Abstract
Introduction
Relatively few studies of breast cancer survivors have included nonwhite women or women who do not speak English.
Methods
We administered a survey to patients who were ≥3 months post-completion of their adjuvant treatment for stage 0–III breast cancer at Columbia University Medical Center in order to assess the prevalence of 16 physical and emotional symptoms and identify sociodemographic factors associated with these symptoms. Univariate analysis, factor analysis, ANOVA, and multiple linear regression analysis were performed.
Results
Of 139 patients surveyed, 58 were white, 63 Hispanic, and 18 black. The symptom most commonly reported was fatigue(76%), and the most common severe symptom was muscle aches(40%). Most patients(70%) complained of ≥6 symptoms. Hispanic women were more likely to report >10 symptoms (p<0.05). Factor analysis reduced the 16 symptoms to 4 underlying symptom clusters that we categorized as ‘depression’, ‘chemotherapy’, ‘hormone’, and ‘pain’-related. In the multiple linear regression models, Hispanic women were more likely to report chemotherapy-related symptoms (p<0.05) and pain-related symptoms (p<0.05). Unemployed women were more likely to report chemotherapy-related symptoms (p<0.05). Women <45 years old were less likely to report chemotherapy (p<0.05) and pain-related symptoms (p<0.05).
Conclusions
The majority of women in this study, particularly those who were Hispanic, elderly, or unemployed, experienced persistent symptoms, most commonly fatigue and muscle aches.
Implications for cancer survivors
Because Hispanic, elderly, or unemployed women experience greater symptom burden, efforts should made to address their unique needs.
Keywords: Breast cancer survivors, Ethnicity, Employment, Elderly
Introduction
More than 2 million women living in the United States today are breast cancer survivors [1]. Increases in survival rates have prompted greater interest in improving the quality of life (QOL) of breast cancer survivors, including those from diverse ethnic, cultural and socioeconomic backgrounds. Studies have found that global quality of life, as measured by physical or mental functioning, subjective measures, or presence of symptoms [2], is generally high among breast cancer survivors and is similar to that of healthy women with no history of cancer [3–6]. However, breast cancer survivors do report deficiencies in physical, psychosocial and sexual function [5, 7–9]. In addition to type of surgery [10, 11] and type of adjuvant therapy [3, 12–16], some patient characteristics, such as age and education, are associated with higher quality of life [17–20].
Hispanics currently comprise 15% of the U.S. population and have become the largest ethnic minority in this country [21]. By 2050, it is projected that Hispanics will comprise 24% of the U.S. population [22]. Although the incidence of breast cancer among Hispanic women is lower compared to non-Hispanic whites, it is increasing more rapidly [23]. Several studies have found that Hispanics present with breast cancer at a younger age and a later stage than non-Hispanic white women [24–27]. Nonwhite women, especially those who do not speak English as a first language, are typically underrepresented in studies that examine QOL and psychosocial functioning of breast cancer survivors. Little information is available on the survivorship experience of Hispanic breast cancer patients. Yoon et al. found that black and Spanish-speaking Hispanic women in Los Angeles were significantly more likely than white women to report an unmet need for symptom management after breast cancer treatment, and patients cited deficiencies in physician-patient communication, such as the doctor not knowing about the problem and not appreciating how much the problem bothered the patient, as reasons for their unmet need [28].
The Columbia University Medical Center (CUMC) serves a diverse population, including both affluent patients from the greater New York City metropolitan area and members of the low-income communities in Inwood and Washington Heights, New York City, where CUMC is located, and Hispanics (mostly from the Dominican Republic) compose approximately 73% of the population [29]. The current study was initiated to assess the prevalence of persistent physical and emotional symptoms and identify sociodemographic factors associated with these symptoms in a cohort of predominantly Hispanic and white breast cancer survivors in order to improve specific aspects of their quality of life and to enhance the quality of care in our rapidly diversifying patient population. We hypothesized that the prevalence of persistent physical and emotional symptoms will be high and that women from economically and socially disadvantaged backgrounds will report experiencing more symptoms.
Patients and methods
Sample
At the Breast Oncology Clinic of CUMC, we conducted a survey of patients with a history of stage 0–III breast cancer who were seen between August and October 2006 and were: 1) at least 3 months post-completion of their primary breast cancer therapy (those receiving adjuvant hormonal therapy were eligible) and 2) able to understand and provide written informed consent in English or Spanish. The study was approved by the CUMC Institutional Review Board.
Potential participants were screened and identified prospectively during routine follow-up visits. They were told that the purpose of the survey was to assess the experiences and concerns of breast cancer survivors. Of 193 consecutive breast cancer patients approached, 20 (10%) declined enrollment, mainly due to time constraints. Of 173 patients who completed the self-administered survey, we excluded three male breast cancer cases, 20 who had metastatic disease, and 11 who self-identified their race/ethnicity as “Asian/Pacific Islander” or “Other.” Because participants were approached consecutively, and race/ethnicity was not specified in the inclusion criteria, “Asian/Pacific Islander” and “Other” were included as options in the survey. However, because they represented such a small group of participants, they were excluded from the analysis. After informed consent was obtained, each participant was given a self-administered survey in either English or Spanish. The research staff is bilingual, and the consent and surveys were translated into Spanish. If the participant was unable to read the questionnaire due to poor vision or literacy, a trained bilingual study coordinator administered the survey to the participant.
Outcome and exposure assessment
Demographic and clinical data were collected using self-report measures and via medical chart review. Self-report data included age, menopausal status, primary language, educational level, current employment status, and annual household income. Participants were asked to define their ethnic background as: 1) White, not of Hispanic origin, 2) Black, not of Hispanic origin, 3) Hispanic, 4) Asian/Pacific Islander, or 5) Other. Medical data were abstracted from the most recent chart notes and included year of breast cancer diagnosis, current stage of disease, and prior cancer treatments (i.e., surgery, chemotherapy, and radiation therapy).
The self-administered questionnaire was adapted from the Memorial Symptoms Assessment Scale Short Form (MSAS-SF), which has been previously validated among cancer patients.[30] The survey obtained information on 16 physical and emotional symptoms that patients had experienced in the last 7 days. Responses were given on a 5-point scale: 1) “Not at all”, 2) “A little bit”, 3) “Somewhat”, 4) “Quite a bit”, or 5) “Very much.” For analyses, each symptom was dichotomized as “None” (“Not at all”) vs. “Any” (“A little bit/Somewhat/Quite a bit/Very much”) as well as divided into “None” (“Not at all”), “Moderate” (“A little bit/Somewhat”), and “Severe” (“Quite a bit/Very much”).
Statistical analysis
We analyzed the distributions of demographic and clinical characteristics by race/ethnicity to describe the sample. Univariate analysis was used to compare differences in each symptom measure among breast cancer survivors by clinical, demographic, and treatment-related factors. In order to identify relationships among symptoms, we computed their Pearson correlations. Because several symptom relationships had correlation coefficients greater than 0.4 in the item–item matrix, we conducted an exploratory factor analysis of all 16 symptoms.
Factor analysis is a statistical tool used to describe the covariance relationships among many inter-related variables by identifying a few underlying factors [31]. This procedure generates factor loadings, which are correlation coefficients between a variable and a factor that assume values between −1 and +1. Maximum likelihood factor analysis of the 16 symptoms using a varimax rotation identified four underlying factors, and symptoms with factor loadings greater than 0.5 were used in further analysis. We assigned labels (pain-related, chemotherapy-related, hormone-related, and depression-related) to the factors based on the commonalities of the symptoms correlated with them based on our clinical impression of the symptom clusters. We then used analyses of variance (ANOVA) to evaluate the associations of each of the sociodemographic and clinical characteristics individually with each of the four factors and multiple linear regression analysis to evaluate the associations of all of the sociodemographic and clinical characteristics (race, age, income, education, language, employment, years since diagnosis, and stage) with each of the four factors. Data were analyzed using SAS (version 9.1; SAS Institute Inc., Cary, NC).
Results
Demographic and clinical characteristics
Of the 139 white, black, and Hispanic women with stage 0-III breast cancer who provided data for this analysis, 58 (42%) were non-Hispanic white, 18 (13%) were non-Hispanic black, and 63 (45%) were Hispanic (Table 1). Their median age was 52.5 years (range, 26–90). Eighty-one (58%) spoke English at home, 54 (39%) spoke Spanish, and 4 (3%) spoke neither English nor Spanish as their primary language at home but were sufficiently fluent in English or Spanish to participate in the study (Table 1). Thirty (22%) had less than a high school education, 30 (22%) had only a high school education, and 78 (56%) had more than a high school education (Table 1).
Table 1.
Demographic and clinical characteristics of breast cancer patients
| Characteristics | White
|
Hispanic
|
Black
|
Total
|
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Totals | N | % | N | % | N | % | N | % | |
| 58 | 42 | 63 | 45 | 18 | 13 | 139 | 100 | ||
| Age group (years) | 0.03 | ||||||||
| <45 | 5 | 9 | 22 | 35 | 4 | 22 | 31 | 22 | |
| 45–54 | 19 | 33 | 16 | 25 | 6 | 33 | 41 | 30 | |
| 55–64 | 18 | 31 | 13 | 21 | 5 | 28 | 36 | 26 | |
| 65+ | 16 | 28 | 9 | 14 | 3 | 17 | 28 | 20 | |
| Primary language | <0.0001 | ||||||||
| English | 53 | 91 | 10 | 16 | 18 | 100 | 81 | 58 | |
| Spanish | 2 | 3 | 52 | 83 | 0 | 0 | 54 | 39 | |
| Other | 3 | 5 | 1 | 2 | 0 | 0 | 4 | 3 | |
| Educational attainment | 0.0001 | ||||||||
| <High school graduate | 3 | 5 | 24 | 38 | 3 | 17 | 30 | 22 | |
| High school graduate | 10 | 17 | 16 | 25 | 4 | 22 | 30 | 22 | |
| Post-high school | 44 | 76 | 23 | 37 | 11 | 61 | 78 | 56 | |
| Employment status | 0.06 | ||||||||
| Not employed | 22 | 38 | 37 | 59 | 11 | 61 | 70 | 50 | |
| Employed | 33 | 57 | 24 | 38 | 7 | 39 | 64 | 46 | |
| Annual household income | <0.0001 | ||||||||
| <$10,000 | 4 | 7 | 29 | 46 | 5 | 28 | 38 | 27 | |
| $10,000–49,999 | 11 | 19 | 11 | 18 | 6 | 33 | 28 | 20 | |
| $50,000–$100,000 | 25 | 43 | 8 | 13 | 2 | 11 | 35 | 25 | |
| >$100,000 | 12 | 21 | 3 | 5 | 2 | 11 | 17 | 12 | |
| Not reporteda | 6 | 10 | 12 | 19 | 3 | 17 | 21 | 15 | |
| Menopausal status | 0.13 | ||||||||
| Premenopausal | 6 | 10 | 15 | 24 | 4 | 22 | 25 | 18 | |
| Postmenopausal | 47 | 81 | 38 | 60 | 13 | 72 | 98 | 71 | |
| Not reporteda | 5 | 9 | 10 | 16 | 1 | 6 | 16 | 12 | |
| Stage | 0.74 | ||||||||
| 0 | 4 | 7 | 2 | 3 | 1 | 6 | 7 | 5 | |
| I | 21 | 36 | 17 | 27 | 6 | 33 | 44 | 32 | |
| II | 25 | 43 | 27 | 43 | 6 | 33 | 58 | 42 | |
| III | 4 | 7 | 8 | 13 | 3 | 17 | 15 | 11 | |
| Not reporteda | 4 | 7 | 9 | 14 | 2 | 11 | 15 | 11 | |
| Years since diagnosis | <0.0001 | ||||||||
| <2 | 12 | 21 | 42 | 67 | 8 | 44 | 62 | 45 | |
| 2 to 5 | 34 | 59 | 18 | 29 | 8 | 44 | 60 | 43 | |
| >5 | 12 | 21 | 3 | 5 | 2 | 11 | 17 | 12 | |
| Cancer treatments | |||||||||
| Surgery | 56 | 97 | 58 | 92 | 15 | 83 | 129 | 93 | 0.21 |
| Radiation therapy | 46 | 79 | 46 | 73 | 9 | 50 | 101 | 73 | 0.07 |
| Chemotherapy | 38 | 66 | 46 | 73 | 11 | 61 | 95 | 68 | 0.59 |
| Hormonal therapy | 31 | 53 | 31 | 49 | 17 | 94 | 79 | 57 | 0.05 |
only total “not reported” >10% included in analysis
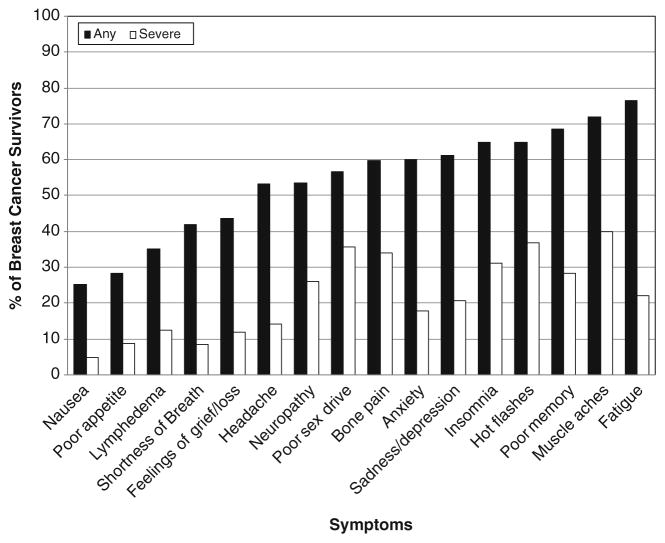
Most patients (70%) reported at least six symptoms. The most common symptom was fatigue (76%), and the most common severe symptom was muscle aches (40%) (Fig. 1). Hispanic women were more likely than others to report >10 symptoms (p<0.05) (Fig. 2).
Figure 1.
Prevalence of any and severe self-reported physical and emotional symptoms among breast cancer patients.
Figure 2.
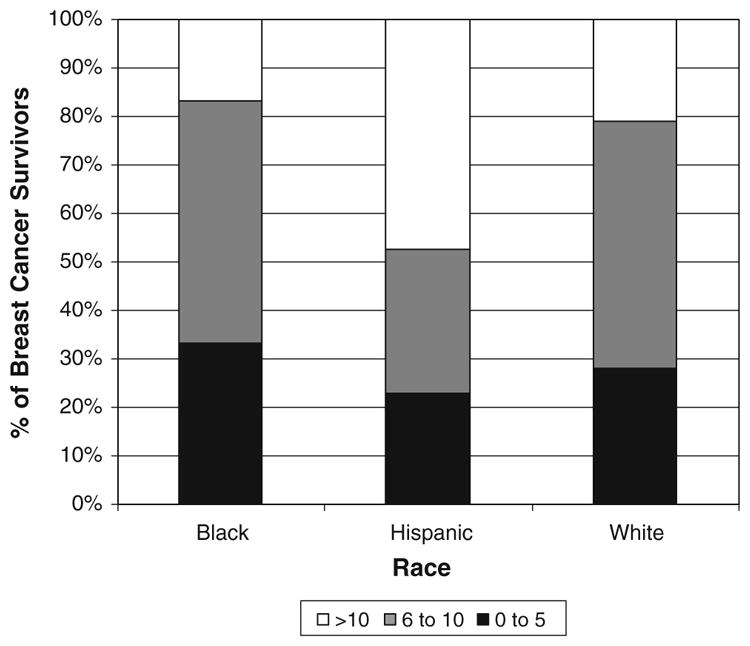
Prevalence of number of self-reported psychological and physical symptoms among breast cancer patients.
Table 2 shows the factor loadings for all 16 symptoms for each factor. No symptom was statistically significant in more than one factor category. For any symptoms, Factor 1 explained 29.93% of the variance; Factor 2 accounted for 11.92% of the variance; Factor 3 accounted for 8.51% of the variance; and Factor 4 accounted for 7.01%. For severe symptoms, Factor 1 explained 33.75% of the variance; Factor 2 accounted for 8.91% of the variance; Factor 3 accounted for 7.17%; and Factor 4 accounted for 10.39% of the variance.
Table 2.
Factor loadings of self-reported psychological and physical symptoms on the four factors derived from exploratory factor analysis
| Factor
|
|||||
|---|---|---|---|---|---|
| ‘Depression’ | ‘Hormone’ | ‘Chemotherapy’ | ‘Pain’ | ||
| Symptom | Poor appetite | −0.12261 | 0.22919 | 0.59518a | 0.16299 |
| Fatigue | −0.01312 | 0.68558a | 0.22116 | 0.17377 | |
| Sadness/Depression | 0.73089a | 0.17351 | 0.06557 | 0.2724 | |
| Anxiety | 0.78222a | 0.1717 | 0.12484 | 0.20124 | |
| Poor sex drive | 0.4569 | 0.55246a | 0.03211 | −0.05862 | |
| Hot flashes | 0.25049 | 0.61372a | 0.1027 | 0.04542 | |
| Headache | 0.04459 | 0.61869a | 0.16925 | 0.3863 | |
| Insomnia | 0.18462 | 0.48276 | −0.26017 | 0.53593a | |
| Muscle aches | 0.28103 | 0.06608 | 0.07852 | 0.76478a | |
| Bone pain | −0.12104 | 0.04634 | 0.34672 | 0.75189a | |
| Shortness of breath | 0.13594 | 0.22548 | 0.49774 | 0.37157 | |
| Nausea | 0.18858 | 0.1342 | 0.57658a | 0.35113 | |
| Lymphedema | 0.04103 | −0.02033 | 0.70494a | −0.08333 | |
| Neuropathy | 0.23578 | 0.157 | 0.67042a | 0.0485 | |
| Poor memory | 0.35597 | 0.6559a | 0.2003 | −0.06035 | |
| Grief/Loss | 0.81468a | 0.17114 | 0.08167 | −0.08368 | |
factor loadings >0.500
In the unadjusted ANOVA (Table 3), several sociodemographic characteristics were associated with ‘chemotherapy-related’ symptoms and ‘pain-related’ symptoms. In a multiple linear regression analysis (Table 4), after controlling for each of the sociodemographic variables, women who were Hispanic (p=0.03), greater than 65 years old (p=0.02), or unemployed (p=0.04) were more likely than women who were white, less than 45 years old, or employed to report ‘chemotherapy-related’ symptoms (R2=0.310). Of note, age and employment were both statistically significant in the multiple linear regression although older women were more likely to be unemployed (chi2 10.2, df: 3, p=0.02). Hispanic women (p=0.03) and those greater than 65 years old (p=0.05) were also more likely to report ‘pain-related’ symptoms (R2=0.285). There were no statistically significant associations for ‘depression’ and ‘hormone-related’ symptoms.
Table 3.
Unadjusted association (ANOVA) between each factor and demographic and clinical characteristics
| Characteristics | Depression-related
|
Hormone-related
|
Chemotherapy-related
|
Pain-related
|
||||
|---|---|---|---|---|---|---|---|---|
| Means | SD | Means | SD | Means | SD | Means | SD | |
| Race/Ethnicity | ||||||||
| Black | 1.1 | 1.1 | 1.7 | 0.9 | 1.0 | 0.9 | 0.9 | 0.7 |
| Hispanic | 1.4 | 0.9 | 2.1 | 1.1 | 1.2 | 0.8 | 1.5 | 0.7 |
| White | 1.3 | 1.0 | 2.0 | 1.0 | 0.6 | 0.7 | 1.3 | 0.7 |
| P-value | 0.5 | 0.4 | 0.005 | 0.01 | ||||
| Age group (years) | ||||||||
| <45 | 1.2 | 0.9 | 2.0 | 1.0 | 0.8 | 0.8 | 1.2 | 0.8 |
| 45–54 | 1.5 | 1.0 | 2.1 | 1.0 | 0.9 | 0.9 | 1.3 | 0.7 |
| 55–64 | 1.2 | 1.0 | 2.0 | 1.0 | 0.8 | 0.8 | 1.5 | 0.8 |
| 65+ | 1.1 | 0.8 | 1.7 | 1.1 | 1.1 | 0.8 | 1.2 | 0.7 |
| P-value | 0.3 | 0.6 | 0.5 | 0.3 | ||||
| Primary language | ||||||||
| English | 1.3 | 1.0 | 2.0 | 1.0 | 0.8 | 0.8 | 1.2 | 0.7 |
| Spanish | 1.4 | 0.9 | 2.0 | 1.1 | 1.1 | 0.9 | 1.6 | 0.7 |
| Other | 1.5 | 1.1 | 1.6 | 1.3 | 1.3 | 1.1 | 1.1 | 0.5 |
| P-value | 0.7 | 0.8 | 0.1 | 0.02 | ||||
| Educational attainment | ||||||||
| < High school graduate | 1.2 | 0.9 | 2.0 | 1.1 | 1.3 | 0.9 | 1.5 | 0.8 |
| High school graduate | 1.5 | 0.9 | 2.0 | 1.0 | 1.1 | 0.8 | 1.5 | 0.7 |
| Post-high school | 1.3 | 1.0 | 2.0 | 1.0 | 0.7 | 0.8 | 1.2 | 0.7 |
| P-value | 0.6 | 1.0 | 0.003 | 0.3 | ||||
| Employment status | ||||||||
| Not employed | 1.4 | 0.9 | 2.2 | 0.9 | 1.2 | 0.8 | 1.4 | 0.7 |
| Employed | 1.2 | 1.0 | 1.8 | 1.1 | 0.6 | 0.7 | 1.3 | 0.8 |
| P-value | 0.4 | 0.1 | 0.001 | 0.4 | ||||
| Annual household income | ||||||||
| <$10,000 | 1.5 | 0.9 | 2.1 | 1.1 | 1.2 | 1.0 | 1.4 | 0.8 |
| $10,000–49,999 | 1.4 | 1.0 | 1.8 | 1.1 | 0.9 | 0.7 | 1.3 | 0.6 |
| >$50,000 | 1.1 | 1.0 | 2.1 | 1.1 | 0.7 | 0.7 | 1.2 | 0.8 |
| Not reported | 1.2 | 0.8 | 2.0 | 1.0 | 1.1 | 0.8 | 1.5 | 0.7 |
| P-value | 0.3 | 0.6 | 0.02 | 0.6 | ||||
| Menopausal status | ||||||||
| Premenopausal | 1.4 | 0.9 | 1.9 | 1.1 | 1.0 | 0.9 | 1.1 | 0.8 |
| Postmenopausal | 1.2 | 1.0 | 2.0 | 1.0 | 0.8 | 0.8 | 1.4 | 0.7 |
| Not reported | 1.5 | 1.0 | 2.4 | 1.0 | 1.1 | 0.8 | 1.5 | 0.8 |
| P-value | 0.5 | 0.3 | 0.5 | 0.2 | ||||
| Years since diagnosis | ||||||||
| <2 | 1.4 | 0.9 | 2.1 | 1.0 | 1.4 | 1.0 | 1.5 | 0.8 |
| 2–5 | 1.1 | 1.0 | 1.9 | 1.0 | 0.9 | 0.8 | 1.2 | 0.7 |
| >5 | 1.5 | 1.0 | 2.0 | 1.1 | 0.5 | 1.0 | 1.4 | 0.7 |
| P-value | 0.1 | 0.9 | 0.005 | 0.2 | ||||
| Stage | ||||||||
| 0–I | 1.1 | 1.0 | 1.8 | 1.1 | 0.8 | 0.9 | 1.2 | 0.8 |
| II | 1.5 | 0.9 | 2.1 | 1.0 | 1.0 | 0.8 | 1.4 | 0.6 |
| III | 1.1 | 0.9 | 2.4 | 0.6 | 1.1 | 0.8 | 1.5 | 0.8 |
| Not reported | 1.5 | 0.8 | 2.1 | 1.1 | 1.0 | 0.7 | 1.1 | 1.0 |
| P-value | 0.2 | 0.5 | 0.7 | 0.6 | ||||
Table 4.
Multiple linear regression models for the association between each factor and demographic and clinical characteristics
| Characteristics | Depression-related
|
Hormone-related
|
Chemotherapy-related
|
Pain-related
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | P-value | Beta | SE | P-value | Beta | SE | P-value | Beta | SE | P-value | |
| Race | ||||||||||||
| White | Referent | Referent | Referent | Referent | ||||||||
| Black | −0.445 | 0.361 | 0.22 | −0.588 | 0.402 | 0.15 | 0.083 | 0.329 | 0.80 | −0.094 | 0.256 | 0.73 |
| Hispanic | 0.511 | 0.509 | 0.32 | 0.292 | 0.568 | 0.61 | 1.06 | 0.465 | 0.03 | 0.778 | 0.362 | 0.03 |
| Employment status | ||||||||||||
| Employed | Referent | Referent | Referent | Referent | ||||||||
| Not employed | 0.137 | 0.249 | 0.21 | 0.453 | 0.278 | 0.11 | 0.472 | 0.228 | 0.04 | 0.148 | 0.177 | 0.41 |
| Age group (years) | ||||||||||||
| 65+ | Referent | Referent | Referent | Referent | ||||||||
| <45 | 0.149 | 0.398 | 0.71 | −0.010 | 0.444 | 0.98 | −0.883 | 0.364 | 0.02 | −0.569 | 0.283 | 0.05 |
| 45–54 | 0.537 | 0.376 | 0.16 | 0.211 | 0.420 | 0.62 | −0.359 | 0.343 | 0.30 | −0.132 | 0.267 | 0.62 |
| 55–64 | 0.002 | 0.367 | 0.99 | 0.088 | 0.409 | 0.83 | −0.621 | 0.335 | 0.07 | 0.119 | 0.261 | 0.65 |
Model controls for education, language, income, years since diagnosis, and stage
Discussion
Most of the female breast cancer survivors in our study, particularly those who were Hispanic, elderly, or unemployed, reported persistent treatment-related physical and psychological symptoms. Other studies have also found that breast cancer survivors report persistent post-treatment physical and psychological symptoms [5, 7–9]. Similar to our finding that the most common severe symptom was muscle aches, other investigators have found that musculoskeletal problems are the most prevalent physical deficits in breast cancer survivors [32]. Symptom assessment in breast cancer survivors is valuable because physical and emotional symptoms affect QOL. It has been found that the number of symptoms patients report is significantly related to QOL [33, 34].
Factor analysis is a useful statistical tool that condenses several inter-related variables (in this case, symptoms) into a few underlying factors. Other studies have implemented this technique to group several patient-reported symptoms by their underlying factors and then use these factors in other statistical analyses [20, 35–37]. For example, in Wilson et al. factor analysis of 12 physical symptoms in patients with fibromyalgia resulted in two distinct factors that the authors distinguished as musculoskeletal and non-musculoskeletal [35]. The factor scores generated from the factor analysis were then used as variables in a k-means clustering procedure to identify symptom profiles among patients [35].
Our factor analysis condensed 16 symptoms into four factors that appeared to be based on symptom etiology and enabled us to sharpen our focus on the associations between outcomes and sociodemographic characteristics. In our sample, Hispanics reported higher rates of pain and chemotherapy-related symptoms than whites. These findings are consistent with those of Eversley et al. which reported that low-income and ethnic minority women reported more post-treatment symptoms than other women [38]. Some studies have shown differences in QOL in cancer survivors based on ethnicity [12, 18, 39–41], while others have not [20, 42]. In a CDC survey of people with no cancer history, Hispanics were twice as likely than whites to report fair or poor health status [43]. Compared to whites, Hispanic and black cancer patients are considered to have a higher risk of adverse effects of treatment [39, 44], but fewer severe symptoms during treatment [45]. In a longitudinal study of cancer-related pain, nonwhites patients reported greater severity of consistent and breakthrough pain than whites [46]. Similarly, in Ashing-Giwa et al.’s study of a multiethnic sample of 703 breast cancer survivors in California, Latina women reported the lowest health-related quality of life [45], while a multiethnic survey of 233 women treated for breast cancer within the prior year found that Hispanic survivors reported significantly higher concerns about pain, survival, and sexuality [44].
In our study, we found that women greater than 65 years old were more likely than women less than 45 years old to report pain and chemotherapy-related symptoms, which has been corroborated by other research findings [47–49]. One explanation for this difference is that the elderly have a greater likelihood of experiencing comorbid medical conditions, which are associated with poor health and diminished quality of life in cancer survivors [50]. Studies have also found that elderly breast cancer survivors are more likely than younger women to report less participatory decision making, fewer supportive interactions, less time spent with physicians as well as lower levels of perceived social support and diminishing size of support networks [51, 52]. These factors also influence symptom experience and management.
Our finding that unemployed women were more likely to report chemotherapy-related symptoms is supported by a few studies that have found similar associations between employment status and quality of life [39, 53, 54]. It is unknown if women were unemployed because their symptoms interfered with their ability to work or if unemployment and its potential consequences, such as lower socioeconomic status, financial instability, or loss of health insurance, exacerbate symptoms and prevent optimal symptom management. It has been found that, over a 5-year study period, breast cancer survivors who were working at the time of their diagnosis experienced significantly larger reductions in annual market earnings than work-matched and age-matched controls with no history of cancer mainly due to functional impairments and, in turn, reduced work effort [55, 56]. Ashing-Giwa et al. demonstrated that after controlling for demographic and medical information, higher health-related quality of life was significantly correlated with high socioeconomic status and low socio-ecological stress in breast cancer survivors [57]. In a large multiethnic study of middle-aged women, those who were married and had low levels of perceived stress reported better QOL than those who were unmarried and had high levels of perceived stress across all racial/ethnic groups [58]. Reports of stress and negative emotion are important predictors of health. Increasing depressive symptoms are associated with lower patient satisfaction [59], less adherence to treatment regimens [60, 61] and poorer health outcomes [62].
The relationship between physical and emotional symptoms and sociodemographic factors provides a promising point for intervention in regards to symptom management: offering new or utilizing pre-existing social support networks for breast cancer survivors. Although studies have shown that group psychosocial support reduces pain and distress [51, 63–68], educated, middle- to upper-class, white women are more likely than non-whites and those of lower socioeconomic status to utilize psychosocial services [39, 69–71]. Adequately educating patients about cancer and presenting linguistically and culturally appropriate psychosocial support services to patients and their family members as a supplement to breast cancer treatment could dispel common misperceptions about cancer and individual or group therapy.
Our findings highlight the importance of contextualizing patient-reported outcomes in symptoms assessment and the problems encountered in symptom control trials that rely on patient-reported outcomes. An analysis of audio recordings of follow-up visits of breast cancer survivors with their oncology providers found that patients spent the majority of their appointment in silence and that more time spent understanding the patient within the context of her life predicted a greater perception of patient-centeredness [72]. Women’s perceived involvement in decision-making about their breast cancer treatment and follow-up care may improve health-related quality of life [73]. Yoon et al. corroborates the importance of improving patient-physician communication in addressing the post-treatment symptoms of breast cancer survivors, especially among Hispanic and black women [28].
A potential limitation of this study is its cross-sectional design which may have resulted in selection bias and recall bias. However, selection bias is unlikely because we recruited consecutive patients during routine follow-up visits in the breast oncology clinic, and relatively few patients (10%) declined enrollment. Our results are consistent with previous studies which found higher rates of physical and emotional symptoms among Hispanics, the elderly, and people of low socioeconomic status.
Another limitation of our study is the absence of a non-cancer control group, so we do not know the prevalence and predictors of these 16 symptoms in healthy women in the CUMC population. However, other studies comparing breast cancer survivors and healthy, age-matched controls have consistently found that despite similar rates of global quality of life, breast cancer survivors reported more physical, sexual and psychological symptoms [2–6].
A strength of the study is the diversity of the study population with respect to socioeconomic status, language, age, and racial/ethnic background. Like other ethnically diverse study populations, we found associations of race/ethnicity, age, and socioeconomic factors with post-treatment symptoms. In addition, while the Memorial Symptoms Questionnaire has been used in other languages, there was no validated translated version available for this study.
This study shows the high prevalence of physical and emotional symptoms in a multiethnic cohort of breast cancer survivors and their associations with ethnicity and employment. Further research is needed to improve the post-treatment experience among all women with a history of breast cancer and to develop and test targeted interventions to best achieve this goal. With an understanding of the interpersonal, cultural, socioeconomic, and institutional factors that affect quality of life in breast cancer survivors, providers can more effectively address their patients’ post-treatment symptoms. Studies have shown that pretreatment emotional distress may be associated with treatment-related effects, such as nausea and pain; assessing such distress may facilitate the prediction and management of treatment-related side effects [74–76]. Assessing the sociodemographic factors that appear to predict post-treatment symptomatology may also provide guidance in the development of preventive interventions.
Acknowledgments
Olivia Fu did this work as part of a Doris Duke Clinical Research Fellowship. Dr. Hershman is the recipient of a grant from the Susan Komen Foundation (DISP0706868).
Contributor Information
Olivia S. Fu, Department of Medicine and the Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA
Katherine D. Crew, Department of Medicine and the Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA, Department of Epidemiology, Mailman School of Public Health, Columbia University, 161 Fort Washington Avenue, 10-1068, New York, NY 10032, USA
Judith S. Jacobson, Department of Epidemiology, Mailman School of Public Health, Columbia University, 161 Fort Washington Avenue, 10-1068, New York, NY 10032, USA
Heather Greenlee, Department of Medicine and the Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA, Department of Epidemiology, Mailman School of Public Health, Columbia University, 161 Fort Washington Avenue, 10-1068, New York, NY 10032, USA.
Gary Yu, Department of Epidemiology, Mailman School of Public Health, Columbia University, 161 Fort Washington Avenue, 10-1068, New York, NY 10032, USA.
Julie Campbell, Department of Medicine and the Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Yvette Ortiz, Department of Medicine and the Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Dawn L. Hershman, Email: dlh23@columbia.edu, Department of Medicine and the Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA, Department of Epidemiology, Mailman School of Public Health, Columbia University, 161 Fort Washington Avenue, 10-1068, New York, NY 10032, USA
References
- 1.American Cancer Society. Cancer Reference Information. 2009 Available at: http://www.cancer.org/docroot/CRI/content/CRI_2_2_1X_How_many_people_get_breast_cancer_5.asp.
- 2.Smith K, Avis NE, Assmann SF. Distinguishing between quality of life and health status in quality of life research: a meta-analysis. Qual Life Res. 1999;8:447–59. doi: 10.1023/a:1008928518577. [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, Rowland J, Meyerowitz B. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 1998;152:396–411. doi: 10.1007/978-3-642-45769-2_38. [DOI] [PubMed] [Google Scholar]
- 4.Lindley C, Vasa S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. 1998;16:1380–7. doi: 10.1200/JCO.1998.16.4.1380. [DOI] [PubMed] [Google Scholar]
- 5.Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol. 1998;16:487–94. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- 6.Schroevers M, Ranchor AV, Sanderman R. Adjustment to cancer in the 8 years following diagnosis: A longitudinal study comparing cancer survivors with healthy individuals. Soc Sci Med. 2006;63:598–610. doi: 10.1016/j.socscimed.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Helgeson V, Tomich P. Surviving cancer: a comparison of 5-year disease-free breast cancer survivors with healthy women. Psychooncology. 2005;14:307–17. doi: 10.1002/pon.848. [DOI] [PubMed] [Google Scholar]
- 8.van der Steeg A, De Vries J, Roukema J. The value of quality of life and health status measurements in the evaluation of the well-being of breast cancer survivors. Eur J Surg Oncol. 2008;34:1225–30. doi: 10.1016/j.ejso.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Ganz P, Rowland JH, Desmond K, Meyerowitz B, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–14. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 10.de Haes J, Curran D, Aaronson N. Quality of life in breast cancer patients aged over 70 years, participating in the EORTC 10850 randomised clinical trial. Eur J Cancer. 2003;39:945–51. doi: 10.1016/s0959-8049(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 11.Lash T, Silliman R. Patient characteristics and treatments associated with a decline in upper-body function following breast cancer therapy. J Clin Epidemiol. 2000;53:615. doi: 10.1016/s0895-4356(99)00176-6. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch M, Flickinger J. Shoulder and arm problems after radiotherapy for primary breast cancer. Am J Clin Oncol. 2001;24:172–6. doi: 10.1097/00000421-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Knobf M, Sun Y. A longitudinal study of symptoms and self-care activities in women treated with primary radiotherapy for breast cancer. Cancer Nurs. 2005;28:210–8. doi: 10.1097/00002820-200505000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Love R, Cameron L, Connell B, Leventhal H. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Int Med. 1991;151:1842–7. [PubMed] [Google Scholar]
- 15.Sadler IJ, Jacobsen P. Progress in understanding fatigue associated with breast cancer treatment. Cancer Invest. 2001;19:723. doi: 10.1081/cnv-100106147. [DOI] [PubMed] [Google Scholar]
- 16.Wood WC, Budman DR, Korzun A, Cooper RM, Younger J, Hart RD, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330:1253. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- 17.Bromberger JT, Meyer PM, Kravitz H. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health. 2001;91:1435–42. doi: 10.2105/ajph.91.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21:4184–93. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz M, Kurt J, Stommel M, Given CW, Given B. The influence of symptoms, age, comorbidity, and cancer site on physical functioning and mental health of geriatric women patients. Women Health. 1999;29:1–12. doi: 10.1300/J013v29n03_01. [DOI] [PubMed] [Google Scholar]
- 20.Stanton AL, Bernaards C, Ganz P. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–56. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein R. US Census Bureau Press Releases. 2008. U.S. Hispanic Population Surpasses 45 Million Now 15 Percent of Total (CB08-67) [Google Scholar]
- 22.Bergman M. US Census Bureau Press Releases. 2004. Census projects tripling of Hispanic and Asian populations in 50 Years; Non-Hispanic whites may drop to half of total population (CB08-67) [Google Scholar]
- 23.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 24.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–12. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 25.Biffl WL, Myers A, Franciose RJ, Gonzalez RJ, Darnell D. Is breast cancer in young Latinas a different disease? Am J Surg. 2001;182:596–600. doi: 10.1016/s0002-9610(01)00789-9. [DOI] [PubMed] [Google Scholar]
- 26.Daly MB, Clark GM, McGuire WL. Breast cancer prognosis in a mixed Caucasian-Hispanic population. J Natl Cancer Inst. 1985;74:753–7. [PubMed] [Google Scholar]
- 27.Frost F, Tollestrup K, Hunt WC, Gilliland F, Key CR, Urbina CE. Breast cancer survival among New Mexico Hispanic, American Indian, and non-Hispanic white women (1973–1992) Cancer Epidemiol Biomarkers Prev. 1996;5:861–6. [PubMed] [Google Scholar]
- 28.Yoon J, Malin JL, Tao ML, Tisnado DM, Adams JL, Timmer MJ, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108:69–77. doi: 10.1007/s10549-007-9580-1. [DOI] [PubMed] [Google Scholar]
- 29.Bergard L. Washington heights/inwood demographic, economic, and social transformations 1990–2005 with a special focus on the Dominican population. Center for Latin America, Caribbean, and Latino Studies; 2008. [Google Scholar]
- 30.Chang V, Hwang SS, Feuerman M, Kasimis BS, Howard H. The memorial symptom assessment scale short form (MSAS-SF) validity and reliability. Cancer. 2000;89:1162–71. doi: 10.1002/1097-0142(20000901)89:5<1162::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 31.Johnson R, Wichern D. Applied multivariate statistical analysis. 4. New Jersey: Prentice-Hall; 1998. [Google Scholar]
- 32.Stein KD, Syrjala KL, MAA Physical and psychological long-term and late effects of cancer. Cancer. 2008;112:2577–92. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portenoy RK, Thaler HT, Kornblith AB, McCarthy Lepore J, Friedlander-Klar H, Coyle N, et al. Symptom prevalence, characteristics, and distress in a cancer population. Qual Life Res. 1994;3:183–9. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 34.Chang VT, Hwang SS, Feuerman M, Kasimis B. Symptom and quality of life survey of medical oncology patients at a Veterans Affairs medical center. Cancer. 2000;88:1175–83. doi: 10.1002/(sici)1097-0142(20000301)88:5<1175::aid-cncr30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Wilson H, Robinson JP, Turk D. Toward the identification of symptom patterns in people with fibromyalgia. Arthritis Rheum. 2009;61:527–34. doi: 10.1002/art.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker A, Thomas JJ, Bainivualiku A, Richards L, Navara K, Roberts AL, et al. Validity and reliability of a Fijian translation and adaptation of the Eating Disorder Examination Questionnaire. Int J Eat Disord. 2009 doi: 10.1002/eat.20675. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharpley C, Christie D. ‘How I was then and how I am now’: current and retrospective self-reports of anxiety and depression in Australian women with breast cancer. Psychooncology. 2007;16:752–62. doi: 10.1002/pon.1125. [DOI] [PubMed] [Google Scholar]
- 38.Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32:250–6. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 39.Bowen DJ, Alfano CM, McGregor BA, Kuniyuki A, Bernstein L, Meeske K, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106:85–95. doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagano IS, Gotay CC. Ethnic differential item functioning in the assessment of quality of life in cancer patients. Health Qual Life Outcomes. 2005;3:60. doi: 10.1186/1477-7525-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotay CC, Holup JL, Pagano I. Ethnic differences in quality of life among early breast and prostate cancer survivors. Psychooncology. 2002;11:103–13. doi: 10.1002/pon.568. [DOI] [PubMed] [Google Scholar]
- 42.Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African–Americans and white long term breast carcinoma survivors. Cancer. 1999;85:418–26. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. Erratum, Cancer 486:732–733. [DOI] [PubMed] [Google Scholar]
- 43.Chowdhury PP, Balluz L, Strine TW. Health-related quality of life among minority populations in the United States, BRFSS 2001–2002. Ethn Dis. 2008;18:483–7. [PubMed] [Google Scholar]
- 44.Spencer SM, Lehman JM, Wynings C, Arena P, Carver CS, Antoni MH, et al. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychol. 1999;18:159–68. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- 45.Yoon J, Malin JL, Tao ML, Tisnado DM, Adams JL, Timmer MJ, et al. Symptoms after breast cancer treatment: are they influenced by patient characteristics? Breast Cancer Res Treat. 2008;108:153–65. doi: 10.1007/s10549-007-9599-3. [DOI] [PubMed] [Google Scholar]
- 46.Green CR, Montague L, Hart-Johnson TA. Consistent and breakthrough pain in diverse advanced cancer patients: a longitudinal examination. J Pain Symptom Manage. 2009;2009:831–47. doi: 10.1016/j.jpainsymman.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Cimprich B, Ronis DL, Martinez-Ramos G. Age at diagnosis and quality of life in breast cancer survivors. Cancer Pract. 2002;10:85–93. doi: 10.1046/j.1523-5394.2002.102006.x. [DOI] [PubMed] [Google Scholar]
- 48.Fehlauer F, Tribius S, Mehnert A, Rades D. Health-related quality of life in long term breast cancer survivors treated with breast conserving therapy: impact of age at therapy. Breast Cancer Res Treat. 2005;92:217–22. doi: 10.1007/s10549-005-2420-2. [DOI] [PubMed] [Google Scholar]
- 49.Paskett E, Herndon J, 2nd, Donohue K, Naughton M, Grubbs S, Pavy M, et al. Health-related quality of life in long-term breast cancer survivors: differences by adjuvant chemotherapy dose in Cancer and Leukemia Group B study 8541. Cancer. 2009;115:1109–20. doi: 10.1002/cncr.24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health and disability. J Gerontol Ser A: Biol Sci Med Sci. 2003;58A:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 51.Sammarco A. Quality of life among older survivors of breast cancer. Cancer Nurs. 2003;26:431–8. doi: 10.1097/00002820-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Silliman RA, Dukes KA, Sullivan LM, Kaplan SK. Breast cancer care in older women. Cancer. 1998;83:706–11. [PubMed] [Google Scholar]
- 53.Mahar KK, BrintzenhofeSzoc K, Shields J. The impact of changes in employment status on psychosocial well-being: a study of breast cancer survivors. J Psychosoc Oncol. 2008;26:1–17. doi: 10.1080/07347330802115400. [DOI] [PubMed] [Google Scholar]
- 54.Salonen P, Tarkka MT, Kellokumpu-Lehtinen PL, Astedt-Kurki P, Luukkaala T, Kaunonen M. Telephone intervention and quality of life in patients with breast cancer. Cancer Nurs. 2009;32:177–90. doi: 10.1097/NCC.0b013e31819b5b65. [DOI] [PubMed] [Google Scholar]
- 55.Chirikos TN, Russell-Jacobs A, Cantor AB. Indirect economic effects of long-term breast cancer survival. Cancer Pract. 2002;10:248–55. doi: 10.1046/j.1523-5394.2002.105004.x. [DOI] [PubMed] [Google Scholar]
- 56.Chirikos TN, Russell-Jacobs A, Jacobsen PB. Functional impairment and the economic consequences of female breast cancer. Women Health. 2002;36:1–20. doi: 10.1300/J013v36n01_01. [DOI] [PubMed] [Google Scholar]
- 57.Ashing-Giwa K, Lim JW. Examining the impact of socioeconomic status and socioecologic stress on physical and mental health quality of life among breast cancer survivors. Oncol Nurs Forum. 2009;36:79–88. doi: 10.1188/09.ONF.79-88. [DOI] [PubMed] [Google Scholar]
- 58.Avis NE, Assmann SF, Kravitz HM, Ganz PA, Ory M. Quality of life in diverse groups of midlife women: assessing the influence of menopause, health status and psychosocial and demographic factors. Qual Life Res. 2004;13:933–46. doi: 10.1023/B:QURE.0000025582.91310.9f. [DOI] [PubMed] [Google Scholar]
- 59.Bui QU, Ostir GV, Kuo YF, Freeman J, Goodwin JS. Relationship of depression to patient satisfaction: findings from the barriers to breast cancer study. Breast Cancer Res Treat. 2005;89:23–8. doi: 10.1007/s10549-004-1005-9. [DOI] [PubMed] [Google Scholar]
- 60.Grol R. Improving the quality of medical care: building bridges among professional pride, payer profit, and patient satisfaction. JAMA. 2001;286:2578–85. doi: 10.1001/jama.286.20.2578. [DOI] [PubMed] [Google Scholar]
- 61.Nagy VT, Wolfe GR. Cognitive predictors of compliance in chronic disease patients. Med Care. 1984;22:912–21. doi: 10.1097/00005650-198410000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52:106–11. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sammarco A. Perceived social support, uncertainty, and quality of life of younger breast cancer survivors. Cancer Nurs. 2001;24:212–9. [PubMed] [Google Scholar]
- 64.Sammarco A, LMK Quality of life, social support, and uncertainty among Latina breast cancer survivors. Oncol Nurs Forum. 2008;35:844–9. doi: 10.1188/08.ONF.844-849. [DOI] [PubMed] [Google Scholar]
- 65.Edelman S, Bell DR, Kidman AD. A group cognitive behaviour therapy programme with metastatic breast cancer patients. Psychooncology. 1999;8:292–305. doi: 10.1002/(SICI)1099-1611(199907/08)8:4<295::AID-PON386>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 66.Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–26. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 67.Gottlieb BH, Wachala ED. Cancer support groups: a critical review of empirical studies. Psychooncology. 2006;16:379–400. doi: 10.1002/pon.1078. [DOI] [PubMed] [Google Scholar]
- 68.Rehse B, Pukrop R. Effects of psychosocial interventions on quality of life in adult cancer patients: Meta analysis of 37 published controlled outcome studies. Patient Educ Couns. 2003;50:179–86. doi: 10.1016/s0738-3991(02)00149-0. [DOI] [PubMed] [Google Scholar]
- 69.Campbell H, Phaneuf MR, Deane K. Cancer peer support programs-do they work? Patient Educ Couns. 2004;55:3–15. doi: 10.1016/j.pec.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Cunningham AJ, Edmonds CV. Group psychological therapy for cancer patients: A point of view, and discussion of the hierarchy of options. Int J Psychiatry Med. 1996;26:51–82. doi: 10.2190/6PG2-GUN8-GK2W-CXAP. [DOI] [PubMed] [Google Scholar]
- 71.Nápoles-Springer A, Ortíz C, O’Brien H, Díaz-Méndez M, Pérez-Stable E. Use of cancer support groups among Latina breast cancer survivors. J Cancer Surviv. 2007;1:193–204. doi: 10.1007/s11764-007-0029-7. [DOI] [PubMed] [Google Scholar]
- 72.Clayton M, Dudley W. Patient-centered communication during oncology follow-up visits for breast cancer survivors: content and temporal structure. Oncol Nurs Forum. 2009;36:E68–79. doi: 10.1188/09.onf.e68-e79. [DOI] [PubMed] [Google Scholar]
- 73.Andersen M, Bowen DJ, Morea J, Stein KD, Baker F. Involvement in decision-making and breast cancer survivor quality of life. Health Psychol. 2009;28:29–37. doi: 10.1037/0278-6133.28.1.29. [DOI] [PubMed] [Google Scholar]
- 74.Higgins SC, Montgomery GH, Bovbjerg DH. Distress before chemotherapy predicts delayed but not acute nausea. Support Care Cancer. 2007;15:171–7. doi: 10.1007/s00520-006-0113-y. [DOI] [PubMed] [Google Scholar]
- 75.Montgomery GH, Bovbjerg DH. Expectations of chemotherapy-related nausea: emotional and experiential predictors. Ann Behav Med. 2003;25:48–54. doi: 10.1207/S15324796ABM2501_07. [DOI] [PubMed] [Google Scholar]
- 76.Sabbioni ME, Bovbjerg DH, Jacobsen PB, Manne SL, Redd WH. Treatment related psychological distress during adjuvant chemotherapy as a conditioned response. Ann Oncol. 1992;3:393–8. doi: 10.1093/oxfordjournals.annonc.a058214. [DOI] [PubMed] [Google Scholar]