Abstract
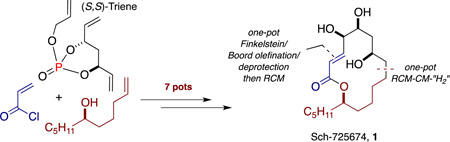
A pot-economical total synthesis of antifungal Sch-725674, 1 is reported. The approach takes advantage of a number of one-pot, sequential transformations, including a phosphate tether-mediated one-pot, sequential RCM/CM/chemoselective hydrogenation protocol, a one-pot tosylation/acrylation sequence, and a one-pot, sequential Finkelstein reaction/Boord olefination/acetonide deprotection procedure to streamline the synthesis route by reducing isolation and purification procedures, thus saving time. Overall, an asymmetric route has been developed that is efficiently accomplished in seven pots from phosphate (S,S)-triene and with minimal purification.
Graphical abstract
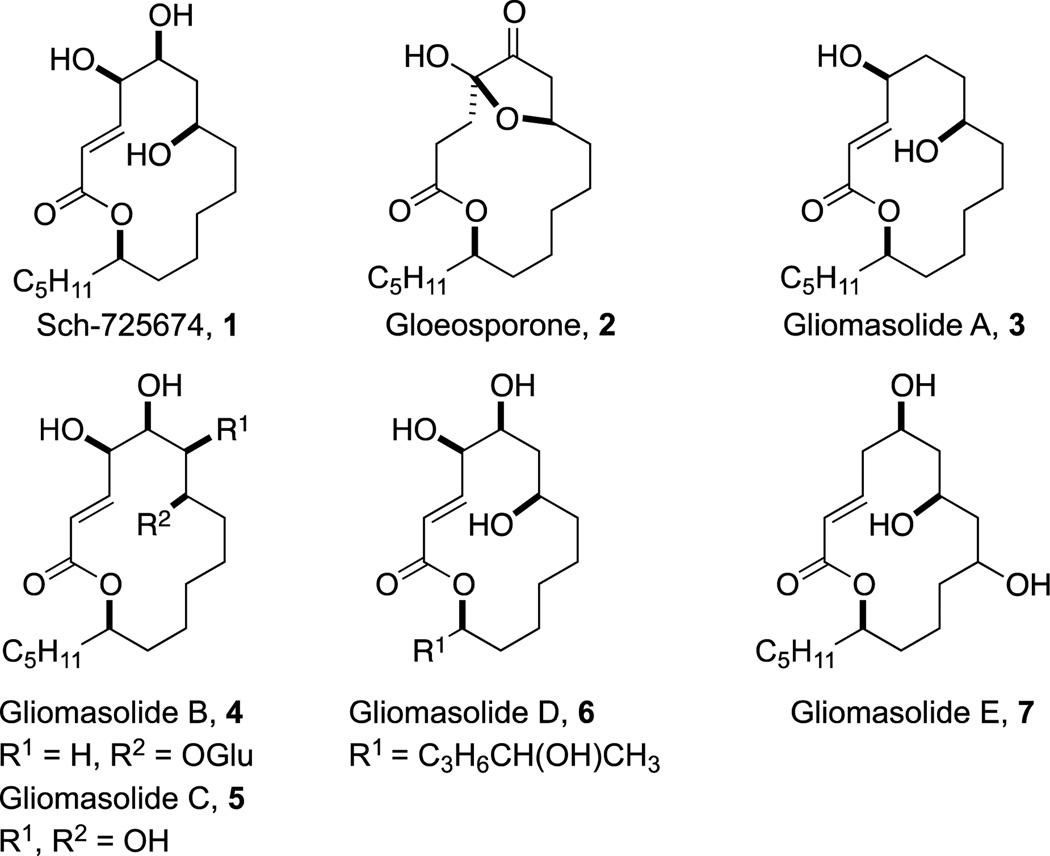
Sch-725674, 1 is an antifungal macrolide that was isolated and structurally elucidated in 2005 by Yang and co-workers from the culture of Aspergillus sp.1 This natural product exhibits activity against Saccharomyces cerevisiae and Candida albicans with MIC values of 8 and 32 µg/mL, respectively. Key structural features of 1 include a 14-membered ring, an E-configured α,β-unsaturated ester, a lipophilic n-pentyl side chain and a 1,3-anti-diol moiety embedded within a four-carbon subunit containing three stereogenic carbinol centers (Figure 1). An intriguing feature of 1 is the absence of commonly found methyl groups on the backbone of macrolides (i.e. erythromycin and derivatives). The closest structural relatives of Sch-725674 are the self-germination inhibitor gloeosporone, 22 and the recently isolated gliomasolides A to E, 3–73 (Figure 1), thus making 1 an attractive biological and synthetic target.
Figure 1.
Natural Product Macrolactones Sch-725674 (1), Gloeosporone, 2 and Gliomasolides A–E, 3–7.
The Curran group reported the first total synthesis of Sch-725674 and a complete library of stereoisomers by using fluorous tagging technology developed in their laboratory, which also established the absolute stereochemistry of 1.4 In 2014, Prasad and co-workers reported an enantioselective synthesis of the macrolactone core,5 followed by the second reported total synthesis featuring a Ley dithiaketalization and ring-closing metathesis (RCM).6 Kaliappan and co-workers later accomplished the total synthesis of 1 employing dithiane alkylation, cross-metathesis (CM) and Yamaguchi macrolactonization as strategic transformations.7 Most recent, a Wacker-type oxidation was showcased in a formal total synthesis of 1 by Reddy and co-workers, along with the first total synthesis of structural relative gliomasolide C, 5.8
Given that 14-membered macrolactones lacking methyl group substitutions are rare in nature and underexplored in biological studies, we wish to provide a streamlined and library amenable synthetic method to access 1. In this regard, pot-economical9 processes have emerged as valuable tools for the synthesis of natural products as they enable the formation of several bonds and stereocenters while using minimal synthesis steps.10 Pot economy is achieved via one-pot reactions, which combine multiple transformations into a single reaction flask without the need for work-up and chromatography operations between sequential reactions. The application of one-pot protocols in natural products and medicinal drugs has recently been reviewed,10b and among several elegant examples contained in this review, seminal efforts by Hayashi11 are highly notable in that they demonstrate use of multiple one-pot transformations to streamline the synthesis of complex molecules.
Taken together, a pot economic route attains a streamlined process that saves operational time and minimizes waste by carrying out successive reactions in one pot. Herein, we report a pot-economical total synthesis of Sch-725674 by incorporating technically simple and effective one-pot, sequential protocols to the route, reducing post-reaction workup and overall purification events.
Previous reports in our group have emphasized the utilization of phosphate tethers to mediate reactions in a chemo- and diastereoselective fashion, with recent work incorporating one-pot, sequential protocols to the synthesis of 1,3-anti-diol containing natural products12 and complex polyols.13 To continue our efforts toward the development of modular and pot-economical approaches for the synthesis of complex molecules, we planned an asymmetric synthesis of Sch-725674 by carrying out a series of one-pot, sequential protocols in an overall minimal number of pots.
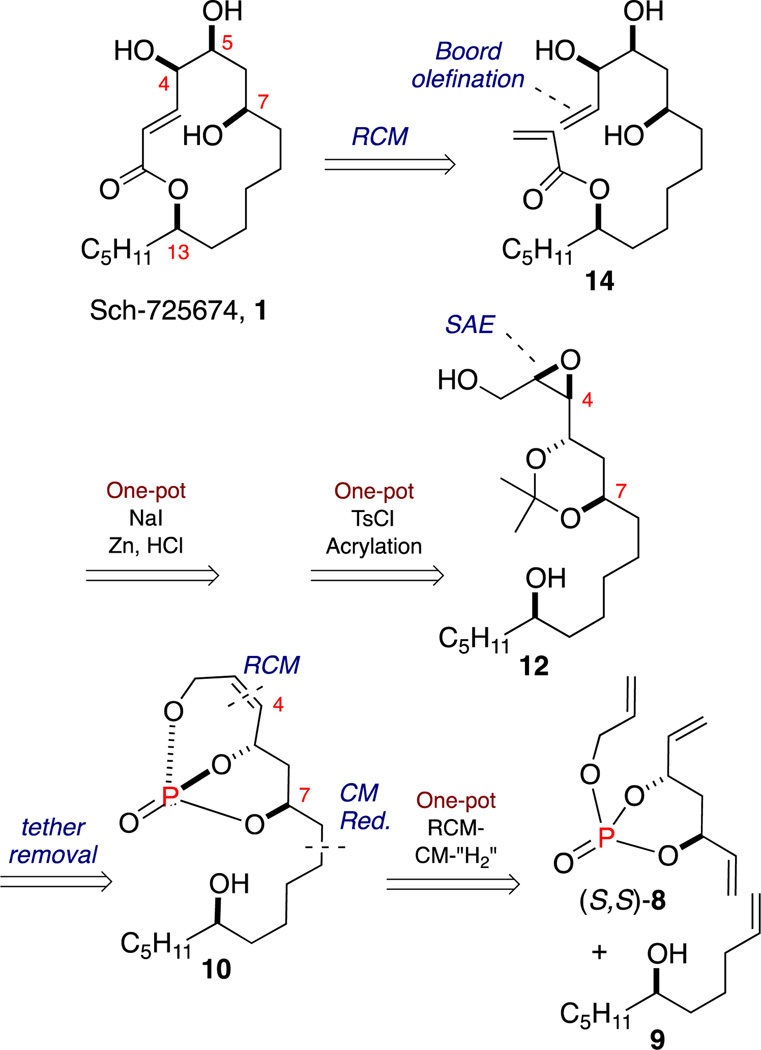
From a retrosynthetic viewpoint, macrocyclization to 1 can be accomplished via RCM of linear diene 14 (Scheme 1). Derivation of triol 14 was planned using two consecutive one-pot, sequential protocols from epoxide 12, namely a Finkelstein substitution/Boord olefination/acetonide deprotection procedure and a two-reaction sequence involving tosylation and acrylation. Epoxide 12 can be synthesized from bicyclic phosphate 10 following reductive tether removal and employing a Sharpless asymmetric epoxidation (SAE) on cis-olefin at C4-C3. The phosphate tethered-triol 10 can be accessed from triene phosphate (S,S)-8 following a one-pot RCM/CM/chemoselective hydrogenation [“H2”] sequence utilizing 9 as the CM partner, simultaneously installing the requisite the C13 n-pentyl side chain and the C5-C7 1,3-anti-diol subunit.14 A salient feature of this approach is the modular installation of the C13–C9 fragment via CM, as well as introduction of the acrylate at a later stage, opening opportunities for future analog generation.
Scheme 1.
Synthesis Plan Toward Sch-725674, 1
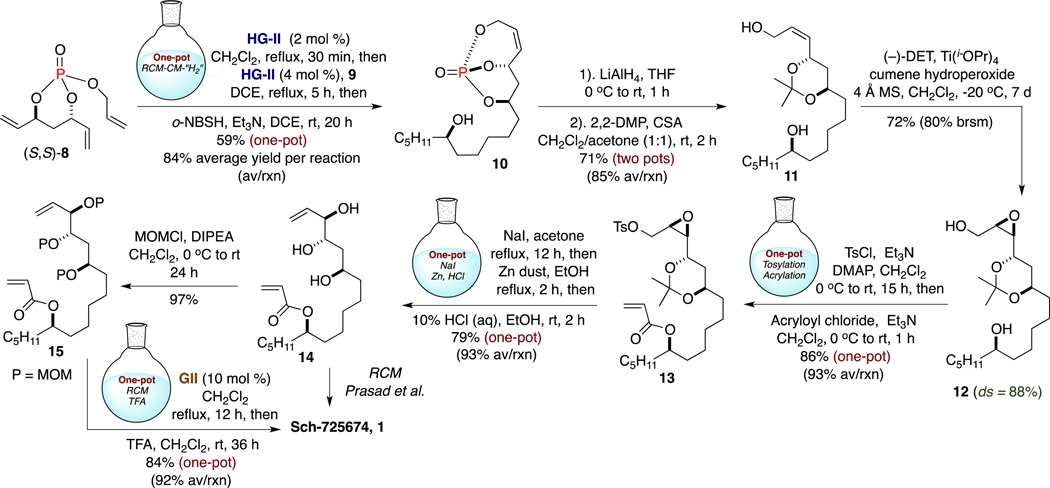
Following optimized conditions for one-pot, sequential RCM/CM/[“H2”],15 triene (S,S)-8 was subjected to an RCM reaction using the second-generation Hoveyda-Grubbs catalyst16 (HG-II) (2 mol %) in refluxing CH2Cl2 (Scheme 2). After RCM completion (30 min), the solvent was changed17 to 1,2-dichloroethane (DCE) and the n-pentyl-substituted CM partner 9 was introduced to the same pot, followed by a second addition of HG-II (4 mol %). The CM event proceeded for 5 h under reflux and subsequent chemoselective diimide reduction at the external olefin was achieved by addition of o-nitrobenzenesulfonylhydrazine (o-NBSH)18 into the reaction mixture. This one-pot, three-reaction, sequential operation provided bicyclic phosphate 10 in 59% yield over one-pot, representing an average yield of 84% per reaction (84% av/rxn).
Scheme 2.
Total Synthesis of Sch-725674, 1
Next, the phosphate tether in 10 was removed under reductive conditions using LiAlH4 (THF, 0 °C) (Scheme 2). The corresponding tetrol was obtained in high purity following the Fieser workup,19 and without chromatography purification the crude 1,3-anti-diol was subsequently subjected to a selective acetonide protection. The crude triol was treated with 2,2-dimethoxypropane (2,2-DMP) and catalytic amounts of camphorsulfonic acid (CSA) as outlined in Scheme 2, providing 1,3-acetonide 11 in 71% yield after two reactions in two pots. The strategy proceeded with a SAE20 event [(−)-diethyl tartrate (DET), Ti(OiPr)4, cumene hydroperoxide]21 on sterically hindered cis-allyl alcohol 11 to give the corresponding diastereomeric products in 72% yield (80% brsm) with 12 as the desired major diastereomer (ds = 88%) (Scheme 2).22
Following the successful assembly of epoxide 12 in scalable quantities, a second one-pot, sequential protocol consisting of tosylation and acrylation was applied. To this end, the primary alcohol in 12 was chemoselectively transformed to tosylate (TsCl, Et3N, DMAP) in the presence of the C13 carbinol following overnight reaction. Next, acryloyl chloride was simply added to the same pot at 0° C to afford acrylate 13 in 86% yield over two reactions in one-pot (93% av/rxn) (Scheme 2).
A subsequent, consecutive one-pot, sequential protocol was established by treating 13 to a Finkelstein substitution, Boord olefination (Zn, EtOH), and acetonide deprotection sequence to assemble triol 14. This three-reaction, one-pot process commenced by exposing the tosyl group in 13 to Finkelstein conditions (NaI, acetone, reflux), followed by a solvent change from acetone to ethanol and addition of activated zinc powder to promote Boord elimination over a 2 h period under reflux. Final addition of HCl at room temperature released the 1,3-anti-diol to deliver triol 14 in 79% yield over three reactions in a single pot operation (93% av/rxn).23
With 14 in hand, Sch-725674, 1 was accessed via a final RCM as reported by Prasad and co-workers, and characterization data was in good accord with that reported by the authors.6 Overall, the total synthesis of 1 was accomplished in seven pots from triene (S,S)-8 and olefin 9. Chromatography isolations were also reduced to six procedures, which saved time and minimized chemical waste generation. While we were unable to match the reported RCM yield (36%),24 we found that simple protection of the alcohols significantly increased the efficiency of this final macrocyclization event. In this regard, we developed a two-reaction, one-pot sequential method that consists of RCM and methoxymethyl (MOM) deprotection to further streamline the synthesis toward 1.
To this end, the carbinols in 14 were protected as MOM ethers, in which the tri-protected diene 15 was obtained in 97% yield after treating with MOMCl in basic conditions (Scheme 2). Next, the one-pot protocol began by treating metathesis precursor 15 with the second-generation Grubbs catalyst25 (GII) (10 mol %) in refluxing CH2Cl2. Following metathesis completion (12 h), the solvent volume was reduced and MOM deprotection proceeded after adding trifluoroacetic acid [TFA (60 v/v%)] to the same pot, delivering natural product 1 in 84% yield over two-reactions in one-pot (92% av/rxn). This alternative approach considerably improved the yield of the RCM event, providing Sch-725674 in 14.6% total yield from triene (S,S)-8 and olefin 9 following eight pots and seven chromatography purifications.
In summary, we have disclosed a pot-economical synthesis route to the antifungal natural product Sch-725674. Overall, a seven-pot route was developed from readily prepared phosphate triene (S,S)-8 and olefin fragment 9, including seven isolations and six chromatography purifications. Key to the strategy is the application of a phosphate tether-mediated one-pot, sequential RCM/CM/hydrogenation process, a one-pot tosylation/acrylation sequence, and a one-pot, sequential Finkelstein reaction/Boord olefination/acetonide deprotection protocol. An alternative approach was introduced at the final stage of the synthesis involving a one-pot, sequential RCM/MOM-deprotection protocol to overcome efficiency challenges during the macrocyclization event. Taken together, the use of sequential reactions in the same pot provided a streamlined synthesis of Sch-725674 in minimal production time by allowing multiple bond transformations in a single flask without the need for purification of several intermediates, thus also reducing waste generation.
We anticipate that the outlined pot-efficient approach can be exploited for the synthesis of related macrocycles, such as 1,3-anti-diol containing gliomasolides (Figure 1) and derivatives in a rapid, efficient, and pot-economical manner, thus augmenting opportunities to explore this class of understudied structures in biological settings. Efforts from our laboratory in this regard will be reported in due course.
Supplementary Material
Acknowledgments
This investigation was generously supported by funds provided by the National Institute of General Medical Sciences (NIH R01GM077309). J.T. acknowledges support from the NIGMS Diversity Program. The authors thank Justin Douglas and Sarah Neuenswander in the University of Kansas NMR Laboratory and Todd Williams for HRMS analysis. Support for the NMR instrumentation was provided by NSF Grant #9512331, #9977422, #0320648 and NIH Center Grant #P20 GM103418, #S10RR024664 and #S10 OD016360. The authors also thank Materia, Inc. for supplying metathesis catalyst.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental details and spectroscopic data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors.
All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): P.R.H. is on the Scientific Advisory Board of Materia, Inc.
REFERENCES
- 1.Yang SW, Chan TM, Terracciano J, Loebenberg D, Pa-tel M, Chu M. J. Antibiot. 2005;58:535–538. doi: 10.1038/ja.2005.74. [DOI] [PubMed] [Google Scholar]
- 2.(a) Meyer WL, Schweizer WB, Beck AK, Scheifele W, Seebach D, Schreiber SL, Kelly SE. Helv. Chim. Acta. 1987;70:281–291. [Google Scholar]; (b) Carling RW, Holmes AB. Tetrahedron Lett. 1986;27:6133–6136. [Google Scholar]
- 3.Zhang J, Lin X-P, Li L-C, Zhong B-L, Liao X-J, Liu Y-H, Xu S-H. RSC Adv. 2015;5:54645–54648. [Google Scholar]
- 4.Moretti JD, Wang X, Curran DP. J. Am. Chem. Soc. 2012;134:7963–7970. doi: 10.1021/ja302260d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunnam SK, Prasad KR. Tetrahedron. 2014;70:2096–2101. [Google Scholar]
- 6.Bali AK, Sunnam SK, Prasad KR. Org. Lett. 2014;16:4001–4008. doi: 10.1021/ol5018678. [DOI] [PubMed] [Google Scholar]
- 7.Ramakrishna K, Kaliappan KP. Org. Biomol. Chem. 2015;13:234–240. doi: 10.1039/c4ob02136c. [DOI] [PubMed] [Google Scholar]
- 8.Seetharamsingh B, Khairnar PV, Reddy DS. J. Org. Chem. 2015 doi: 10.1021/acs.joc.5b02318. Article ASAP. [DOI] [PubMed] [Google Scholar]
- 9.(a) Clarke PA, Santos S, Martin WHC. Green Chem. 2007;9:438–440. [Google Scholar]; (b) Clarke PA, Zaytzav AV, Whitwood AC. Tetrahedron Lett. 2007;48:5209–5212. [Google Scholar]; (c) Ishikawa H, Suzuki T, Hayashi Y. Angew. Chem. Int. Ed. 2009;48:1304–1307. doi: 10.1002/anie.200804883. [DOI] [PubMed] [Google Scholar]
- 10.For reviews on pot economy, see: Vaxelaire C, Winter P, Christmann M. Angew. Chem. Int. Ed. 2011;50:3605–3607. doi: 10.1002/anie.201100059. Hong B-C, Raja A, Sheth VM. Synthesis. 2015;47:3257–3285. Kashinath K, Reddy DS. Org. Biomol. Chem. 2015;13:970–973. doi: 10.1039/c4ob02143f. For reviews on step economy, see: Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc. Chem. Soc. 2008;41:40–49. doi: 10.1021/ar700155p. Wender PA. Nat. Prod. Rep. 2014;31:433–440. doi: 10.1039/c4np00013g. For reviews on atom economy, see: Trost BM. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. Trost BM. Angew. Chem. Int. Ed. 1995;34:259–281. For a review on redox economy, see: Burns NZ, Baran PS, Hoff-mann RW. Angew. Chem. Int. Ed. 2009;48:2854–2867. doi: 10.1002/anie.200806086.
- 11.(a) Ishikawa H, Honma M, Hayashi Y. Angew. Chem. Int. Ed. 2011;50:2824–2827. doi: 10.1002/anie.201006204. [DOI] [PubMed] [Google Scholar]; (b) Umemiya S, Hayashi Y. Angew. Chem. Int. Ed. 2013;52:3450–3452. doi: 10.1002/anie.201209380. [DOI] [PubMed] [Google Scholar]; (c) Hayashi Y, Sakamoto D, Okamura D. Org. Lett. 2015 doi: 10.1021/acs.orglett.5b02839. Article ASAP. [DOI] [PubMed] [Google Scholar]
- 12. Venukadasula PKM, Chegondi R, Maitra S, Hanson PR. Org. Lett. 2010;12:1556–1559. doi: 10.1021/ol1002913. Hanson PR, Chegondi R, Nguyen J, Thomas CD, Waetzig J, Whitehead AJ. J. Org. Chem. 2011;76:4358–4370. doi: 10.1021/jo2003506. Chegondi R, Tan MML, Han-son PR. J. Org. Chem. 2011;76:3909–3916. doi: 10.1021/jo200337v. Jayasinghe S, Venukadasula PKM, Hanson PR. Org. Lett. 2014;16:122–125. doi: 10.1021/ol403110p. Chegondi R, Hanson PR. Tetrahedron Lett. 2015;56:3330–3333. doi: 10.1016/j.tetlet.2015.01.109. For a review, see: Hanson PR, Jayasinghe S, Maitra S, Markley JL. Top. Curr. Chem. 2015;361:253–271. doi: 10.1007/128_2014_572.
- 13.(a) Whitehead A, McReynolds MD, Moore JD, Hanson PR. Org. Lett. 2005;7:3375–3378. doi: 10.1021/ol0512886. [DOI] [PubMed] [Google Scholar]; (b) Thomas CD, McParland JM, Hanson PR. Eur. J. Org. Chem. 2009:5487–5500. doi: 10.1002/ejoc.200900560. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Che-gondi R, Maitra S, Markley JL, Hanson PR. Chem. Eur. J. 2013;19:8088–8093. doi: 10.1002/chem.201300913. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hanson PR, Jayasinghe S, Maitra S, Ndi CN, Chegondi R. Beilstein J. Org. Chem. 2014;10:2332–2337. doi: 10.3762/bjoc.10.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triene (S,S)-8 is readily prepared via a two-step coupling of the corresponding C2-symmetric 1,3-anti-diene diol and allyl alcohol with POCl3 (see ref. 13a,b), or in one step by employing phospho-ramidite chemistry (see ref. 12a). The C2-symmetric 1,3-anti-diene diol can be prepared in two steps from bis(1,5-dichloro-2,4-pentanedione)copper(II)complex Matsui K, Motoi M, Nojiri T. Bull. Chem. Soc. Jpn. 1973;46:562–565. Rychnovsky SD, Griesgraber G, Powers JP. Organic Syntheses. 2000;77:1–11. Rychnovsky SD, Griesgraber G, Zeller S, Skalitzky DJ. J. Org. Chem. 1991;56:5161–5169. We have routinely prepared the starting copper (II) salt >100 gram batches and have found that if it is washed appropriately with diethyl ether, it can be stored for long-term (>2 years) at room temperature on the benchtop.. The CM partner 9 can be generated from (S)-epichlorohydrin in two steps, see: Kubizna P, Špánik I, Kožíšek J, Szolcsányi P. Tetrahedron. 2010;66:2351–2355.
- 15.Venukadasula PKM, Chegondi R, Suryn GM, Hanson PR. Org. Lett. 2012;14:2634–2637. doi: 10.1021/ol301007h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Kingsbury JS, Harrity JPA, Bonitatebus PJ, Jr, Hoveyda AH. J. Am. Chem. Soc. 1999;121:791–799. [Google Scholar]; (b) Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168–8179. [Google Scholar]; (c) Gessler S, Randl S, Blechert S. Tetrahedron Lett. 2000;41:9973–9976. [Google Scholar]
- 17.Switching from CH2Cl2 (40 °C) to DCE (70 °C) is necessary in order to reduce homodimerization of the CM partner 9 (type I olefin), see ref. 15.
- 18.(a) Myers AG, Zheng B, Movassaghi M. J. Org. Chem. 1997;62:7507–7507. doi: 10.1021/jo9710137. [DOI] [PubMed] [Google Scholar]; (b) O’Doherty GA, Haukaas MH. Org. Lett. 2002;4:1771–1774. doi: 10.1021/ol025844x. [DOI] [PubMed] [Google Scholar]; (c) Buszek KR, Brown NJ. J. Org. Chem. 2007;72:3125–3128. doi: 10.1021/jo0622173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fieser LF, Fieser M. Reagents for Organic Synthesis. Vol. 1. New York: Wiley; 1967. pp. 581–595. Mićović VM, Mihailović ML. J. Org. Chem. 1953;18:1190–1200. (c) See Supporting Information.
- 20.Katsuki T, Sharpless KB. J. Am. Chem. Soc. 1980;102:5974–5976. [Google Scholar]
- 21.Krishna PR, Reddy VVR, Sharma GVM. Synthesis. 2004;13:2107–2114. [Google Scholar]
- 22.The minor diastereomer was isolated in 8.6%; see Supporting Information.
- 23.The spectroscopic data of diene 14 matched in all aspects with literature data, see ref. 6.
- 24.Despite several trials of conditions attempted, in our hands the RCM yields from 14 to 1 were no more than 20%. For the reported procedure, see ref. 6.
- 25.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.