Abstract
Importance
Although intermittent androgen deprivation therapy (ADT) has not been associated with better overall survival in prostate cancer (PC), it has the potential for lower side effects. The incidence of long-term adverse health events has not been reported.
Objective
Given that older patients are more likely to suffer long-term complications from ADT, we examined long-term late events in elderly patients randomized to intermittent or continuous ADT. Our hypothesis was that late cardiovascular and endocrine events would be lower in patients on intermittent ADT.
Design
Linkage between patient trial data and corresponding Medicare claims.
Setting
Multicenter clinical trial.
Participants
Patients from S9346, a randomized SWOG trial of intermittent vs. continuous ADT in men with metastatic PC.
Main Outcomes and Measures
The main outcome was to identify long-term adverse health events by treatment arm. Patients were classified as having an adverse health event if they had any hospital claim – or at least 2 physician or outpatient claims at least 30 days apart – for any of the following diagnoses: ischemic and thrombotic events; endocrine events; sexual dysfunction, dementia and depression. To incorporate time from beginning of observation through evidence of an event, we determined the cumulative incidence of each event. Competing risks Cox regression was used, adjusting for covariates.
Results
In total, n=1134 eligible U.S.-based patients with metastatic PC were randomized to continuous vs. intermittent ADT on S9346. A total of 636 (56%) of trial participants had ≥1 year of continuous Medicare parts A & B coverage and no HMO participation. The median age was 71.3 years. The most common long-term events were hypercholesterolemia (31%) and osteoporosis (19%). The 10-year cumulative incidence of ischemic and thrombotic events differed by arm; 24% for continuous and 33% for intermittent ADT (Hazard Ratio=0.69, p=.02). There were no statistically significant differences by arm in any other adverse health events.
Conclusions and Relevance
Contrary to our hypothesis that intermittent ADT would reduce long-term health-related events compared to continuous ADT, we found that older men assigned to intermittent ADT had no apparent reduction in bone, endocrine, or cognitive events and an increased incidence of ischemic and thrombotic events.
INTRODUCTION
The methodology to compare experimental to standard therapies through large, randomized clinical trials in a network of large cooperative oncology groups has been refined and improved over decades. The result has been significant improvements in patient survival, and a dramatic increase in the number of cancer survivors in the U.S, especially survivors of breast and prostate cancer.(1) Such trials routinely capture detailed patient information on prognostic factors for eligibility, detailed treatment information, short-term acute toxicity and adverse effect information, cancer recurrence dates and dates of death. However, the ascertainment of long-term adverse health events following treatment is often a challenge. Ascertainment of adverse health events require the evaluation of large numbers of patients for many years following primary treatment and is typically prohibitively expensive. Additionally, some events may not be anticipated or recognized, and therefore not attributed to the intervention.
Androgen deprivation therapy (ADT) with gonadotropin-releasing hormone (GnRH) agonists or bilateral orchiectomy, is the mainstay of treatment of metastatic prostate cancer, and its use has been increasing steadily over time.(2) Although ADT improves outcomes in patients with prostate cancer, it has an array of side effects including sexual dysfunction, bone demineralization, cardiovascular disease, metabolic complications, cognitive changes, and diminished quality-of-life.(3) To study these outcomes, investigators have used large administrative databases such as the Surveillance, Epidemiology and End-Results (SEER)-Medicare database, that contains clinical, demographic, and medical claims data. With regard to skeletal complications, the incidence of vertebral fractures increases by 40% in men on ADT, with longer duration associated with increased risk.(4) Incident diabetes is likely a side effect of ADT, with 2 reports showing risks of new onset diabetes ranging from 36%–49%.(5, 6) Increased cardiovascular mortality has also been reported. In a recent study, GnRH agonist use was associated with increased risk of coronary heart disease (adjusted HR, 1.16; P < .001), myocardial infarction (adjusted HR, 1.11; P = .03), and sudden cardiac death (adjusted HR, 1.16; P = .004).(5) In addition, an increased risk of acute kidney injury has recently been reported with ADT. (7, 8) These observational studies are often limited due to selection bias and lack of detailed information on treatments and prognostic factors.
Efforts to minimize toxicity have focused on the use of intermittent androgen deprivation (IAD) in which the GnRH agonist is started and stopped cyclically to allow androgen levels to return to normal, reducing the hypogonadal period and potentially reducing side effects. In general, patients can experience improvements in quality-of-life, sexual function, anemia, weight gain, hot flashes, and psychological well-being while off ADT; symptoms generally return when ADT is restarted.(9) In 2013, results of two randomized trials intensified the controversy with regard to these two therapies. In patients with metastatic prostate cancer, SWOG 9346 randomized 1535 subjects to continuous or intermittent ADT; while there was a short-term benefit for intermittent therapy in terms of impotence and mental health, intermittent ADT was inferior in terms of survival.(10) In non-metastatic cancer, a randomized trial found the two treatments equivalent for cancer control but that intermittent ADT had improved endocrine symptoms. Long-term outcomes were not reported.(11)
To overcome some of the challenges of selection and treatment indication bias from observational studies, we have linked the clinical records for prostate cancer patients in SWOG trials to their Medicare administrative claims. Given that older patients are more likely to suffer long-term complications from ADT, we examined long-term late events in elderly patients randomized to intermittent or continuous ADT. Our hypothesis was that late cardiovascular and endocrine events would be lower in patients on intermittent ADT.
PATIENTS AND METHODS
Clinical trial details
We studied patients from SWOG study S9346, a randomized trial of intermittent vs. continuous androgen deprivation therapy; the study design was previously described.(10) In brief, men with newly diagnosed, metastatic, hormone-sensitive prostate cancer were initially registered if they had a performance status of 0–2 and a prostate specific antigen (PSA) of ≥5 ng/m. After 7 months of induction GnRH and an antiandrogen, patients whose PSA fell to ≤4 ng/m were randomized to continuous vs. intermittent androgen deprivation therapy. Patients randomized to the intermittent group discontinued ADT and then resumed once the PSA increased, and discontinued again if, after 7 months, PSA fell again to ≤4 ng/m. Patients were excluded if they had active medical illness that precluded protocol treatment.
Prospective clinical trial data were obtained according to protocol specifications and included demographic factors (age (<65 years vs. ≥65 years), race (black vs. white vs. other) and data on prognostic factors used in stratification performance status (based on Zubrod 0–1 vs. 2), extent of disease (minimal vs. extensive), and type of prior hormone therapy (neoadjuvant vs. finasteride vs. neither).(10) Patients on the trial were followed for 10 years after initial registration or until death, whichever came first.
Methods
To identify long-term adverse health events following treatment, we established a linkage between the SWOG clinical records and Medicare claims data. To establish the link, Medicare claims must have matched the SWOG clinical record according to social security number, sex, and date of birth. To be included, patients must have had ≥1 year of continuous Medicare Parts A & B coverage, in order to ensure a sufficient minimum amount of coverage to identify potential adverse health events. While enrolled in Medicare, patients must not have had HMO coverage for 1 continuous year, as HMO providers do not submit data to CMS. To increase the amount of available follow-up claims information, we included patients with ≥1 year of Medicare claims at any time in the follow-up period; thus patients may not have been ≥65 years old at randomization, but instead, could have aged into the Medicare claims cohort. Identification of late effects of treatment relied on physician supplier Part B (i.e. carrier), hospital outpatient, and hospital inpatient Medicare claims records. Long-term events were determined using HCPCS and ICD9 diagnosis codes. The set of codes used to identify adverse events was established by the authors prior to the analysis; codes were chosen based on the literature and clinical relevance. The date of death was based on the SWOG clinical record when available, and on Medicare records when SWOG data were not available. Although S9346 was an international clinical trial, since the study relies on a linkage to Medicare claims data, we analyzed only U.S.-based patients. Approval to conduct this research was obtained from the Institutional Review Board of Cancer Research And Biostatistics (Seattle, WA).
Adverse Health Events
Patients were classified as having an adverse health event if they had any hospital claim (Current Procedural Terminology (CPT) codes)– or at least 2 physician or outpatient claims at least 30 days apart – for any of the following diagnoses: ischemic and thrombotic events (acute myocardial infarction; ischemic heart disease, thrombosis); endocrine events (diabetes, diabetes with sequelae, hypercholesterolemia, obesity, osteoporosis, fractures, any bone event); sexual dysfunction, dementia and depression; and acute kidney injury (Appendix)(7, 12, 13). Superficial thrombosis was removed due to a small number of events. Prior history of a specific event was defined as any claim within 12 months before study registration. For the analysis of a given adverse event, patients with evidence that the event occurred within 1 year prior to study randomization (i.e. pre-existing comorbid conditions) were excluded.(14) This was done to emphasize the incidence of new events.
Statistical Analysis
To incorporate time from beginning of observation till evidence of an event, and to account for potential competing risks of death, we analyzed the cumulative incidence from randomization. Data were left-truncated given there may have been a gap between randomization and the initiation of Medicare claims coverage, especially for patients <65 years of age at randomization. For each event, multivariable competing risk regression analysis was conducted, based on Cox regression (procedure proc phreg, SAS Version 9.4), to examine time-to-event adjusting for covariates and incorporating left truncated and right censored data.(15, 16) The subdistribution hazard ratios, 95% confidence intervals, and p-values were generated for each event. In each model, the adverse health event was considered the event of interest and deaths were analyzed as a competing risk; otherwise patients were censored at their date of last contact if no death was observed. Model covariates included demographic and stratification factors as previously specified. To examine event rates alone by arm (rather than time to event), multivariable logistic regression models were used, adjusting for the same demographic and stratifications as covariates.
Results
A total of 1134 eligible patients from the U.S. were randomized to continuous or intermittent therapy on S9346. We linked 748 of these patients to Medicare claims, of which 636 (56% of total U.S. based patients) had ≥1 year of continuous Medicare parts A & B coverage and no HMO participation. This cohort comprised the evaluable patient set for this analysis.
Patient Characteristics
Patient characteristics by intervention assignment are shown in Table 1. No notable imbalances by arm were observed by age, ethnicity, weight, performance status, extent of disease or prior hormonal therapy. The majority of patients had a body mass index of >25 (76%), took no prior hormonal therapy (86%), and had a performance status of 0–1 (98%). Patients in this subset had a similar profile to the parent study population. At baseline, there was a small difference in the proportion of patients with diabetes with sequalae (4% on the continuous arm vs. 1% on the intermittent arm, p=.02). Baseline event rates were otherwise similar between the treatment groups for all other events examined.
Table 1.
Baseline characteristics of patients on Continuous and Intermittent Androgen Deprivation Therapy, S9346
| Current Medicare Study | p- value1 |
S9346 Parent Population |
|||
|---|---|---|---|---|---|
| Continuous | Intermittent | All | |||
| n=311 | n=325 | n=636 | n=1,535 | ||
| Age, yr | .56 | ||||
| Mean | 71.5 | 71.1 | 71.3 | 70 | |
| Median | 71.2 | 71 | 71.1 | 70.4 | |
| <65 | 70 (23%) | 80 (25%) | 151 (24%) | 476 (31%) | |
| 65+ | 241 (77%) | 245 (75%) | 490 (76%) | 1057 (69%) | |
| Weight, kg | .46 | ||||
| Mean | 88 | 90.3 | 89.1 | 89.6 | |
| Median | 87.4 | 85 | 86.6 | 85 | |
| BMI, kg/m2 | .96 | ||||
| Mean | 30.7 | 30.6 | 30.6 | 29.6 | |
| Median | 27.8 | 27.8 | 27.8 | 27.6 | |
| <25 | 69 (24%) | 72 (24%) | 143 (24%) | 371 (27%) | |
| 25+ | 218 (76%) | 232 (76%) | 453 (76%) | 995 (73%) | |
| Performance Status | .70 | ||||
| 0–1 | 305 (98%) | 320 (98%) | 630 (98%) | 1474 (96%) | |
| 2 | 6 (2%) | 5 (2%) | 11 (2%) | 59 (4%) | |
| Severity of Disease | .30 | ||||
| Minimal | 175 (56%) | 170 (52%) | 345 (54%) | 791 (52%) | |
| Extensive | 135 (44%) | 155 (48%) | 296 (46%) | 742 (48%) | |
| Prior Hormone Therapy | .40 | ||||
| Finasteride | 3 (<1%) | 2 (<1%) | 5 (1%) | 10 (1%) | |
| Neoadjuvant | 34 (11%) | 47 (14%) | 85 (13%) | 176 (11%) | |
| Neither | 274 (88%) | 276 (85%) | 551 (86%) | 1347 (88%) | |
| Race | .68 | ||||
| White | 253 (81%) | 262 (81%) | 520 (81%) | 1024 (67%) | |
| Black | 51 (16%) | 52 (16%) | 103 (16%) | 189 (12%) | |
| Asian/PI | 4 (1%) | 3 (1%) | 7 (1%) | 16 (1%) | |
| Native | 1 (1%) | 3 (1%) | 4 (1%) | 6 (1%) | |
| Unknown | 2 (1%) | 5 (1%) | 7 (1%) | 298 (19%)3 | |
| Prior comorbidities2 | |||||
| Diabetes | 44 (14%) | 43 (14%) | 87 (14%) | .73 | |
| Hypercholesterolemia | 66 (21%) | 75 (24%) | 141 (22%) | .59 | |
| Osteoperosis | 40 (13%) | 39 (12%) | 79 (12%) | .73 | |
| All Bone | 97 (31%) | 85 (26%) | 182 (28%) | .15 | |
| Organic Sexual Dysfunction |
13 (4%) | 15 (5%) | 28 (4%) | .79 | |
| Depression | 14 (4%) | 12 (4%) | 26 (4%) | .60 | |
T-tests were conducted to compare continuous measures between treatment arms, and chi-square tests to compare categorical variables between treatment arms.
Based on claims prior to enrollment. Selected conditions are shown. In addition to the conditions listed in the table, there was no difference by arm in the proportion of patients with acute MI (p=.93; overall baseline rate = 2%), ischemic heart disease (p=.74; overall baseline rate = 3%), severe thrombosis (p=.95; overall baseline rate = 1%), obesity (p=.59; overall baseline rate = 2%), fracture (p=.11; overall baseline rate = 4%), dementia (p=.69; overall baseline rate <1%), and acute kidney injury (p=.15; overall baseline rate = 2%). Diabetes with sequelae was somewhat higher on the continuous arm (p=.02; overall baseline rate = 3%).
The large number of patients reporting unknown race from the parent trial was due to the absence of race reporting from international collaborators.
We examined whether survival patterns in the subset of patients with Medicare claims could have influenced ascertainment of adverse health events by arm. The hazard ratio of death for intermittent to continuous therapy was 1.14 (95% CI: 0.94–1.38, p=.18) in those with Medicare claims and 1.09 (95% CI: 0.93–1.27, p=.31) in those without Medicare claims. There was no evidence that these survival patterns by arm differed by Medicare claims group (interaction p-value=.79).
Adverse Events Following ADT
The adverse health events following ADT therapy observed through Medicare claims are shown in Table 2. Overall, the most common events were endocrine (41%), especially hypercholesterolemia (31%). Bone related events were also common with 19% of men diagnosed with osteoporosis and 14% having a fracture. Ischemic and thrombotic events were also common (27%) with 10% having a claim for ischemic heart disease.
Table 2.
Estimates of Long-Term Adverse Health Events Following Treatment with Continuous (C) vs. Intermittent (I) Androgen Deprivation Therapy for Patients on S9346
| Logistic Regression Results | Cumulative Incidence Results | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | C | I | 5-Year | 10-Year | |||||||||||
| Late Effect | N | % | N | % | N | % | OR (95% CI) | p- value |
Total | C | I | C | I | HR* | p- value |
|
Ischemic and Thrombotic Events |
153 | 24 | 64 | 20 | 89 | 27 | 0.69 (0.47–0.99) | .05 | 30 | 16 | 23 | 24 | 33 | 0.69 | .02 |
| Acute MI | 51 | 8 | 23 | 7 | 28 | 9 | 0.82 (0.46–1.47) | .51 | 10 | 5 | 6 | 8 | 10 | 0.80 | .42 |
| Ischemic Heart Disease |
49 | 8 | 18 | 6 | 31 | 10 | 0.56 (0.31–1.03) | .06 | 9 | 5 | 8 | 7 | 12 | 0.55 | .05 |
| Thrombosis (severe) | 85 | 13 | 37 | 12 | 48 | 15 | 0.79 (0.5–1.26) | .33 | 17 | 9 | 12 | 14 | 17 | 0.79 | .28 |
| Endocrine | 273 | 43 | 138 | 44 | 135 | 41 | 1.15 (0.83–1.59) | .39 | 52 | 44 | 41 | 51 | 48 | 1.06 | .69 |
| Diabetes | 123 | 19 | 62 | 20 | 61 | 19 | 1.12 (0.75–1.67) | .58 | 24 | 19 | 18 | 23 | 22 | 1.08 | .69 |
| Diabetes with Sequelae |
56 | 9 | 26 | 8 | 30 | 9 | 0.9 (0.52–1.57) | .72 | 12 | 8 | 8 | 9 | 10 | 0.89 | .66 |
| Hypercholesterolemia | 200 | 31 | 99 | 32 | 101 | 31 | 1.04 (0.74–1.45) | .83 | 39 | 30 | 29 | 37 | 37 | 1.00 | 1.00 |
| Obesity | 42 | 7 | 25 | 8 | 17 | 5 | 1.59 (0.83–3.01) | .16 | 8 | 7 | 5 | 9 | 6 | 1.49 | .20 |
| Osteoporosis | 135 | 21 | 73 | 23 | 62 | 19 | 1.29 (0.87–1.9) | .20 | 26 | 21 | 18 | 27 | 22 | 1.17 | .35 |
| Fracture | 99 | 15 | 53 | 17 | 46 | 14 | 1.24 (0.8–1.92) | .34 | 19 | 12 | 10 | 20 | 17 | 1.17 | .43 |
| All Bone | 182 | 28 | 97 | 31 | 85 | 26 | 1.28 (0.9–1.82) | .17 | 35 | 27 | 23 | 36 | 31 | 1.16 | .32 |
|
SEXUAL DYSFUNCTION |
|||||||||||||||
| Organic | 36 | 6 | 20 | 6 | 16 | 5 | 1.32 (0.67–2.61) | .42 | 7 | 6 | 5 | 8 | 6 | 1.29 | .46 |
| MISCELLANEOUS | |||||||||||||||
| Dementia | 33 | 5 | 22 | 7 | 11 | 3 | 2.05 (0.97–4.36) | .06 | 7 | 5 | 2 | 8 | 4 | 1.97 | .07 |
| Depression | 89 | 14 | 43 | 14 | 46 | 14 | 0.94 (0.59–1.47) | .77 | 18 | 11 | 12 | 15 | 16 | 0.92 | .69 |
| Acute Kidney Injury | 122 | 19 | 60 | 19 | 62 | 19 | 1.02 (0.68–1.52) | .93 | 30 | 14 | 14 | 21 | 21 | 0.98 | .91 |
Regression analyses were conducted separately for each adverse event type in multivariable regression models, adjusting for demographic and stratification factors as covariates as specified in the Methods.
Represents the subdistribution hazard ratios based on competing risk regression analyses.
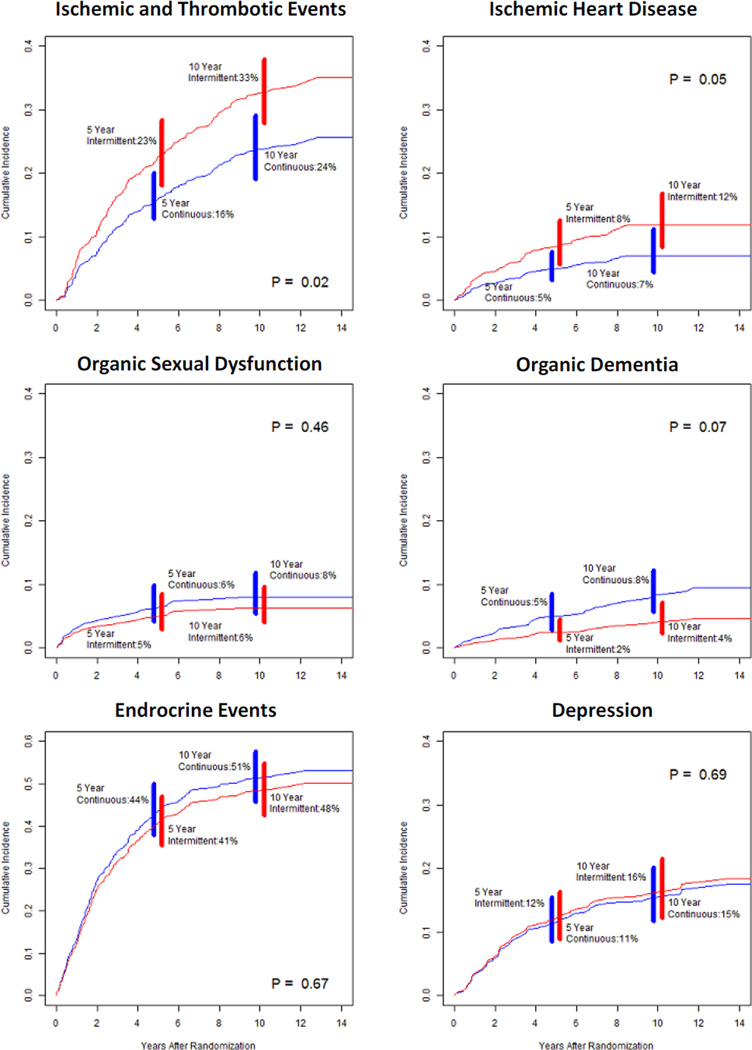
The 10-year cumulative incidence of any ischemic or thrombotic event was 24% for continuous therapy and 33% for intermittent therapy (HR=0.69, p=.02). The 10-year cumulative incidence of ischemic heart disease alone was 7% for continuous therapy and 12% for intermittent therapy (HR=0.55, p=.05). There were no statistically significant differences by arm in the cumulative incidence of any other events (Table 2). Dementia was observed in 8% of patients on continuous therapy and 4% on intermittent therapy (HR=1.98, p=.07), however these results should be interpreted with caution given the low number of reported events. Plots of the predicted cumulative incidence functions for the common adverse health events are shown in Figure 2.
Sensitivity Analyses
To rule out the impact of post-progression interventions on patterns of adverse health events, we examined the average impact of randomized treatment on the cumulative incidence of adverse events prior to progression. The median time to progression among patients who progressed was 2.2 years. To avoid selection bias, we truncated follow-up at this timepoint for all patients. The results were consistent with the primary results (Table 3), suggesting that observed differences by arm were consistent throughout follow-up.
Table 3.
Cumulative Incidence of Long-Term Adverse Health Events Following Treatment with Continuous (C) vs. Intermittent (I) Androgen Deprivation Therapy for Patients on S9346, events prior to median progression-free survival (812 days)
| Cumulative Incidence Results | |||||
|---|---|---|---|---|---|
| Adverse Health Event | Total CI | C | I | HR* | p-value |
| Ischemic and Thrombotic Events | 10 | 7 | 14 | 0.48 | .02 |
| Acute MI | 4 | 3 | 5 | 0.57 | .22 |
| Ischemic Heart Disease | 4 | 2 | 6 | 0.35 | .04 |
| Thrombosis (severe) | 5 | 4 | 6 | 0.79 | .57 |
| Endocrine | 28 | 30 | 26 | 1.16 | .42 |
| Diabetes | 12 | 13 | 12 | 1.09 | .73 |
| Diabetes with Sequelae | 4 | 5 | 4 | 1.34 | .49 |
| Hypercholesterolemia | 21 | 22 | 20 | 1.13 | .56 |
| Obesity | 3 | 3 | 2 | 1.57 | .45 |
| Osteoporosis | 11 | 12 | 10 | 1.09 | .74 |
| Skeletal related event and fracture | 5 | 7 | 4 | 1.83 | .14 |
| All Bone | 14 | 15 | 13 | 1.15 | .56 |
| Sexual Dysfunction | |||||
| Organic | 4 | 4 | 4 | 1.16 | .75 |
| Miscellaneous | |||||
| Depression | 7 | 7 | 8 | 0.85 | .62 |
| Acute Kidney Injury | 7 | 6 | 9 | 0.62 | .18 |
Regression analyses were conducted separately for each adverse event type in multivariable regression models, adjusting for demographic and stratification factors as covariates as specified in the Methods. In addition to the events listed in the table, there was no difference by arm in the cumulative incidence of dementia (overall cumulative incidence = 2%; OR=2.91, p=.12).
Represents the subdistribution hazard ratios based on competing risk regression analyses.
One concern about the primary analysis by randomized assignment is that patients on the intermittent arm may not have received any additional therapy. As a sensitivity analysis, we excluded the 12% of intermittent arm patients with no evidence of receipt of any anti-androgen deprivation consolidation therapy. No substantive differences compared to the primary analysis results were evident (see Appendix Table 2), however the increased incidence of dementia in the continuous arm was now significant (HR=2.51, p=.03). To assess whether potential differences by arm in the duration of ADT influenced the results, we included a time-dependent covariate in the multivariable Cox regression models indicating whether the event occurred within six months of the completion of ADT. The regression model results by arm were similar (data not shown). Also, on the IAD arm, there was no difference in the mean percentage of time receiving therapy between those with and without ischemic or thrombotic events (47% vs. 47%, p=.86). Lastly, for severe thrombosis, fracture, and acute kidney injury, there was no substantial differences in the results when only procedure codes, rather than the combination of diagnosis and procedure codes, were used to identify events (data not shown).
Discussion
Using a novel linkage between elderly patients enrolled in SWOG S9346 and Medicare claims, we have shown that known complications from androgen deprivation therapy were common in men on both treatment arms. Despite a belief that intermittent androgen deprivation would reduce complications from androgen deprivation therapy, we found no difference between arms for the endocrine, psychiatric, sexual or neurologic adverse events. In addition we found an increased cumulative incidence of ischemic and thrombotic events on intermittent therapy.
Prior population based observational studies have shown an increased risk of cardiovascular disease among men with prostate cancer treated with ADT. In a study of patients treated in the Veterans Healthcare Administration, ADT was associated with an increased risk of coronary heart disease (adjusted HR=1.19), myocardial infarction (adjusted HR=1.28), and sudden cardiac death (adjusted HR=1.35).(17) Similar results have been seen with SEER-Medicare.(5) We found a higher cumulative incidence of these events among men receiving intermittent ADT compared to continuous. This result was unexpected. In two prior trials comparing intermittent therapy to continuous therapy, patients in the continuous arm had a higher risk of cardiovascular mortality.(18, 19) Of interest, however, results from a Phase II study found patients treated with intermittent therapy to have an increased incidence of myocardial infarction during their off treatment period (4.6%) than during their on-treatment period (2.8%), but these differences were not statistically significant, and that patients were only evaluated until disease progression.(20) The biologic reasons for an increase in ischemic events in elderly patients treated with intermittent therapy are unclear, but changes in the coagulation cascade have been reported with lowering of testosterone during ADT as well as with increasing estrogen (after stopping ADT).(21) The risk of vascular events is highest initially, before coagulation cascade homeostasis is reached.(21, 22) In addition, a large study from the Swedish national health registry reported that the risk of incident cardiovascular disease was highest in the first 6 months after initiation of ADT in men with prostate cancer, particularly in those with a prior history of cardiovascular disease.(23) While patients in our study had a high rate of baseline diabetes and hypercholesterolemia, there was no difference in claims between the treatment groups.
Compared to no ADT, the incidence of vertebral fractures increases by 40% in men on ADT, with longer duration associated with increased risk.(4) In our study, bone events (osteoporosis or fracture) were common in both intermittent and continuous ADT, but were not different between arms. The cumulative incidence of these events was slightly higher than previous reports with shorter follow up.(24) The rates of skeletal events may be an underestimate of actual rates as all patients were randomized after receiving 7 months of ADT. It is also possible that events were misclassified as prostate cancer progression. Prior studies have shown that the largest decline in bone mineral density occurs during the first ADT on-treatment period with substantial heterogeneity in subsequent cycles.(25) It is also possible that there were inaccuracies in the submission of the claims. Finally, it was not known which patients were receiving bisphosphonate therapy, which may have affected the fracture rate.
It’s assumed that the main advantage of intermittent therapy appears to be a reduction in short-term symptoms while on therapy and reduced ADT cost. In a recent systematic review of trials comparing intermittent to continuous ADT, six of the nine trials evaluated quality of life and treatment related adverse effects. In some of the trials patients on intermittent therapy had improved sexual function, physical activity and general well being, but these results often did not persist over time, and there was only significant superiority of overall quality of life of men on intermittent therapy in one study.(26) In the results from the primary study there were no differences in grade 3 or 4 treatment-related adverse events between the intermittent (30.4%) and continuous (32.7%) groups, and no difference in cardiovascular events(10). It is possible that differences in severity may have resulted in the submission of claims for events that were not captured by event reporting, however we would expect that bias to be similar by arm given the random assignment of treatments. It is also possible that not all toxicities are captured in clinical trials, especially if the event was unknown to the primary investigative team. This may increase as a patient is further out from randomization and study monitoring becomes less frequent.
We acknowledge several limitations of our study and of the Medicare database in general. Although we required subjects to have two claims to reduce misclassification bias, a process routinely performed in studies using administrative claims, it is still possible that not all patients with Medicare claims had the complication we assigned.(27–29) It is also possible that patients with the toxicity did not have claims associated with it. Medicare lacks data on the severity of the toxicity; therefore, it is possible that these complications were mild and not life-threatening, and therefore not known to research staff reporting adverse events. Alternatively, as mild complications are sometimes not captured with billing data, our findings may have underestimated some complications in these older men. However we would anticipate this misclassification bias would not differ between randomly assigned treatments. We did not adjust for multiple comparisons; thus more marginal results may be more likely due to chance. Any positive findings should be confirmed in other studies. It is also possible that our results are not generalizable to younger patients in whom complication rates may be lower, however prostate cancer is more common in elderly men. Finally, all the patients were enrolled in a clinical trial, and therefore these results may not be generalizable to all patients who did not meet eligibility criteria for S9346, as the majority of patients had a performance status of 0 or 1.
This study has several unique strengths. Unlike previous observational studies of late-effects, our study benefited from random assignment between the arms, reducing the potential for unmeasured confounders to influence the treatment decisions as well as the outcomes. In addition, extent of disease and prior therapy were known on all participants. Also, evaluation of intermittent therapy with observational data would be complex, as PSA results and the reason for the treatment gap would not be captured in billing claims. Finally, we used similar methodology in defining outcome events as many prior investigators using claims data.
In conclusion, we did not find that patients randomly assigned to intermittent androgen deprivation had consistently fewer long-term adverse health events compared to those on continuous ADT. In fact, unexpectedly, we observed that elderly men assigned to intermittent therapy had an increased incidence of ischemic and thrombotic events. If these finding are confirmed, given the failure of the parent study to prove its non-inferiority endpoint, clinicians should be cautious about using intermittent ADT therapy in older men with metastatic prostate cancer given our inability to demonstrate a reduction in long-term adverse health events, the primary rationale for intermittent ADT.
Figure 1.
Predicted cumulative incidence of each individual adverse health event by treatment arm*
The p-values associated with each curve represent the multivariate competing risk regression p-values for the association of treatment and each individual adverse event, adjusting for demographic and stratification
AT A GLANCE.
Given that older patients are more likely to suffer long-term complications from ADT, we examined long-term late events in elderly patients randomized to intermittent or continuous ADT. Our hypothesis was that late cardiovascular and endocrine events would be lower in patients on intermittent ADT.
We have shown that known complications from androgen deprivation therapy are common in men on both intermittent and continuous ADT treatment arms.
Despite a belief that intermittent androgen deprivation would reduce complications from androgen deprivation therapy, we found no difference between arms for the endocrine, psychiatric, sexual or neurologic adverse events.
In fact, unexpectedly, we observed that elderly men assigned to intermittent therapy had an increased incidence of ischemic and thrombotic events.
Acknowledgments
Dr. Hershman (NCI R01CA134964) and Dr. Wright (NCI R01CA169121-01A1) are recipients of grants from the National Cancer Institute. Division of Cancer Prevention, NCORP Research Base grant 1UG1CA189974-01
Appendix
Appendix Table 1.
Codes for Toxicity
| Adverse Health Event | ICD –9 Diagnostic Codes | HCPCS Procedure Code | |
|---|---|---|---|
| Ischemic and Thrombotic Events | |||
| Acute MI | 410.XX | ||
| Ischemic Heart Disease | 411.0, 411.1, 411.81, 411.89 | ||
| Thrombosis (severe) | 325, 415.11, 415.12, 415.13, 415.19, 416.2, 437.6, 444.01, 444.09, 444.1, 444.21, 444.22, 444.81, 444.89, 444.9, 445.01, 445.02, 445.81, 445.89, 451.0, 451.11, 451.19, 451.2, 451.81, 451.82, 451.83, 451.84, 451.89, 451.9, 452, 453.0, 453.x, V12.51, V12.55 |
34101, 34111, 34203 | |
| Thrombosis (cautionary) |
451.0, 451.82 | ||
| Endocrine | |||
| Diabetes | 249.00, 249.01, 250.00, 250.01, 250.02, 250.03 | ||
| Diabetes with Sequelae | 249.1X-9X, 250.1X-9X, 253.5 | ||
| Hypercholesterolemia | 272.2, 272.4 | ||
| Obesity | 278.00, 278.01 | ||
| Osteoporosis | 733.00, 733.01, 733.02, 733.03, 733.09, 733.90, 733.99 | ||
| Fracture | 275.42, 336.9, 733.10, 733.11, 733.12, 733.13, 733.14, 733.15, 733.16, 733.19, 733.81, 733.82, 733.93, 733.94, 733.95, 733.96, 733.97, 733.98, 800.XX, 801.XX, 803.XX, 804.XX, 805.00, 805.01, 805.0X, 805.1X, 805.X, 806.XX, V54.17, V54.27 |
21310, 21470, 21800, 22305, 22310, 22318, 23500, 23515, 23570, 23575, 23600, 23615, 23620, 24500, 24515, 24516, 24685, 25545, 25560, 25600, 25605, 25606, 25607, 25620, 25622, 25630, 26600, 26720, 26725, 26740, 26746, 26765, 26770, 26776, 27193, 27215, 27228, 27235, 27506, 27511, 27520, 27524, 27530, 27750, 27758, 27759, 27786, 27814, 28400, 28470, 28490, 28510, 28630 |
|
| All Bone | Includes codes for osteoporosis and fracture. | ||
| SEXUAL DYSFUNCTION | |||
| Organic | 607.84 | ||
| MISCELLANEOUS | |||
| Dementia | 290.XX, 294.10, 294.11, 294.20, 294.21, 331.19, 331.2, 331.82 | ||
| Depression | 296.2X, 296.3X, 296.80, 298.0, 300.4, 301.12, 309.0, 309.1, 309.21, 311 |
||
| Acute Kidney Injury | 584.5, 584.6, 584.7, 584.8, 584.9, E879.1, V45.1, V56.1, V56.2, V56.8 |
90935, 90937 | |
Appendix Table 2.
Estimates of Long Term Adverse Health Events Following Treatment with Continuous (C) vs. Intermittent (I) Androgen Deprivation Therapy for Patients on S9346, Excluding Intermittent Arm Patients with No Evidence of Receipt of Any Consolidation Therapy
| Logistic Regression Results | Cumulative Incidence Results | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | C | I | 5-Year | 10-Year | |||||||||||
| Adverse Health Event | N | % | N | % | N | % | OR (95% CI) | p-value | Total | C | I | C | I | HR* | p-value |
| ISCHEMIC AND THROMBOTIC EVENTS |
146 | 24 | 64 | 20 | 82 | 28 | 0.67 (0.46–0.98) | .04 | 31 | 16 | 23 | 23 | 33 | 0.67 | .02 |
| Acute MI | 47 | 8 | 23 | 7 | 24 | 8 | 0.89 (0.49–1.62) | .70 | 10 | 5 | 6 | 8 | 9 | 0.86 | .60 |
| Ischemic Heart Disease | 49 | 8 | 18 | 6 | 31 | 11 | 0.5 (0.27–0.93) | .03 | 10 | 5 | 9 | 7 | 13 | 0.50 | .02 |
| Thrombosis (severe) | 82 | 14 | 37 | 12 | 45 | 15 | 0.76 (0.47–1.21) | .25 | 18 | 9 | 12 | 14 | 18 | 0.75 | .20 |
| ENDOCRINE | 259 | 43 | 138 | 44 | 121 | 41 | 1.15 (0.83–1.6) | .41 | 52 | 44 | 41 | 51 | 48 | 1.03 | .81 |
| Diabetes | 117 | 19 | 62 | 20 | 55 | 19 | 1.13 (0.75–1.72) | .55 | 24 | 18 | 18 | 23 | 22 | 1.09 | .67 |
| Diabetes with Sequelae | 52 | 9 | 26 | 8 | 26 | 9 | 0.97 (0.54–1.72) | .91 | 12 | 7 | 8 | 9 | 10 | 0.95 | .84 |
| Hypercholesterolemia | 194 | 32 | 99 | 32 | 95 | 32 | 0.97 (0.69–1.37) | .89 | 40 | 30 | 31 | 37 | 38 | .94 | .66 |
| Obesity | 42 | 7 | 25 | 8 | 17 | 6 | 1.44 (0.76–2.73) | .27 | 9 | 7 | 5 | 9 | 7 | 1.35 | .34 |
| Osteoporosis | 130 | 21 | 73 | 23 | 57 | 19 | 1.25 (0.84–1.85) | .28 | 26 | 21 | 18 | 26 | 23 | 1.13 | .49 |
| Skeletal related event and fracture |
96 | 16 | 53 | 17 | 43 | 15 | 1.18 (0.76–1.85) | .45 | 20 | 12 | 11 | 20 | 18 | 1.12 | .58 |
| All Bone | 176 | 29 | 97 | 31 | 79 | 27 | 1.22 (0.85–1.75) | .28 | 35 | 27 | 24 | 36 | 32 | 1.11 | .52 |
| SEXUAL DYSFUNCTION | |||||||||||||||
| Organic | 35 | 6 | 20 | 6 | 15 | 5 | 1.29 (0.65–2.58) | .47 | 7 | 6 | 5 | 8 | 6 | 1.25 | .52 |
| MISCELLANEOUS | |||||||||||||||
| Depression Acute Kidney Injury |
83 117 |
14 19 |
43 60 |
14 19 |
40 57 |
14 19 |
0.99 (0.62–1.58) 1.01 (0.67–1.52) |
.97 .97 |
18 31 |
11 13 |
12 14 |
15 21 |
15 21 |
0.97 0.98 |
.87 .90 |
Regression analyses were conducted separately for each adverse event type in multivariable regression models, adjusting for demographic and stratification factors as covariates as specified in the Methods. In addition to the events listed in the table, patients on the continuous ADT arm had higher observed rates of dementia (overall percent = 5%; OR = 2.65, p=.02; overall cumulative incidence = 7%, hazard ratio = 2.51, p=.03).
Represents the subdistribution hazard ratios based on competing risk regression analyses.
REFERENCES
- 1.Cancer facts and figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 2.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103(8):1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 3.Buchan NC, Goldenberg SL. Intermittent versus continuous androgen suppression therapy: do we have consensus yet? Curr Oncol. 2010;17(Suppl 2):S45–S48. doi: 10.3747/co.v17i0.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23(31):7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 5.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 6.Lage MJ, Barber BL, Markus RA. Association between androgen-deprivation therapy and incidence of diabetes among males with prostate cancer. Urology. 2007;70(6):1104–1108. doi: 10.1016/j.urology.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Gandaglia G, Sun M, Hu JC, Novara G, Choueiri TK, Nguyen PL, et al. Gonadotropin-releasing hormone agonists and acute kidney injury in patients with prostate cancer. European urology. 2014;66(6):1125–1132. doi: 10.1016/j.eururo.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Lapi F, Azoulay L, Niazi MT, Yin H, Benayoun S, Suissa S. Androgen deprivation therapy and risk of acute kidney injury in patients with prostate cancer. Jama. 2013;310(3):289–296. doi: 10.1001/jama.2013.8638. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsson PA. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2010;57(1):49–59. doi: 10.1016/j.eururo.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368(14):1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crook J. The role of intermittent androgen suppression in biochemically recurrent or newly diagnosed metastatic prostate cancer. Current opinion in supportive and palliative care. 2013;7(3):258–264. doi: 10.1097/SPC.0b013e328363602e. [DOI] [PubMed] [Google Scholar]
- 12.SEER-Medicare macro for the calculation of comorbidity weights. [Accessed on 2/25/2013]; available at: http://appliedresearch.cancer.gov/seermedicare/program/comorbidity.html.
- 13.Tollefson MK, Gettman MT, Karnes RJ, Frank I. Administrative data sets are inaccurate for assessing functional outcomes after radical prostatectomy. J Urol. 2011;185(5):1686–1690. doi: 10.1016/j.juro.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Medical care. 2002;40(8 Suppl) doi: 10.1097/00005650-200208001-00009. IV-62-8. [DOI] [PubMed] [Google Scholar]
- 15.Cox D. Regression models and life tables. Stat Soc Serv. 1972;34:187–220. [Google Scholar]
- 16.Fine J, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Jounal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 17.Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. Journal of the National Cancer Institute. 2010;102(1):39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calais da Silva FE, Bono AV, Whelan P, Brausi M, Marques Queimadelos A, Martin JA, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. European urology. 2009;55(6):1269–1277. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Silva FC, Silva FM, Goncalves F, Santos A, Kliment J, Whelan P, et al. Locally advanced and metastatic prostate cancer treated with intermittent androgen monotherapy or maximal androgen blockade: results from a randomised phase 3 study by the South European uroncological group. European urology. 2014;66(2):232–239. doi: 10.1016/j.eururo.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 20.Bruchovsky N, Klotz L, Crook J, Phillips N, Abersbach J, Goldenberg SL. Quality of life, morbidity, and mortality results of a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen relapse after radiation therapy for locally advanced prostate cancer. Clin Genitourin Cancer. 2008;6(1):46–52. doi: 10.3816/CGC.2008.n.008. [DOI] [PubMed] [Google Scholar]
- 21.Cano A, Van Baal WM. The mechanisms of thrombotic risk induced by hormone replacement therapy. Maturitas. 2001;40(1):17–38. doi: 10.1016/s0378-5122(01)00270-5. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Li X, Li J, Deng X, Li Y, Cong Y. Experimental arterial thrombosis regulated by androgen and its receptor via modulation of platelet activation. Thrombosis research. 2007;121(1):127–134. doi: 10.1016/j.thromres.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 23.O'Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33(11):1243–1251. doi: 10.1200/JCO.2014.59.1792. [DOI] [PubMed] [Google Scholar]
- 24.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 25.Yu EY, Kuo KF, Gulati R, Chen S, Gambol TE, Hall SP, et al. Long-term dynamics of bone mineral density during intermittent androgen deprivation for men with nonmetastatic, hormone-sensitive prostate cancer. J Clin Oncol. 2012;30(15):1864–1870. doi: 10.1200/JCO.2011.38.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niraula S, Le LW, Tannock IF. Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol. 2013;31(16):2029–2036. doi: 10.1200/JCO.2012.46.5492. [DOI] [PubMed] [Google Scholar]
- 27.Smith GL, Smith BD, Buchholz TA, Giordano SH, Garden AS, Woodward WA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26(31):5119–5125. doi: 10.1200/JCO.2008.16.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giordano SH, Lee A, Kuo YF, Freeman J, Goodwin JS. Late gastrointestinal toxicity after radiation for prostate cancer. Cancer. 2006;107(2):423–432. doi: 10.1002/cncr.21999. [DOI] [PubMed] [Google Scholar]
- 29.Hassett MJ, O'Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. Journal of the National Cancer Institute. 2006;98(16):1108–1117. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]