
11. Conclusions
The CNS is a tightly regulated milieu that can be perturbed by alteration in peripheral conditions, such as pH altering systemic acid–base disorders. Moreover, neuronal activity is associated with localized fluctuations between acidic and alkaline pHo, which act as feedback mechanisms to regulate firing activity by modulating neuronal excitability. Therefore, it is conceivable that dysregulation of pH homeostasis alters neuronal excitability and leads to seizure development. pH regulatory mechanisms, including sodium-bicarbonate transporters, chloride-bicarbonate exchangers, carbonic anhydrases, and sodium-proton exchangers, are important to regulate neuronal excitability. The picture of how pH regulation alters excitability becomes more complex when we consider the role of pH on inhibitory neurons and the pathological bursting activity seen during seizures.
This review summarizes newly emerging research findings of dysfunctions of NHE1, NHE6 and NHE9 in the development of epilepsy. In summary, the most abundant plasma membrane isoform in the CNS, NHE1, is highly sensitive to changes of pHi and regulates intracellular H+ homeostasis through H+ extrusion and Na+ influx across the cell membrane. Lack of NHE1 function fails to regulate pHi and produces an alkaline pHo which may reduce the threshold for the initiation or propagation of synchronous epileptic discharges (Fig. 3). On the other hand, intracellular organelle NHE members NHE6 and NHE9 are located in early and late endosomes, respectively, and mediate H+ leakage from the organelles to maintain the optimal pH homeostasis between the cytosol and components of the endocytic pathway. Therefore, NHE6 and NHE9 are important for regulating protein trafficking, enzyme activity, endosome degradation and recycling, and uptake of molecules like the neurotransmitter glutamate (Fig. 3). NHE6 and/or NHE9 mutations result in abnormal glutamate accumulation, which may lead to epilepsy. Meanwhile, dysfunction of NHE6 in patients with SLC9A6 mutations is also related to the X-linked Angelman-like condition and manifests with epilepsy, autistic behavior, intellectual disability, and ataxia (Fig. 3). Taken together, these new findings illustrate the significance of NHE family members in regulating H+ homeostasis in the CNS and the causative relationship between their dysfunctions and development of the epilepsy and related brain disorders. A better understanding of the mechanisms underlying NHE1, 6, 9 dysfunctions in epilepsy formation has the potential to identify new strategies for epilepsy therapy development.
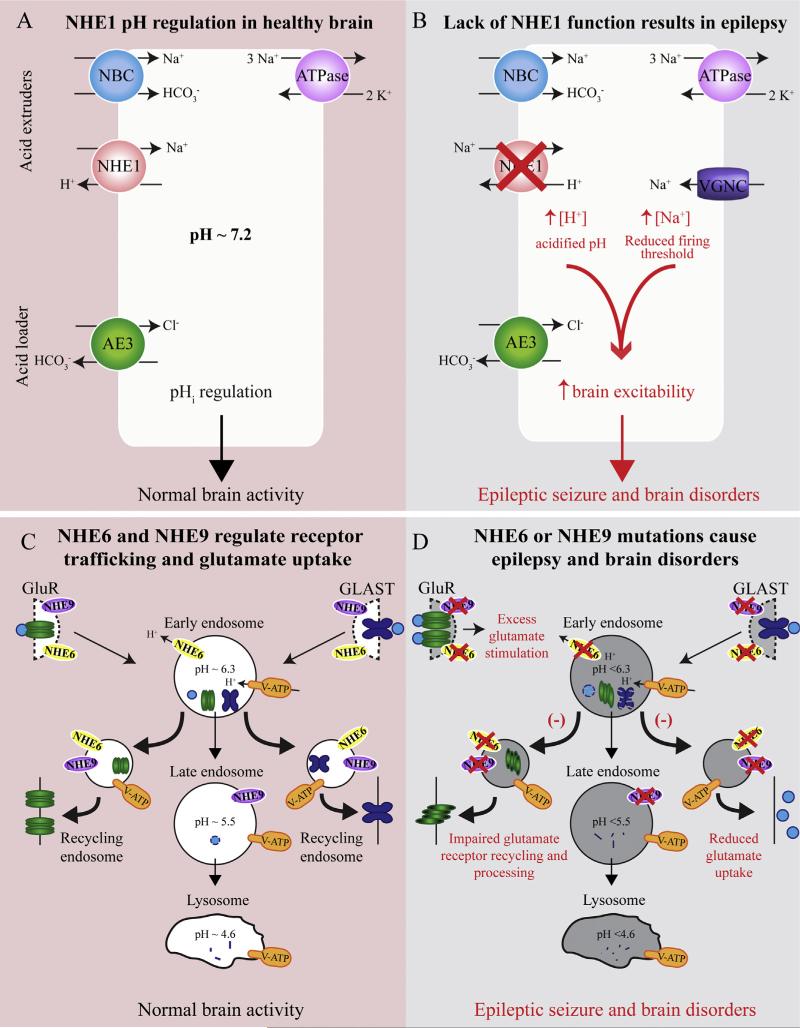
Fig. 3.
Schematic view of impaired functions of NHE1, NHE6, and NHE9 in brain disorders. A cell's pH is regulated by the balance of acid extruders, NHE1 and the sodium coupled bicarbonate transporter (NBC), and the primary acid loader the chloride-bicarbonate exchanger AE3. The sodium-potassium ATPase removes excess Na+ in order to balance ion concentrations. Proper function of all these components is crucial for pHi regulation and normal brain activity.
Mutations that cause NHE1 loss of function result in an acidified intracellular compartment. NHE1 deletion also results in increased expression of voltage sensitive Na+ channels (VGNC), which increases intracellular Na+ concentration and lowers the action potential firing threshold. Together, these ionic changes increase neuronal excitability and facilitate epileptic activity. Intracellular organellar NHE6 and NHE9 are located in early and late endosomes, respectively. Vesicular ATPases (V-ATP)-mediated H+ influx and the NHEs-mediated H+ leakage from the organelles maintain the optimal pH homeostasis between the cytosol, endosome, and lysosome. Glutamate receptors (GluR) and glutamate are endocytosed in endosomes and are either degraded or returned to the membrane. Glutamate transporters, such as GLAST, are also recycled using NHE9 dependent mechanisms. Thus, appropriate pHi regulates protein trafficking, glutamate uptake, and normal brain function. When NHE6 or NHE9 are nonfunctional, the hyperacidified endosomes inhibit appropriate protein processing mechanisms, meaning receptors that are reinserted into the membrane may be misfolded or mislocalized. NHE9 mutations decrease surface expression of GLAST, resulting from hyperacidified endosomes and degradation of the transporter before it can be recycled to the membrane. Decreased GLAST expression will increase extracellular glutamate concentrations, exacerbating the glutamate receptor over stimulation which may lead to epilepsy. Disruption of membrane trafficking and glutamate signaling during development likely contributes to the epilepsy, and other brain disorders including autistic behavior, intellectual disability, ataxia, and attention-deficit/hyperactivity disorder seen in patients with NHE family mutations.
Acknowledgments
The research was supported by NIH R01NS048216 (DS).
Abbreviations
- CNS
central nervous system
- NHE
Na+/H+ exchanger
- ADHD
attention deficit hyperactivity disorder
- CA
carbonic anhydrase
- pHi
intracellular pH
- pHo
extracellular pH
- BBB
blood–brain barrier
- pCO2
partial pressure of carbon dioxide
- V-ATPase
vacuolar-type H+ ATPase
- Swe
slow-wave epilepsy
- ECoG
electrocorticographic
- XLID
X-chromosome linked intellectual disability
- ID
intellectual disability
- CS
Christianson syndrome
- CaM
calmodulin
- CHP
calcineurin homologous protein
- ASD
autism spectrum disorder
- GABA
γ-aminobutyric acid
- GABAR
γ-aminobutyric acid receptor
- GABAT
GABA transaminase
- GAT
GABA transporter
- AED
antiepileptic drug
- NBC
sodium-coupled bicarbonate transporter
- AE
anion exchanger
- KO
knockout
- PMCAs
Ca2+/H+ ATPases
- DIA
depolarization-induced alkalization
- PTZ
pentylenetetrazole
- MCT
monocarboxylate transporter
- RACK1
receptor for activated C-kinase
- XLMR
X-linked mental retardation
References
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Abend NS, Bearden D, Helbig I, McGuire J, Narula S, Panzer JA, Topjian A, Dlugos DJ. Status epilepticus and refractory status epilepticus management. Semin. Pediatr. Neurol. 2014;21:263–274. doi: 10.1016/j.spen.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DJ, Lueders H. Hyperventilation and 6-hour EEG recording in evaluation of absence seizures. Neurology. 1981;31:1175–1177. doi: 10.1212/wnl.31.9.1175. [DOI] [PubMed] [Google Scholar]
- Aggarwal M, Kondeti B, McKenna R. Anticonvulsant/antiepileptic carbonic anhydrase inhibitors: a patent review. Expert Opin. Ther. Pat. 2013;23:717–724. doi: 10.1517/13543776.2013.782394. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Tinner B, Staines WA, Vaananen K, Fuxe K. On the cellular localization and distribution of carbonic anhydrase II immunoreactivity in the rat brain. Brain Res. 1995;676:10–24. doi: 10.1016/0006-8993(95)00026-m. [DOI] [PubMed] [Google Scholar]
- Ali A, Ahmad FJ, Dua Y, Pillai KK, Vohora D. Seizures and sodium hydrogen exchangers: potential of sodium hydrogen exchanger inhibitors as novel anticonvulsants. CNS Neurol. Disord. Drug Targets. 2008;7:343–347. doi: 10.2174/187152708786441830. [DOI] [PubMed] [Google Scholar]
- Ali A, Ahmad FJ, Pillai KK, Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy Behav. 2004;5:322–328. doi: 10.1016/j.yebeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Alka K, Casey JR. Bicarbonate transport in health and disease. IUBMB Life. 2014;66:596–615. doi: 10.1002/iub.1315. [DOI] [PubMed] [Google Scholar]
- Alvarez BV, Fujinaga J, Casey JR. Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circ. Res. 2001;89:1246–1253. doi: 10.1161/hh2401.101907. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Angehagen M, Ben-Menachem E, Shank R, Ronnback L, Hansson E. Topiramate modulation of kainate-induced calcium currents is inversely related to channel phosphorylation level. J. Neurochem. 2004;88:320–325. doi: 10.1046/j.1471-4159.2003.02186.x. [DOI] [PubMed] [Google Scholar]
- Armijo JA, Shushtarian M, Valdizan EM, Cuadrado A, de las Cuevas I, Adin J. Ion channels and epilepsy. Curr. Pharm. Des. 2005;11:1975–2003. doi: 10.2174/1381612054021006. [DOI] [PubMed] [Google Scholar]
- Attaphitaya S, Park K, Melvin JE. Molecular cloning and functional expression of a rat Na+/H+ exchanger (NHE5) highly expressed in brain. J. Biol. Chem. 1999;274:4383–4388. doi: 10.1074/jbc.274.7.4383. [DOI] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog. Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NR, Orlowski J, Szabo EZ, Zaun HC, Schultheis PJ, Menon AG, Shull GE. Molecular cloning, genomic organization, and functional expression of Na+/H+ exchanger isoform 5 (NHE5) from human brain. J. Biol. Chem. 1999;274:4377–4382. doi: 10.1074/jbc.274.7.4377. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am. J. Physiol. 1999;276:C788–C795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- Bertrand B, Wakabayashi S, Ikeda T, Pouyssegur J, Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J. Biol. Chem. 1994;269:13703–13709. [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bobulescu IA, Di Sole F, Moe OW. Na+/H+ exchangers: physiology and link to hypertension and organ ischemia. Curr. Opin. Nephrol. Hypertens. 2005;14:485–494. doi: 10.1097/01.mnh.0000174146.52915.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet U, Bingmann D, Leniger T, Scherbaum N, Widman G, Hufnagel A, Wiemann M. Valproate acidifies hippocampal CA3-neurons –a novel mode of action. Eur. Neuropsychopharmacol. 2002;12:279–285. doi: 10.1016/s0924-977x(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Bingmann D, Wiemann M. Intracellular pH modulates spontaneous and epileptiform bioelectric activity of hippocampal CA3-neurones. Eur. Neuropsychopharmacol. 2000;10:97–103. doi: 10.1016/s0924-977x(99)00063-2. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Bingmann D, Wiltfang J, Scherbaum N, Wiemann M. Modula-tory effects of neuropsychopharmaca on intracellular pH of hippocampal neurones in vitro. Br. J. Pharmacol. 2010;159:474–483. doi: 10.1111/j.1476-5381.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein C, Musch MW, DePaoli A, Xie Y, Rabenau K, Villereal M, Rao MC, Chang EB. Characterization of the rat Na+/H+ exchanger isoform NHE4 and localization in rat hippocampus. Am. J. Physiol. 1996;271:C1629–C1638. doi: 10.1152/ajpcell.1996.271.5.C1629. [DOI] [PubMed] [Google Scholar]
- Borden LA, Smith KE, Hartig PR, Branchek TA, Weinshank RL. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J. Biol. Chem. 1992;267:21098–21104. [PubMed] [Google Scholar]
- Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/ proton exchangers. Am. J. Physiol. Cell Physiol. 2005a;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- Brett CL, Tukaye DN, Mukherjee S, Rao R. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell. 2005b;16:1396–1405. doi: 10.1091/mbc.E04-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett CL, Wei Y, Donowitz M, Rao R. Human Na(+)/H(+) exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am. J. Physiol. Cell Physiol. 2002;282:C1031–C1041. doi: 10.1152/ajpcell.00420.2001. [DOI] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, Anney R, Franke B, Gill M, Ebstein R, Buitelaar J, Sham P, Campbell D, Knight J, Andreou P, Altink M, Arnold R, Boer F, Buschgens C, Butler L, Christiansen H, Feldman L, Fleischman K, Fliers E, Howe-Forbes R, Goldfarb A, Heise A, Gabriels I, Korn-Lubetzki I, Johansson L, Marco R, Medad S, Minderaa R, Mulas F, Muller U, Mulligan A, Rabin K, Rommelse N, Sethna V, Sorohan J, Uebel H, Psychogiou L, Weeks A, Barrett R, Craig I, Banaschewski T, Sonuga-Barke E, Eisenberg J, Kuntsi J, Manor I, McGuffin P, Miranda A, Oades RD, Plomin R, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Taylor E, Thompson M, Faraone SV, Asherson P. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol. Psychiatry. 2006;11:934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal A. Epilepsy and autism spectrum disorders: are there common developmental mechanisms? Brain Dev. 2010;32:731–738. doi: 10.1016/j.braindev.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Butler LS, Silva AJ, Abeliovich A, Watanabe Y, Tonegawa S, McNamara JO. Limbic epilepsy in transgenic mice carrying a Ca2+/calmodulin-dependent kinase II alpha-subunit mutation. Proc. Natl. Acad. Sci. U. S. A. 1995;92:6852–6855. doi: 10.1073/pnas.92.15.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu. Rev. Pharmacool. Toxicol. 2014;54:317–338. doi: 10.1146/annurev-pharmtox-011112-140232. [DOI] [PubMed] [Google Scholar]
- Cengiz P, Kintner DB, Chanana V, Yuan H, Akture E, Kendigelen P, Begum G, Fidan E, Uluc K, Ferrazzano P, Sun D. Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation. PLOS ONE. 2014;9:e84294. doi: 10.1371/journal.pone.0084294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Chesler M. Autocrine boost of NMDAR current in hippocampal CA1 pyramidal neurons by a PMCA-dependent, perisynaptic, extracellular pH shift. J. Neurosci. 2015;35:873–877. doi: 10.1523/JNEUROSCI.2293-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Bezprozvanny I, Tsien RW. Molecular basis of proton block of L-type Ca2+ channels. J. Gen. Physiol. 1996;108:363–374. doi: 10.1085/jgp.108.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol. Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A. The dynamic synapse. Neuron. 2013;80:691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Christianson AL, Stevenson RE, van der Meyden CH, Pelser J, Theron FW, van Rensburg PL, Chandler M, Schwartz CE. X linked severe mental retardation, craniofacial dysmorphology, epilepsy, ophthalmoplegia, and cerebellar atrophy in a large South African kindred is localised to Xq24-q27. J. Med. Genet. 1999;36:759–766. doi: 10.1136/jmg.36.10.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res. Brain Res. Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson RT, Fu A, Aronson PS, Noebels JL, Frankel WN. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91:139–148. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R2136–R2146. doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Prasad V, Hubner CA, Praetorius J. Nhe1 is a luminal Na+/H+ exchanger in mouse choroid plexus and is targeted to the basolateral membrane in Ncbe/Nbcn2-null mice. Am. J. Physiol. Cell Physiol. 2009;296:C1291–C1300. doi: 10.1152/ajpcell.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Manfridi A, Biella G. Activity-dependent pH shifts and periodic recurrence of spontaneous interictal spikes in a model of focal epileptogenesis. J. Neurosci. 1998;18:7543–7551. doi: 10.1523/JNEUROSCI.18-18-07543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deda G, Ekim M, Guven A, Karagol U, Tumer N. Hypopotassemic paralysis: a rare presentation of proximal renal tubular acidosis. J. Child Neurol. 2001;16:770–771. doi: 10.1177/088307380101601013. [DOI] [PubMed] [Google Scholar]
- Deitmer JW. Strategies for metabolic exchange between glial cells and neurons. Respir. Physiol. 2001;129:71–81. doi: 10.1016/s0034-5687(01)00283-3. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Prog. Neurobiol. 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Sombati S, Coulter DA. Effects of topiramate on sustained repetitive firing and spontaneous recurrent seizure discharges in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl. 1):S40–S44. doi: 10.1111/j.1528-1157.2000.tb06048.x. [DOI] [PubMed] [Google Scholar]
- Deshpande LS, Delorenzo RJ. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front. Neurol. 2014;5:11. doi: 10.3389/fneur.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41(Suppl. 1):S35–S39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to corticalexcitabilityvia pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla CG, Frenguelli BG, Staley KJ, Masino SA. Intracellular acidification causes adenosine release during states of hyperexcitability in the hippocampus. J. Neurophysiol. 2009;102:1984–1993. doi: 10.1152/jn.90695.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Achkar CM, Olson HE, Poduri A, Pearl PL. The genetics of the epilepsies. Curr. Neurol. Neurosci. Rep. 2015;15:39. doi: 10.1007/s11910-015-0559-8. [DOI] [PubMed] [Google Scholar]
- Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plas. 2013;2013:429815. doi: 10.1155/2013/429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichou Y, Bahi-Buisson N, Nectoux J, Chelly J, Heron D, Cuisset L, Bienvenu T. Mutation in the SLC9A6 gene is not a frequent cause of sporadic Angel-man-like syndrome. Eur. J. Hum. Genet. 2009;17:1378–1380. doi: 10.1038/ejhg.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SE, Francks C, McCracken JT, McGough JJ, Marlow AJ, MacPhie IL, Newbury DF, Crawford LR, Palmer CG, Woodward JA, Del'Homme M, Cantwell DP, Nelson SF, Monaco AP, Smalley SL. A genome wide scan for loci involved in attention-deficit/hyperactivity disorder. Am. J. Hum. Genet. 2002;70:1183–1196. doi: 10.1086/340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe –a systematic review. Eur. J. Neurol. 2005;12:245–253. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/ H+ exchangers. Pflugers Arch. 2014;466:61–76. doi: 10.1007/s00424-013-1408-8. [DOI] [PubMed] [Google Scholar]
- Futai M, Oka T, Sun-Wada G, Moriyama Y, Kanazawa H, Wada Y. Luminal acidification of diverse organelles by V-ATPase in animal cells. J. Exp. Biol. 2000;203:107–116. doi: 10.1242/jeb.203.1.107. [DOI] [PubMed] [Google Scholar]
- Gabis L, Pomeroy J, Andriola MR. Autism and epilepsy: cause, consequence, comorbidity, or coincidence? Epilepsy Behav. 2005;7:652–656. doi: 10.1016/j.yebeh.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Neumann M, Trojanowski JQ, Lee VM, Feldman G, Norris JW, Friez MJ, Schwartz CE, Stevenson R, Sima AA. A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition. Brain. 2010;133:1391–1402. doi: 10.1093/brain/awq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J. Biol. Chem. 2007;282:9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- Gilfillan GD, Selmer KK, Roxrud I, Smith R, Kyllerman M, Eiklid K, Kroken M, Mattingsdal M, Egeland T, Stenmark H, Sjoholm H, Server A, Samuelsson L, Christianson A, Tarpey P, Whibley A, Stratton MR, Futreal PA, Teague J, Edkins S, Gecz J, Turner G, Raymond FL, Schwartz C, Stevenson RE, Undlien DE, Stromme P. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angel-man syndrome. Am. J. Hum. Genet. 2008;82:1003–1010. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi AC, Di Sole F. Deciphering the mechanisms of the Na+/H+ exchanger-3 regulation in organ dysfunction. Am. J. Physiol. Cell Physiol. 2012;302:C1569–C1587. doi: 10.1152/ajpcell.00017.2012. [DOI] [PubMed] [Google Scholar]
- Goren MZ, Onat F. Ethosuximide: from bench to bedside. CNS Drug Rev. 2007;13:224–239. doi: 10.1111/j.1527-3458.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemo-sensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Greenfield LJ., Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure. 2013;22:589–600. doi: 10.1016/j.seizure.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone BP, Baraban SC. Animal models in epilepsy research: legacies and new directions. Nat. Neurosci. 2015;18:339–343. doi: 10.1038/nn.3934. [DOI] [PubMed] [Google Scholar]
- Gu XQ, Yao H, Haddad GG. Increased neuronal excitability and seizures in the Na(+)/H(+) exchanger null mutant mouse. Am. J. Physiol. Cell Physiol. 2001;281:C496–C503. doi: 10.1152/ajpcell.2001.281.2.C496. [DOI] [PubMed] [Google Scholar]
- Guaranha MS, Garzon E, Buchpiguel CA, Tazima S, Yacubian EM, Sakamoto AC. Hyperventilation revisited: physiological effects and efficacy on focal seizure activation in the era of video-EEG monitoring. Epilepsia. 2005;46:69–75. doi: 10.1111/j.0013-9580.2005.11104.x. [DOI] [PubMed] [Google Scholar]
- Gupta PP, Thacker AK, Haider J, Dhawan S, Pandey N, Pandey AK. Assessment of topiramate's efficacy and safety in epilepsy. J. Neurosci. Rural Pract. 2014;5:144–148. doi: 10.4103/0976-3147.131657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmi P, Parkkila S, Honkaniemi J. Expression of carbonic anhydrases II, IV, VII, VIII and XII in rat brain after kainic acid induced status epilepticus. Neurochem. Int. 2006;48:24–30. doi: 10.1016/j.neuint.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hamidi S, Avoli M. Carbonic anhydrase inhibition by acetazolamide reduces in vitro epileptiform synchronization. Neuropharmacology. 2015;95:377–387. doi: 10.1016/j.neuropharm.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy MM, Ruusuvuori E, Watkins PV, Voipio J, Kanold PO, Kaila K. Acid extrusion via blood–brain barrier causes brain alkalosis and seizures after neonatal asphyxia. Brain. 2012;135:3311–3319. doi: 10.1093/brain/aws257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl−/HCO3− exchanger AE3 display a reduced seizure threshold. Mol. Cell. Biol. 2006;26:182–191. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser D, Astrup J, Lassen NA, Betz BE. Brain carbonic acid acidosis after acetazolamide. Acta Physiol. Scand. 1975;93:385–390. doi: 10.1111/j.1748-1716.1975.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Xu X, Trask RB, Maddatu TP, Johnson BA, Naggert JK, Nishina PM, Ikeda A. Carbonic anhydrase related protein 8 mutation results in aberrant synaptic morphology and excitatory synaptic function in the cerebellum. Mol. Cell. Neurosci. 2007;35:161–170. doi: 10.1016/j.mcn.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hubner CA. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc. Natl. Acad. Sci. U. S. A. 2008;105:311–316. doi: 10.1073/pnas.0705487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean T, Frelin C, Vigne P, Barbry P, Lazdunski M. Biochemical properties of the Na+/H+ exchange system in rat brain synaptosomes. Interdependence of internal and external pH control of the exchange activity. J. Biol. Chem. 1985;260:9678–9684. [PubMed] [Google Scholar]
- Johnston GA. GABAc receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol. Sci. 1996;17:319–323. [PubMed] [Google Scholar]
- Jung MJ, Lippert B, Metcalf BW, Bohlen P, Schechter PJ. gamma-Vinyl GABA (4-amino-hex-5-enoic acid), a new selective irreversible inhibitor of GABA-T: effects on brain GABA metabolism in mice. J. Neurochem. 1977;29:797–802. doi: 10.1111/j.1471-4159.1977.tb10721.x. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat. Clin. Pract. Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Kaila K, Paalasmaa P, Taira T, Voipio J. pH transients due to monosynaptic activation of GABAA receptors in rat hippocampal slices. Neuroreport. 1992;3:105–108. doi: 10.1097/00001756-199201000-00028. [DOI] [PubMed] [Google Scholar]
- Kaila K, Ransom BR. pH and Brain Function. Wiley-Liss; New York: 1998. [Google Scholar]
- Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987;330:163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kang TC, An SJ, Park SK, Hwang IK, Bae JC, Won MH. The evidence for GABAB receptor-mediated regulation of acid–base balance: involvement of Na+/H+ exchanger and Na+/HCO3− cotransporter. Brain Res. Mol. Brain Res. 2003;114:86–90. doi: 10.1016/s0169-328x(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Kang TC, An SJ, Park SK, Hwang IK, Suh JG, Oh YS, Bae JC, Won MH. Alterations in Na+/H+ exchanger and Na+/HCO3− cotransporter immunoreactivities within the gerbil hippocampus following seizure. Brain Res. Mol. Brain Res. 2002;109:226–232. doi: 10.1016/s0169-328x(02)00559-4. [DOI] [PubMed] [Google Scholar]
- Kerr MP. The impact of epilepsy on patients' lives. Acta Neurol. Scand. Suppl. 2012:1–9. doi: 10.1111/ane.12014. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Su G, Lenart B, Ballard AJ, Meyer JW, Ng LL, Shull GE, Sun D. Increased tolerance to oxygen and glucose deprivation in astrocytes from Na(+)/H(+) exchanger isoform 1 null mice. Am. J. Physiol. Cell Physiol. 2004;287:C12–C21. doi: 10.1152/ajpcell.00560.2003. [DOI] [PubMed] [Google Scholar]
- Klanke CA, Su YR, Callen DF, Wang Z, Meneton P, Baird N, Kandasamy RA, Orlowski J, Otterud BE, Leppert M, et al. Molecular cloning and physical and genetic mapping of a novel human Na+/H+ exchanger (NHE5/ SLC9A5) to chromosome 16q22.1. Genomics. 1995;25:615–622. doi: 10.1016/0888-7543(95)80002-4. [DOI] [PubMed] [Google Scholar]
- Klier M, Andes FT, Deitmer JW, Becker HM. Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters. J. Biol. Chem. 2014;289:2765–2775. doi: 10.1074/jbc.M113.537043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl(−)/HCO(−)(3) exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am. J. Physiol. Cell Physiol. 2002;283:C1206–C1218. doi: 10.1152/ajpcell.00512.2001. [DOI] [PubMed] [Google Scholar]
- Kondapalli KC, Hack A, Schushan M, Landau M, Ben-Tal N, Rao R. Functional evaluation of autism-associated mutations in NHE9. Nat. Commun. 2013;4:2510. doi: 10.1038/ncomms3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapalli KC, Prasad H, Rao R. An inside job: how endosomal Na(+)/H(+) exchangers link to autism and neurological disease. Front. Cell. Neurosci. 2014;8:172. doi: 10.3389/fncel.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac S, Walker MC. Neuropeptides in epilepsy. Neuropeptides. 2013;47:467–475. doi: 10.1016/j.npep.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Krafte DS, Kass RS. Hydrogen ion modulation of Ca channel current in cardiac ventricular cells. Evidence for multiple mechanisms. J. Gen. Physiol. 1988;91:641–657. doi: 10.1085/jgp.91.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Kwan P, Sills GJ, Brodie MJ. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol. Ther. 2001;90:21–34. doi: 10.1016/s0163-7258(01)00122-x. [DOI] [PubMed] [Google Scholar]
- Lacerda G, Krummel T, Sabourdy C, Ryvlin P, Hirsch E. Optimizing therapy of seizures in patients with renal or hepatic dysfunction. Neurology. 2006;67:S28–S33. doi: 10.1212/wnl.67.12_suppl_4.s28. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, Vasquez AA, Chen W, Asherson P, Buitelaar J, Banaschewski T, Ebstein R, Gill M, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Daly M, Laird N, Lange C, Faraone SV. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am. J. Med. Genet. B: Neuropsychiatr. Genet. 2008;147B:1345–1354. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- Leach JP, Sills GJ, Majid A, Butler E, Carswell A, Thompson GG, Brodie MJ. Effects of tiagabine and vigabatrin on GABA uptake into primary cultures of rat cortical astrocytes. Seizure. 1996;5:229–234. doi: 10.1016/s1059-1311(96)80041-0. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kibby MY, Cohen MJ, Stanford L, Park Y, Strickland S. Differences in memory functioning between children with attention-deficit/hyper-activity disorder and/or focal epilepsy. Child Neuropsychol. 2015:1–22. doi: 10.1080/09297049.2015.1060955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leniger T, Thone J, Wiemann M. Topiramate modulates pH of hippocampal CA3 neurons by combined effects on carbonic anhydrase and Cl−/HCO3− exchange. Br. J. Pharmacol. 2004;142:831–842. doi: 10.1038/sj.bjp.0705850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leniger T, Wiemann M, Bingmann D, Widman G, Hufnagel A, Bonnet U. Carbonic anhydrase inhibitor sulthiame reduces intracellular pH and epilepti-form activity of hippocampal CA3 neurons. Epilepsia. 2002;43:469–474. doi: 10.1046/j.1528-1157.2002.32601.x. [DOI] [PubMed] [Google Scholar]
- Letts VA. Stargazer –a mouse to seize! Epilepsy Curr. 2005;5:161–165. doi: 10.1111/j.1535-7511.2005.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Alvarez B, Casey JR, Reithmeier RA, Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J. Biol. Chem. 2002;277:36085–36091. doi: 10.1074/jbc.M111952200. [DOI] [PubMed] [Google Scholar]
- Lin X, Barber DL. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12631–12636. doi: 10.1073/pnas.93.22.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lu QW, Chen LM. [Physiology and pathophysiology of Na(+)/HCO(3)(−) cotransporter NBCe1]. Sheng li xue bao (Acta Physiolog. Sin.) 2012;64:729–740. [PubMed] [Google Scholar]
- Loscher W, Rogawski MA. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia. 2012;53(Suppl. 8):12–25. doi: 10.1111/epi.12025. [DOI] [PubMed] [Google Scholar]
- Luckermann M, Trapp S, Ballanyi K. GABA- and glycine-mediated fall of intracellular pH in rat medullary neurons in situ. J. Neurophysiol. 1997;77:1844–1852. doi: 10.1152/jn.1997.77.4.1844. [DOI] [PubMed] [Google Scholar]
- Lukashova V, Jinadasa T, Ilie A, Verbich D, Cooper E, Orlowski J. The Na(+)/H (+) exchanger NHE5 is sorted to discrete intracellular vesicles in the central and peripheral nervous systems. Adv. Exp. Med. Biol. 2013;961:397–410. doi: 10.1007/978-1-4614-4756-6_34. [DOI] [PubMed] [Google Scholar]
- Ma E, Haddad GG. Expression and localization of Na+/H+ exchangers in rat central nervous system. Neuroscience. 1997;79:591–603. doi: 10.1016/s0306-4522(96)00674-4. [DOI] [PubMed] [Google Scholar]
- MacDonald RL, Rogers CJ, Twyman RE. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J. Physiol. (Lond.) 1989;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KK, White HS, Schousboe A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol. Ther. 2010;125:394–401. doi: 10.1016/j.pharmthera.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Majumdar D, Bevensee MO. Na-coupled bicarbonate transporters of the solute carrier 4 family in the nervous system: function, localization, and relevance to neurologic function. Neuroscience. 2010;171:951–972. doi: 10.1016/j.neuroscience.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, Harkins LE, Boron WF, Roth KA, Bevensee MO. Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuro-science. 2008;155:818–832. doi: 10.1016/j.neuroscience.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani S, Chesler M. Rapid rise of extracellular pH evoked by neural activity is generated by the plasma membrane calcium ATPase. J. Neurophysiol. 2010;103:667–676. doi: 10.1152/jn.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markunas CA, Quinn KS, Collins AL, Garrett ME, Lachiewicz AM, Sommer JL, Morrissey-Kane E, Kollins SH, Anastopoulos AD, Ashley-Koch AE. Genetic variants in SLC9A9 are associated with measures of attention-deficit/hyperactivity disorder symptoms in families. Psychiatr. Genet. 2010;20:73–81. doi: 10.1097/YPG.0b013e3283351209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur. J. Med. Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- Masino SA, Kawamura M, Jr., Ruskin DN. Adenosine receptors and epilepsy: current evidence and future potential. Int. Rev. Neurobiol. 2014;119:233–255. doi: 10.1016/B978-0-12-801022-8.00011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. The bicarbonate transport metabolon. J. Enzyme Inhib. Med. Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- Meldrum BS, Chapman AG. Basic mechanisms of gabitril (tiagabine) and future potential developments. Epilepsia. 1999;40(Suppl. 9):S2–S6. doi: 10.1111/j.1528-1157.1999.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Mellman I. The importance of being acid: the role of acidification in intracellular membrane traffic. J. Exp. Biol. 1992;172:39–45. doi: 10.1242/jeb.172.1.39. [DOI] [PubMed] [Google Scholar]
- Mignot C, Heron D, Bursztyn J, Momtchilova M, Mayer M, Whalen S, Legall A, Billette de Villemeur T, Burglen L. Novel mutation in SLC9A6 gene in a patient with Christianson syndrome and retinitis pigmentosum. Brain Dev. 2013;35:172–176. doi: 10.1016/j.braindev.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Millichap JG, Woodbury DM, Goodman LS. Mechanism of the anticonvulsant action of acetazoleamide, a carbonic anhydrase inhibitor. J. Pharmacol. Exp. Ther. 1955;115:251–258. [PubMed] [Google Scholar]
- Moriyama Y, Maeda M, Futai M. Energy coupling of L-glutamate transport and vacuolar H(+)-ATPase in brain synaptic vesicles. J. Biochem. 1990;108:689–693. doi: 10.1093/oxfordjournals.jbchem.a123264. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science (New York, N.Y.) 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Kouri N, Lin WL, Jack CR, Jr., Dickson DW, Vemuri P. Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimer's Res. Ther. 2014;6:1. doi: 10.1186/alzrt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- Novik TS, Hervas A, Ralston SJ, Dalsgaard S, Rodrigues Pereira R, Lorenzo MJ, Group AS. Influence of gender on attention-deficit/hyperactivity disorder in Europe–ADORE. Eur. Child Adolesc. Psychiatry. 2006;15(Suppl. 1):I15–I24. doi: 10.1007/s00787-006-1003-z. [DOI] [PubMed] [Google Scholar]
- Obara M, Szeliga M, Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem. Int. 2008;52:905–919. doi: 10.1016/j.neuint.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Ohgaki R, Fukura N, Matsushita M, Mitsui K, Kanazawa H. Cell surface levels of organellar Na+/H+ exchanger isoform 6 are regulated by interaction with RACK1. J. Biol. Chem. 2008;283:4417–4429. doi: 10.1074/jbc.M705146200. [DOI] [PubMed] [Google Scholar]
- Ohgaki R, Matsushita M, Kanazawa H, Ogihara S, Hoekstra D, van Ijzendoorn SC. The Na+/H+ exchanger NHE6 in the endosomal recycling system is involved in the development of apical bile canalicular surface domains in HepG2 cells. Mol. Biol. Cell. 2010;21:1293–1304. doi: 10.1091/mbc.E09-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki R, van ISC, Matsushita M, Hoekstra D, Kanazawa H. Organellar Na+/H+ exchangers: novel players in organelle pH regulation and their emerging functions. Biochemistry. 2011;50:443–450. doi: 10.1021/bi101082e. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Avoli M. GABA and epileptogenesis. Epilepsia. 1997;38:399–407. doi: 10.1111/j.1528-1157.1997.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Olson HE, Poduri A, Pearl PL. Genetic forms of epilepsies and other paroxysmal disorders. Semin. Neurol. 2014;34:266–279. doi: 10.1055/s-0034-1386765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J. Biol. Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr. Opin. Cell Biol. 2007;19:483–492. doi: 10.1016/j.ceb.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Q, Lizarraga SB, Schmidt M, Yang U, Gong J, Ellisor D, Kauer JA, Morrow EM. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol. Rev. 2013;93:803–959. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Munzenmayer G, Catalan MA, Kondo Y, Jaramillo Y, Liu F, Shull GE, Melvin JE. Ae4 (Slc4a9) anion exchanger drives Cl− uptake-dependent fluid secretion by mouse submandibular gland acinar cells. J. Biol. Chem. 2015;290:10677–10688. doi: 10.1074/jbc.M114.612895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescosolido MF, Stein DM, Schmidt M, El Achkar CM, Sabbagh M, Rogg JM, Tantravahi U, McLean RL, Liu JS, Poduri A, Morrow EM. Genetic and phenotypic diversity of NHE6 mutations in Christianson syndrome. Ann. Neurol. 2014;76:581–593. doi: 10.1002/ana.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzonia JH, Biemesderfer D, Abu-Alfa AK, Wu MS, Exner M, Isenring P, Igarashi P, Aronson PS. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am. J. Physiol. 1998;275:F510–F517. doi: 10.1152/ajprenal.1998.275.4.F510. [DOI] [PubMed] [Google Scholar]
- Prasad H, Rao R. The Na+/H+ exchanger NHE6 modulates endosomal pH to control processing of amyloid precursor protein in a cell culture model of Alzheimer disease. J. Biol. Chem. 2015;290:5311–5327. doi: 10.1074/jbc.M114.602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. Am. J. Physiol. Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Raley-Susman KM, Cragoe EJ, Jr., Sapolsky RM, Kopito RR. Regulation of intracellular pH in cultured hippocampal neurons by an amiloride-insensitive Na+/H+ exchanger. J. Biol. Chem. 1991;266:2739–2745. [PubMed] [Google Scholar]
- Rho JM, Donevan SD, Rogawski MA. Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons. J. Physiol. (Lond.) 1996;497(Pt 2):509–522. doi: 10.1113/jphysiol.1996.sp021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess A, Rossier E, Kruger R, Dufke A, Beck-Woedl S, Horber V, Alber M, Glaser D, Riess O, Tzschach A. Novel SLC9A6 mutations in two families with Christianson syndrome. Clin. Genet. 2013;83:596–597. doi: 10.1111/j.1399-0004.2012.01948.x. [DOI] [PubMed] [Google Scholar]
- Robertson J, Hatton C, Emerson E, Baines S. Prevalence of epilepsy among people with intellectual disabilities: a systematic review. Seizure. 2015;29:46–62. doi: 10.1016/j.seizure.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Rogalski R, Rogalski A. Benzodiazepine selection in the management of status epilepticus: a review. Adv. Emerg. Nurs. J. 2015;37:83–94. doi: 10.1097/TME.0000000000000064. quiz E82. [DOI] [PubMed] [Google Scholar]
- Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO(3)(−)) transporters. Mol. Aspects Med. 2013;34:159–182. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Rotin D, Grinstein S. Impaired cell volume regulation in Na(+)-H+ exchange-deficient mutants. Am. J. Physiol. 1989;257:C1158–C1165. doi: 10.1152/ajpcell.1989.257.6.C1158. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Marsden CA, Martin KF. Differential effects of phenytoin and sodium valproate on seizure-induced changes in gamma-aminobutyric acid and glutamate release in vivo. Eur. J. Pharmacol. 1995;294:541–546. doi: 10.1016/0014-2999(95)00589-7. [DOI] [PubMed] [Google Scholar]
- Rowley NM, Madsen KK, Schousboe A, Steve White H. Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem. Int. 2012;61:546–558. doi: 10.1016/j.neuint.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Roxrud I, Raiborg C, Gilfillan GD, Stromme P, Stenmark H. Dual degradation mechanisms ensure disposal of NHE6 mutant protein associated with neurological disease. Exp. Cell Res. 2009;315:3014–3027. doi: 10.1016/j.yexcr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Ruffin VA, Salameh AI, Boron WF, Parker MD. Intracellular pH regulation by acid–base transporters in mammalian neurons. Front. Physiol. 2014;5:43. doi: 10.3389/fphys.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusuvuori E, Huebner AK, Kirilkin I, Yukin AY, Blaesse P, Helmy M, Kang HJ, El Muayed M, Hennings JC, Voipio J, Sestan N, Hubner CA, Kaila K. Neuronal carbonic anhydrase VII provides GABAergic excitatory drive to exacerbate febrile seizures. EMBO J. 2013;32:2275–2286. doi: 10.1038/emboj.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusuvuori E, Kaila K. Carbonic anhydrases and brain pH in the control of neuronal excitability. Subcell. Biochem. 2014;75:271–290. doi: 10.1007/978-94-007-7359-2_14. [DOI] [PubMed] [Google Scholar]
- Ruusuvuori E, Li H, Huttu K, Palva JM, Smirnov S, Rivera C, Kaila K, Voipio J. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J. Neurosci. 2004;24:2699–2707. doi: 10.1523/JNEUROSCI.5176-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Toliat MR, Heils A, Leschik G, Becker C, Ruschendorf F, Rohde K, Mundlos S, Nurnberg P. Association of the 867Asp variant of the human anion exchanger 3 gene with common subtypes of idiopathic generalized epilepsy. Epilepsy Res. 2002;51:249–255. doi: 10.1016/s0920-1211(02)00152-3. [DOI] [PubMed] [Google Scholar]
- Schechter PJ, Tranier Y, Jung MJ, Bohlen P. Audiogenic seizure protection by elevated brain GABA concentration in mice: effects of gamma-acetylenic gaba and gamma-vinyl GABA, two irreversible GABA-T inhibitors. Eur. J. Pharmacol. 1977;45:319–328. doi: 10.1016/0014-2999(77)90270-9. [DOI] [PubMed] [Google Scholar]
- Schellenberg GD, Anderson L, Swanson PD. Inhibition of Na+-Ca2+ exchange in rat brain by amiloride. Mol. Pharmacol. 1983;24:251–258. [PubMed] [Google Scholar]
- Schroer RJ, Holden KR, Tarpey PS, Matheus MG, Griesemer DA, Friez MJ, Fan JZ, Simensen RJ, Stromme P, Stevenson RE, Stratton MR, Schwartz CE. Natural history of Christianson syndrome. Am. J. Med. Genet. A. 2010;152a:2775–2783. doi: 10.1002/ajmg.a.33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchmann S, Hauck S, Henning S, Gruters-Kieslich A, Vanhatalo S, Schmitz D, Kaila K. Respiratory alkalosis in children with febrile seizures. Epilepsia. 2011;52:1949–1955. doi: 10.1111/j.1528-1167.2011.03259.x. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Schmitz D, Rivera C, Vanhatalo S, Salmen B, Mackie K, Sipila ST, Voipio J, Kaila K. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat. Med. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueler C, Becker HM, McKenna R, Deitmer JW. Transport activity of the sodium bicarbonate cotransporter NBCe1 is enhanced by different isoforms of carbonic anhydrase. PLoS ONE. 2011;6:e27167. doi: 10.1371/journal.pone.0027167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol. Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- Schwede M, Garbett K, Mirnics K, Geschwind DH, Morrow EM. Genes for endosomal NHE6 and NHE9 are misregulated in autism brains. Mol. Psychiatry. 2014;19:277–279. doi: 10.1038/mp.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah GN, Ulmasov B, Waheed A, Becker T, Makani S, Svichar N, Chesler M, Sly WS. Carbonic anhydrase IV and XIV knockout mice: roles of the respective carbonic anhydrases in buffering the extracellular space in brain. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16771–16776. doi: 10.1073/pnas.0508449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kim D, Caldwell M, Sun D. The role of Na(+)/h (+) exchanger isoform 1 in inflammatory responses: maintaining H(+) homeostasis of immune cells. Adv. Exp. Med. Biol. 2013;961:411–418. doi: 10.1007/978-1-4614-4756-6_35. [DOI] [PubMed] [Google Scholar]
- Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 2012;287:40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinning A, Hubner CA. Minireview: pH and synaptic transmission. FEBS Lett. 2013;587:1923–1928. doi: 10.1016/j.febslet.2013.04.045. [DOI] [PubMed] [Google Scholar]
- Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem. J. 2007;401:623–633. doi: 10.1042/BJ20061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K. Molecular mechanisms of epilepsy. Nat. Neurosci. 2015;18:367–372. doi: 10.1038/nn.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff BJ. The AMPA receptor antagonist perampanel in the adjunctive treatment of partial-onset seizures: clinical trial evidence and experience. Ther. Adv. Neurol. Disord. 2015;8:137–147. doi: 10.1177/1756285615575696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA. The effects of benzodiazepines on cognition. J. Clin. Psychiatry. 2005;66(Suppl. 2):9–13. [PubMed] [Google Scholar]
- Stridh MH, Alt MD, Wittmann S, Heidtmann H, Aggarwal M, Riederer B, Seidler U, Wennemuth G, McKenna R, Deitmer JW, Becker HM. Lactate flux in astrocytes is enhanced by a non-catalytic action of carbonic anhydrase II. J. Physiol. (Lond.) 2012;590:2333–2351. doi: 10.1113/jphysiol.2011.220152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromme P, Dobrenis K, Sillitoe RV, Gulinello M, Ali NF, Davidson C, Micsenyi MC, Stephney G, Ellevog L, Klungland A, Walkley SU. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain. 2011;134:3369–3383. doi: 10.1093/brain/awr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer J, Bartsch C, Haas C. Sodium/proton exchanger 3 (NHE3) and sudden infant death syndrome (SIDS). Int. J. Legal Med. 2014;128:939–943. doi: 10.1007/s00414-014-0978-0. [DOI] [PubMed] [Google Scholar]
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- Supuran CT. Carbonic anhydrase inhibitors: an editorial. Expert Opin. Ther. Pat. 2013;23:677–679. doi: 10.1517/13543776.2013.778246. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De Jonghe P, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G. Defective membrane expression of the Na(+)-HCO(3)( ) cotransporter NBCe1 is associated with familial migraine. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15963–15968. doi: 10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Farrell K, Simon RP. Acidosis causes failure of astrocyte glutamate uptake during hypoxia. J. Cereb. Blood Flow Metab. 1995;15:417–424. doi: 10.1038/jcbfm.1995.52. [DOI] [PubMed] [Google Scholar]
- Takahashi KI, Copenhagen DR. Modulation of neuronal function by intra-cellular pH. Neurosci. Res. 1996;24:109–116. doi: 10.1016/0168-0102(95)00989-2. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Hosoki K, Matsushita M, Funatsuka M, Saito K, Kanazawa H, Goto Y, Saitoh S. A loss-of-function mutation in the SLC9A6 gene causes X-linked mental retardation resembling Angelman syndrome. Am. J. Med. Genet. B: Neuropsychiatr. Genet. 2011;156B:799–807. doi: 10.1002/ajmg.b.31221. [DOI] [PubMed] [Google Scholar]
- Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, Hardy C, O'Meara S, Latimer C, Dicks E, Menzies A, Stephens P, Blow M, Greenman C, Xue Y, Tyler-Smith C, Thompson D, Gray K, Andrews J, Barthorpe S, Buck G, Cole J, Dunmore R, Jones D, Maddison M, Mironenko T, Turner R, Turrell K, Varian J, West S, Widaa S, Wray P, Teague J, Butler A, Jenkinson A, Jia M, Richardson D, Shepherd R, Wooster R, Tejada MI, Martinez F, Carvill G, Goliath R, de Brouwer AP, van Bokhoven H, Van Esch H, Chelly J, Raynaud M, Ropers HH, Abidi FE, Srivastava AK, Cox J, Luo Y, Mallya U, Moon J, Parnau J, Mohammed S, Tolmie JL, Shoubridge C, Corbett M, Gardner A, Haan E, Rujirabanjerd S, Shaw M, Vandeleur L, Fullston T, Easton DF, Boyle J, Partington M, Hackett A, Field M, Skinner C, Stevenson RE, Bobrow M, Turner G, Schwartz CE, Gecz J, Raymond FL, Futreal PA, Stratton MR. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat. Genet. 2009;41:535–543. doi: 10.1038/ng.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauboll E, Sveberg L, Svalheim S. Interactions between hormones and epilepsy. Seizure. 2015;28:3–11. doi: 10.1016/j.seizure.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Terbach N, Williams RS. Structure–function studies for the panacea, valproic acid. Biochem. Soc. Trans. 2009;37:1126–1132. doi: 10.1042/BST0371126. [DOI] [PubMed] [Google Scholar]
- Thiry A, Rolin S, Vullo D, Frankart A, Scozzafava A, Dogne JM, Wouters J, Supuran CT, Masereel B. Indanesulfonamides as carbonic anhydrase inhibitors and anticonvulsant agents: structure–activity relationship and pharmacological evaluation. Eur. J. Med. Chem. 2008;43:2853–2860. doi: 10.1016/j.ejmech.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Thomas RC. The plasma membrane calcium ATPase (PMCA) of neurones is electroneutral and exchanges 2 H+ for each Ca2+or Ba2+ ion extruded. J. Physiol. (Lond.) 2009;587:315–327. doi: 10.1113/jphysiol.2008.162453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolner EA, Hochman DW, Hassinen P, Otahal J, Gaily E, Haglund MM, Kubova H, Schuchmann S, Vanhatalo S, Kaila K. Five percent CO(2) is a potent, fast-acting inhalation anticonvulsant. Epilepsia. 2011;52:104–114. doi: 10.1111/j.1528-1167.2010.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Differential sensitivity to intracellular pH among high- and low-threshold Ca2+ currents in isolated rat CA1 neurons. J. Neurophysiol. 1997;77:639–653. doi: 10.1152/jn.1997.77.2.639. [DOI] [PubMed] [Google Scholar]
- Tong CK, Chen K, Chesler M. Kinetics of activity-evoked pH transients and extracellular pH buffering in rat hippocampal slices. J. Neurophysiol. 2006;95:3686–3697. doi: 10.1152/jn.01312.2005. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-d-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl. 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann. Neurol. 1989;25:213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- Tzschach A, Ullmann R, Ahmed A, Martin T, Weber G, Decker-Schwering O, Pauly F, Shamdeen MG, Reith W, Oehl-Jaschkowitz B. Christianson syndrome in a patient with an interstitial Xq26.3 deletion. Am. J. Med. Genet. A. 2011;155a:2771–2774. doi: 10.1002/ajmg.a.34230. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P, Hoogenraad CC. New insights in endosomal dynamics and AMPA receptor trafficking. Semin. Cell Dev. Biol. 2011;22:499–505. doi: 10.1016/j.semcdb.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Velisek L, Dreier JP, Stanton PK, Heinemann U, Moshe SL. Lowering of extracellular pH suppresses low-Mg(2+)-induces seizures in combined entorhinal cortex-hippocampal slices. Exp. Brain Res. 1994;101:44–52. doi: 10.1007/BF00243215. [DOI] [PubMed] [Google Scholar]
- Velisek L, Moshe SL, Cammer W. Developmental changes in seizure susceptibility in carbonic anhydrase II-deficient mice and normal littermates. Brain Res. Dev. Brain Res. 1993;72:321–324. doi: 10.1016/0165-3806(93)90200-t. [DOI] [PubMed] [Google Scholar]
- Velly J, Grima M, Decker N, Cragoe EJ, Jr., Schwartz J. Effects of amiloride and its analogues on [3H]batrachotoxinin-A 20-alpha benzoate binding, [3H]tetracaine binding and 22Na influx. Eur. J. Pharmacol. 1988;149:97–105. doi: 10.1016/0014-2999(88)90047-7. [DOI] [PubMed] [Google Scholar]
- Verpelli C, Galimberti I, Gomez-Mancilla B, Sala C. Molecular basis for prospective pharmacological treatment strategies in intellectual disability syndromes. Dev. Neurobiol. 2014;74:197–206. doi: 10.1002/dneu.22093. [DOI] [PubMed] [Google Scholar]
- Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J. Physiol. (Lond.) 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas GL, Johnson DE, Freund P, Casey JR. Characterization of an epilepsy-associated variant of the human Cl /HCO3(−) exchanger AE3. Am. J. Physiol. Cell Physiol. 2009;297:C526–C536. doi: 10.1152/ajpcell.00572.2008. [DOI] [PubMed] [Google Scholar]
- Vink JM, Smit AB, de Geus EJ, Sullivan P, Willemsen G, Hottenga JJ, Smit JH, Hoogendijk WJ, Zitman FG, Peltonen L, Kaprio J, Pedersen NL, Magnusson PK, Spector TD, Kyvik KO, Morley KI, Heath AC, Martin NG, Westendorp RG, Slagboom PE, Tiemeier H, Hofman A, Uitterlinden AG, Aulchenko YS, Amin N, van Duijn C, Penninx BW, Boomsma DI. Genome-wide association study of smoking initiation and current smoking. Am. J. Hum. Genet. 2009;84:367–379. doi: 10.1016/j.ajhg.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley SU. Secondary accumulation of gangliosides in lysosomal storage disorders. Semin. Cell Dev. Biol. 2004;15:433–444. doi: 10.1016/j.semcdb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kaida Y, Takechi K, Kamei C. Anticonvulsant effect of (RS)-1-aminoindan-1,5-dicarboxylic acid on pentetrazol-induced kindled seizures in mice. Biol. Pharm. Bull. 2010;33:647–652. doi: 10.1248/bpb.33.647. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Li W, Chen JF. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim. Biophys. Acta. 2011;1808:1358–1379. doi: 10.1016/j.bbamem.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Whitten ST, Garcia-Moreno EB, Hilser VJ. Local conformational fluctuations can modulate the coupling between proton binding and global structural transitions in proteins. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4282–4287. doi: 10.1073/pnas.0407499102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Epilepsy Fact Sheet Ed. W.H. Organization: 2015. [Google Scholar]
- Xia Y, Zhao P, Xue J, Gu XQ, Sun X, Yao H, Haddad GG. Na+ channel expression and neuronal function in the Na+/H+ exchanger 1 null mutant mouse. J. Neurophysiol. 2003;89:229–236. doi: 10.1152/jn.00488.2002. [DOI] [PubMed] [Google Scholar]
- Xinhan L, Matsushita M, Numaza M, Taguchi A, Mitsui K, Kanazawa H. Na+/H+ exchanger isoform 6 (NHE6/SLC9A6) is involved in clathrin-dependent endocytosis of transferrin. Am. J. Physiol. Cell Physiol. 2011;301:C1431–C1444. doi: 10.1152/ajpcell.00154.2011. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen H, Li J, Zhao Y, Ghishan FK. Disruption of NHE8 expression impairs Leydig cell function in the testes. Am. J. Physiol. Cell Physiol. 2015;308:C330–C338. doi: 10.1152/ajpcell.00289.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Shi XY, Ju J, Zhang WN, Liu YJ, Li XY, Zou LP. 5% CO(2) inhalation suppresses hyperventilation-induced absence seizures in children. Epilepsy Res. 2014;108:345–348. doi: 10.1016/j.eplepsyres.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289:49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Yoon D, Frick KD, Carr DA, Austin JK. Economic impact of epilepsy in the United States. Epilepsia. 2009;50:2186–2191. doi: 10.1111/j.1528-1167.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- Zhang-James Y, DasBanerjee T, Sagvolden T, Middleton FA, Faraone SV. SLC9A9 mutations, gene expression, and protein-protein interactions in rat models of attention-deficit/hyperactivity disorder. Am. J. Med. Genet. B: Neuropsychiatr. Genet. 2011;156b:835–843. doi: 10.1002/ajmg.b.31229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang-James Y, Middleton FA, Sagvolden T, Faraone SV. Differential expression of SLC9A9 and interacting molecules in the hippocampus of rat models for attention deficit/hyperactivity disorder. Dev. Neurosci. 2012;34:218–227. doi: 10.1159/000338813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Velumian AA, Jones OT, Carlen PL. Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia. 2000;41(Suppl. 1):S52–S60. doi: 10.1111/j.1528-1157.2000.tb02173.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chernova MN, Stuart-Tilley AK, Jiang L, Alper SL. The cytoplasmic and transmembrane domains of AE2 both contribute to regulation of anion exchange by pH. J. Biol. Chem. 1996;271:5741–5749. doi: 10.1074/jbc.271.10.5741. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nguyen KT, Barrett EF, David G. Vesicular ATPase inserted into the plasma membrane of motor terminals by exocytosis alkalinizes cytosolic pH and facilitates endocytosis. Neuron. 2010;68:1097–1108. doi: 10.1016/j.neuron.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]