Abstract
Background
The Questionnaire for Verifying Stroke-free Status (QVSFS) has been validated in Western populations as a method for verifying stroke-free status in participants of clinical, epidemiological and genetic studies. This instrument has not been validated in low-income settings where populations have limited knowledge of stroke symptoms and literacy levels are low.
Objective
To simultaneously validate the 8-item QVSFS in 3 languages spoken in West Africa (Yoruba, Hausa and Akan) for ascertainment of stroke-free status of control subjects in SIREN.
Methods
Using a cross-sectional study design, 100 participants each from the 3 linguistic groups will be consecutively recruited from neurology and general medicine clinics of 5 tertiary referral hospitals in Nigeria and Ghana. Ascertainment of stroke status will be determined by neurologists using structured neurological examination, review of case records and neuro-imaging (Gold standard). The relative performance of QVSFS without and with pictures of stroke symptoms (pictograms) will be assessed using sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).
Conclusion
The proposed study will provide valuable data on the performance of the QVSFS in resource-limited settings.
Keywords: Stroke-free phenotype, West Africa, Accuracy, Pictogram
Highlights
-
•
This study seeks to evaluate the performance of the 8-item QVSFS in Akan, Yoruba and Hausa for the ascertainment of stroke-free status among West Africans.
-
•
A line pictogram has been developed to determine its impact on the relative performance of the 8-item QVSFS.
1. Introduction
There is an epidemic of stroke in low and middle-income countries due to rapidly increasing prevalence of vascular risk factors such as hypertension, diabetes mellitus, dyslipidaemia [1], [2]. The need has thus arisen to conduct studies aimed at epidemiologic, genetic and phenotypic characterisation of stroke in sub-Saharan Africa to provide evidence-based information to confront this menace. To this end, it is important that controls selected for comparison with cases are recruited with a high degree of certainty that they indeed do not have stroke or TIA to allow for valid comparisons to be made. In view of this simple, quick and accurate assessment of symptoms of stroke is needed for epidemiologic studies particularly those of the case–control type.
In 2000, Meschia et al. developed the Questionnaire for Verifying Stroke-free Status (QVSFS) as a method for verifying the stroke-free phenotype in participants of clinical, epidemiological and genetic studies [3]. Having one or more affirmative answers to any of the questionnaire was associated with finding stroke, TIA, or either stroke or TIA on medical record review [3]. Jones et al. showed by directly interviewing participants and review of medical records that the QVSFS had a NPPV and PPV of 0.96 and 0.71 respectively making the 8-item QVSFS a sensitive and valid tool [4], and a structured telephone interview format of questionnaire administration still had an excellent sensitivity of 1.0, specificity of 0.86, positive predictive value of 0.36 and negative predictive value of 1.0 among participants aged 60 years and above. The QVSFS is also reliable with intra-rater and inter-rater agreement of stroke-free status classification of 0.90 and 0.94 respectively [5]. The QVSFS is easy to administer as a self-report pencil-and-paper questionnaire or by an interview in person or by telephone and has been translated into Spanish by Castillo et al. for use among Hispanic populations [6].
Two questions in the instrument assess previous diagnosis of either stroke/TIA together with six questions about stroke symptoms. Thus not only is the 8-item QVSFS a useful tool in identifying stroke-free individuals but it could be used to screen for undiagnosed stroke. The sensitivity and specificity of having any of the six symptom questions for stroke detection was 0.82 and 0.62 respectively [7]. Indeed the QVSFS has been successfully used in the recruitment of controls involved genetic studies into ischaemic stroke [8], [9], to screen for cerebrovascular end points in the Carotid Revascularisation Endarterectomy versus Stent Trial (CREST) [10] and in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study [11].
This instrument could have tremendous potential in developing countries where stroke prevalence is increasing, awareness of stroke symptoms low and the confirmation of stroke diagnosis either missed by health workers or constrained by the pervasive lack of neuro-imaging modalities. In these low literacy settings the lack of knowledge of stroke symptoms might impact negatively on the performance of stroke symptom questionnaires. Furthermore there is a lack of specific medical terminology for symptoms of stroke in local dialects across sub-Saharan Africa. The performance of the questionnaire could be enhanced further by the introduction of culturally acceptable pictures of stroke symptoms. Hence the performance of the 8-item QVSFS needs validation in developing countries.
One of the key comments regarding the 8-item QVSFS questionnaire is that the benchmark for verification of strokes has relied on review of medical records of patients but this approach may be problematic in settings where medical records are kept under less than optimal circumstances and where patients may not always seek health services for ailments from medical facilities but from herbalists, chemical shops and so forth. An approach to overcome this limitation would be to examine participants by neurologists or to use a cranial CT scan for verification in a subset of participants. This study seeks to validate the 8-item QVSFS at 5 centers in Nigeria and Ghana which are involved in the on-going NIH supported Stroke Investigative Research and Educational Networks (SIREN) study.
2. Methods
2.1. Study sites
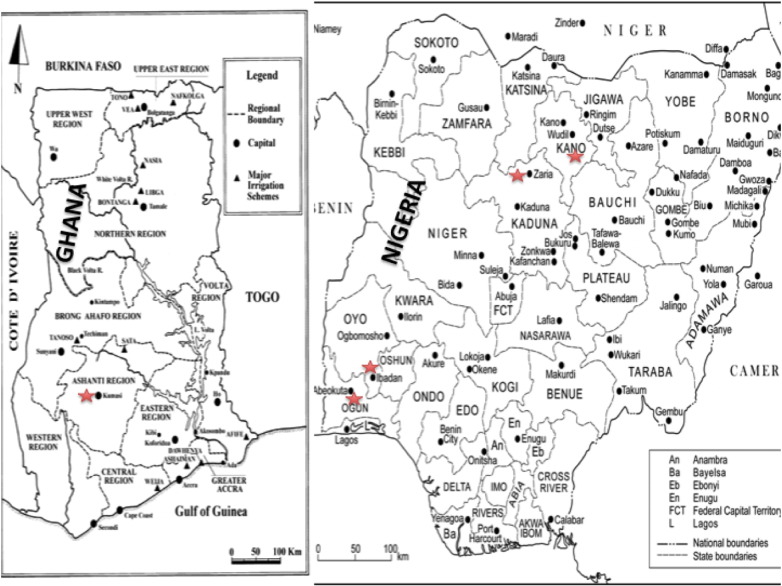
Participants will be recruited from five Tertiary referral medical centers in West Africa namely the Komfo Anokye Teaching Hospital (KATH) in Kumasi, Ghana; the University College Hospital (UCH), Ibadan, Nigeria; Federal Medical Centre (FMC), Abeokuta, Nigeria; Ahmadu Bello University Hospital (ABUH), Zaria, Nigeria and Aminu Kano Teaching Hospital (AKTH), Kano, Nigeria (Fig. 1). Kumasi where KATH is situated is the principal city of the Akan tribe in central Ghana and serves a population of 4 million. The Akan language is spoken by 50% of the Ghanaian population. UCH and FMC situated in Ibadan and Abeokuta respectively are located in Southern Nigeria and serve a combined population of 4 million Nigerians of predominantly Yoruba ethnicity. Yoruba is a trans-border language spoken by 30 million Nigerians. ABUH and AKTH in Zaria and Kano provide health care services to 15 million Nigerians of mainly Hausa and Fulani descent. All these study sites have consultant neurologists. Ethical approval for the study will be sought from the ethics committees of the 5 participating sites.
Fig. 1.
Map showing 5 study sites (indicated by red stars).
2.2. Translation of QVSFS into 3 languages
The original questionnaire developed by Meschia et al. [3] will be translated into the local languages (Yoruba — southern Nigeria, Hausa — Northern Nigeria, Akan — middle & lower belts of Ghana), pre-tested and back-translated into English language to establish semantic equivalence. At each of the study sites, a panel comprising of a neurologist, three to five doctors and nurses, and public health practitioners will translate the questionnaire into the local dialect by consensus. The translated versions of the questionnaires in 3 languages are shown in supplementary information section (S1).
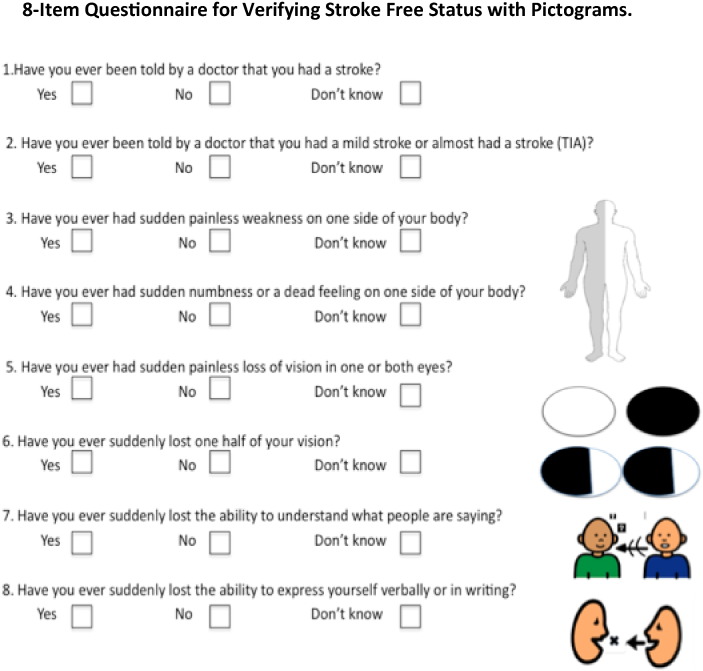
Another version of the questionnaire will be developed where question items 3 to 8 will have line pictures of the neurological symptoms being elicited by the interviewer (Fig. 2).
Fig. 2.
8-Item QVSFS with line pictures of stroke symptoms.
2.2.1. Inclusion criteria
-
1.
Participants should be aged 18 years and above.
-
2.
Willingness to complete study protocol.
2.2.2. Exclusion criteria
-
1.
Patients with hearing impairment.
-
2.
Severe dysphasia or severe cognitive impairment.
2.3. Procedure for administration of questionnaires
Participants attending General Medicine and Neurology Out-Patient Clinic appointments will be approached and enrolled into the study after obtaining informed consent. Baseline demographic details will be obtained and the questionnaire administered by a medicine resident in the local dialect and the answers recorded as “yes”, “no”, or “don't know” to each question item. The version of the questionnaire without pictures will be administered first followed by the version with pictograms by the same interviewer. Furthermore, participant awareness of known vascular risk factors such as hypertension, diabetes mellitus, dyslipidaemia, cigarette smoking, excessive alcohol intake, physical inactivity, known heart disorders, family history of stroke will be assessed using 8 questions assessing knowledge of the presence of these specific risk factors.
2.4. Evaluation of participants by neurologist
After the interview participants will undergo a 3-staged evaluation by a neurologist. The first stage will involve a review of the medical records of patients to determine if a previous diagnosis of stroke has ever been made, the duration of stroke diagnosis, whether neuro-imaging information on stroke type is available. The second stage entails an examination for presence of hemiparesis, hemi-anesthesia, mono-ocular/binocular visual loss, hemianopsia, receptive or expressive aphasia, and cerebellar deficits. The third stage will comprise of an evaluation of a cranial CT scan in a subset of participants. This neuro-imaging validation will be performed within 48 h among a subset of subjects (at least 4 stroke and 4 stroke-free) per site. They shall be selected by random sampling. After this thorough evaluation the neurologist makes a diagnosis which is our Gold standard.
2.5. ROC curve power analysis for sample size calculation
Assuming that the standard test has an accuracy of 75% as measured by the area under the ROC curve (AUC), a sample of 35 cases and 35 controls achieve 82% power to detect an extra accuracy by the proposed test of 15% (AUC = 90% in the proposed test) using a two-sided test at a significance level of 0.05. The sample size required increases to 80 per group in order to detect a difference of 10% (AUC = 85% in the proposed test) under the same conditions. Thus, the sample size of 100 per linguistic category (Akan, Yoruba, Hausa) will be sufficient detect an effect size as small as 10% allowing up to 10% false positive rates and control to case ratio of the standard deviation of the responses to be 0.5 as suggested by Obuchowski and McClish [12]. (See Fig. 3.)
Fig. 3.
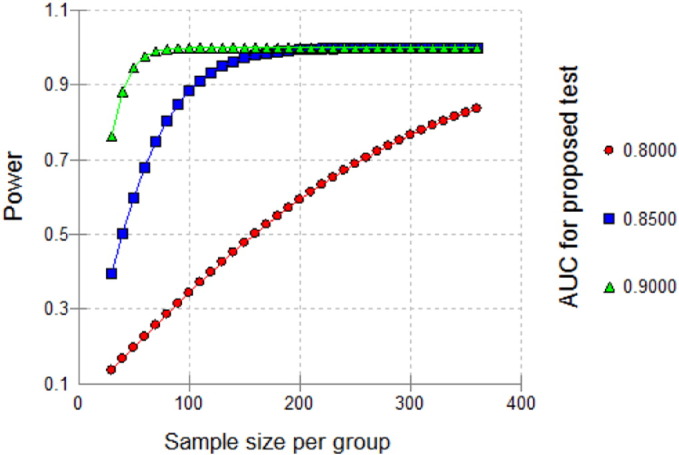
Sample size and power calculation.
Given that the 8 items QVSFS is going to be translated into three (Yoruba, Hausa, Chi) different languages, we will evaluate the reliability of the translation before it is implemented to predict stroke status using a sample size of 50 consistency and validity of the translation for each language using exploratory factor analysis and Cronbach's alpha [13], [14].
2.6. Proposed analysis
2.6.1. Interpretation of results
Yes = 1, No = 0, Don't know = 0. Patients who score 0/8 will be deemed as stroke free whereas those with any positive score will be deemed as not stroke free according to the 8-item QVSFS. Sensitivity analysis will be performed where “Don't know” will be assumed to be = 1.
2.6.2. Statistical analysis
Baseline demographic characteristics between patients who are symptom free for stroke and those with stroke will be compared using either chi-squared test (categorical variables) or student's t-test or Mann–Whitney's U-test for means/medians respectively (continuous variables). The sensitivity, specificity, positive predictive value, negative predictive value, positive and negative likelihood ratios as well as the accuracy and reliability of the 8-item QVSFS will be assessed in comparison with the benchmark stroke determination by history and examination by a neurologist. Furthermore, the performance of the questionnaire will be compared with neuro-imaging results in a small randomly selected subset. The sensitivity and specificity of the 6 stroke symptom questions will be determined for stroke diagnosis among patients with stroke. The performance of the questionnaire will also be compared with the modified version of the questionnaire with images illustrating the neurological deficits being elicited by items 3 to 6. We will use factor analysis approach to assess and confirm the structure relationship between each item and the QVSFS score overall. Kappa statistics will be employed to compare inter-rater and intra-rater performance of the tool administered by medicine residents, at each site and also between study sites.
3. Discussion
The Questionnaire for Verifying Stroke-free Status (QVSFS), developed and validated primarily in English-speaking Western World populations, is a method for verifying stroke-free status in participants of clinical, epidemiological and genetic studies. However, the performance of this tool in settings where levels of education and awareness of stroke symptoms are low such as sub-Saharan Africa has not been conducted. The tool has tremendous potential for widespread use in regions such as ours where stroke incidence, prevalence and mortality are escalating and questions on the epidemiology and genetic underpinnings of the stroke epidemic remain unresolved.
We propose to validate QVSFS for ascertainment of stroke-free status of control subjects enrolled in an ongoing stroke epidemiological study in West Africa funded by the National Institute of Health for H3Africa. The novel aspects of our validation study are: firstly, to carry out this validation study simultaneously at five tertiary hospitals across Nigeria and Ghana involving participants speaking three different languages (Yoruba, Hausa and Akan). The QVSFS will be translated into these languages and administered to participants enrolled from neurology and general clinics in these hospitals. Secondly, given the low levels of education, the lack of awareness of stroke symptoms, and general paucity of semantically equivalent medical terminology in the local dialects for stroke symptoms in our settings, we will evaluate the performance of the tool with and without pictograms. Finally, ascertainment of stroke status will be conducted by neurologists across the 5 sites using a combination of structured medical history, neurological examination, review of medical records and neuro-imaging (Gold Standard) compared with previously published studies which relied on medical records review and neurological examination.
4. Conclusion
The 8-item QVSFS is a simple, accurate and cheap tool which could have widespread use for ascertaining stroke status in clinical, epidemiological and genetic studies in settings where facilities for stroke confirmation are lacking.
Conflict of interest
None to declare.
Acknowledgments
The SIREN study is supported by U01 NS079179, and U54 HG007479 from the National Institutes of Health as part of the H3Africa Consortium.
Footnotes
Study Protocol Article
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ensci.2016.03.004.
Appendix A. Other contributing authors
Komfo Anokye Teaching Hospital, Ghana — Sheila Adamu, MD; Vida Obese, MD; Nana Boa-Antwi, MD; Lambert Appiah, MD; Arti Singh MD, MSc.; Aham Okorozo, MD; Dominic Awuah, MD.
Medical University of South Carolina, USA — Dan Lackland MD, PhD.
Aminu Kano Teaching Hospital, Nigeria — Suleiman Isah, BSc; Nasir Tabari, BSc; Aliyu Mande, MPH; Abdul Dambatta, MD; Naser Ishaq, MD.
Ahmadu Bello University, Zaria, Nigeria — Eric Tabi-Ajayi, MD; Ibinaiye Phillip, MD; Paschal Azuh, MD.
University of Ibadan — Onoja Akpa, PhD; Abiodun Adeoye, MD; Aridegbe Tosin, BSc; Osimhiarherhuo Adeleye, MSc; Atinuke Agunloye, MD; Godwin Ogbole, MD; Joshua Akinyemi, PhD; Ezinne Uvere, MPH; Gregory Adekunle, MPH; Salaam Kehinde, MSc.
Korle-Bu Teaching Hospital — Ruth Laryea, BSc.
Appendix B. Supplementary data
Supplementary material
References
- 1.Mensah G., Roth G., Sampson U. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the global burden of disease study 2013. Cardiovasc. J. Afr. 2015;26:S6–S10. doi: 10.5830/CVJA-2015-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owolabi M.O., Mensah G.A., Kimmel P.L. Understanding the rise in cardiovascular diseases in Africa: harmonizing H3Africa genomic epidemiological teams and tools. Cardiovasc. J. Afr. 2014;25(3):134–136. doi: 10.5830/CVJA-2014-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meschia J.F., Brott T.G., Chukwudelunzu F.E. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31:1076–1080. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 4.Jones W.J., Williams L.S., Meschia J.F. Validating the questionnaire for verifying stroke-free status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- 5.Meschia J.F., Lojacono M.A., Miller M.J. Reliability of the questionnaire for verifying stroke-free status. Cerebrovasc. Dis. 2004;17:218–223. doi: 10.1159/000075794. [DOI] [PubMed] [Google Scholar]
- 6.Castillo P.R., Brott T.G., Alvarez S. Creation of a bilingual Spanish-English version of the questionnaire for verifying stroke-free status. Neuroepidemiology. 2004;23:236–239. doi: 10.1159/000079949. [DOI] [PubMed] [Google Scholar]
- 7.Sung V.W., Johnson N., Granstaff U.S. Sensitivity and specificity of stroke symptom questions to detect stroke or transient ischaemic attack. Neuroepidemiology. 2011;36:100–104. doi: 10.1159/000323951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meschia J.F., Brown R.D., Jr., Brott T.G. The siblings with ischaemic stroke study (SWISS) protocol. BMC Med. Genet. 2002;3:1. doi: 10.1186/1471-2350-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meschia J.F., Brott T.G., Brown R.D., Jr. The ischaemic stroke genetics study (ISGS) protocol. BMC Neurol. 2003;3:4. doi: 10.1186/1471-2377-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobson R.W., II Update on the Carotid Revascularisation Endartectomy versus Stent Trial (CREST) protocol. J. Am. Coll. Surg. 2002;194:S9–S14. doi: 10.1016/s1072-7515(01)01078-x. (suppl) [DOI] [PubMed] [Google Scholar]
- 11.Howard G., Cushman M., Gomez C.R. A test of an innovative approach to develop a national cohort to address racial and geographic disparities in stroke. Circulation. 2003;109:e7001–e7039. [Google Scholar]
- 12.Obuchowski N., McClish D. Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat. Med. 1997;16:1529–1542. doi: 10.1002/(sici)1097-0258(19970715)16:13<1529::aid-sim565>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Bornstedt G.W. Reliability and validity in attitude measurement. In: Summers G.F., editor. Attitude Measurement. Kershaw Publishing Company; London: 1977. pp. 80–99. [Google Scholar]
- 14.Rattray J.C., Jones M.C. Essential elements of questionnaire design and development. J Clin Nurs. 2007;16:234–243. doi: 10.1111/j.1365-2702.2006.01573.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material