Abstract
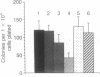
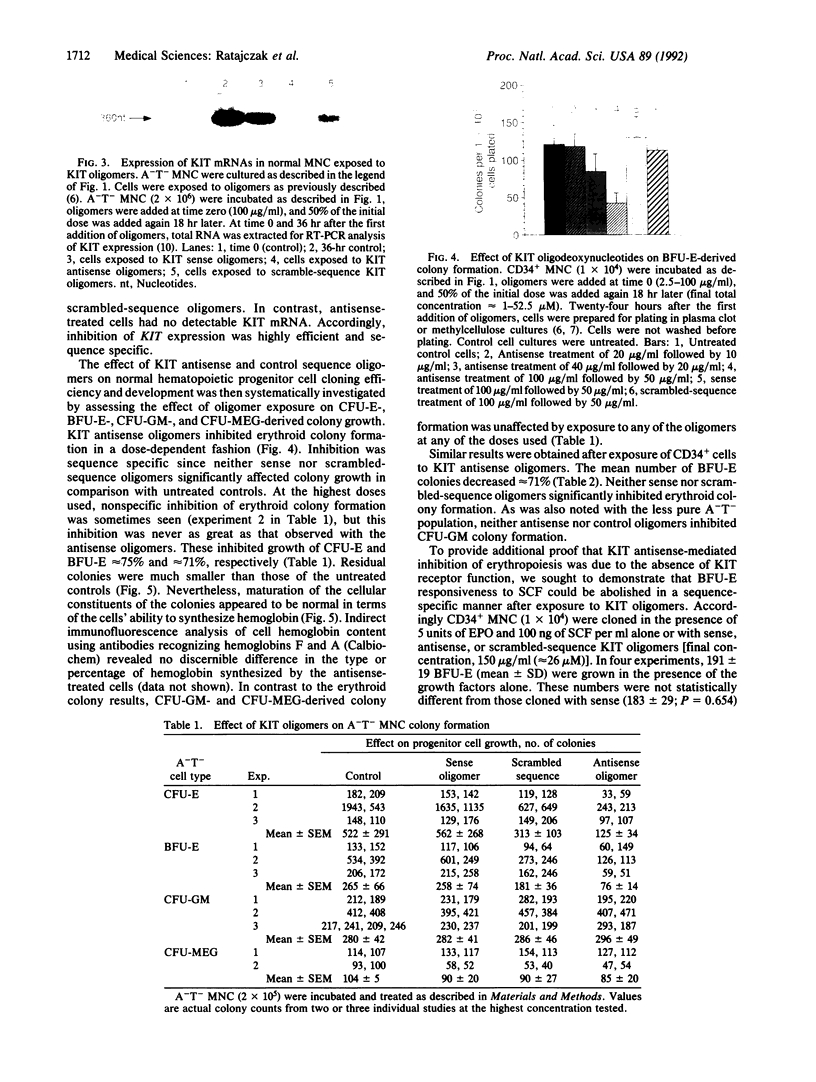
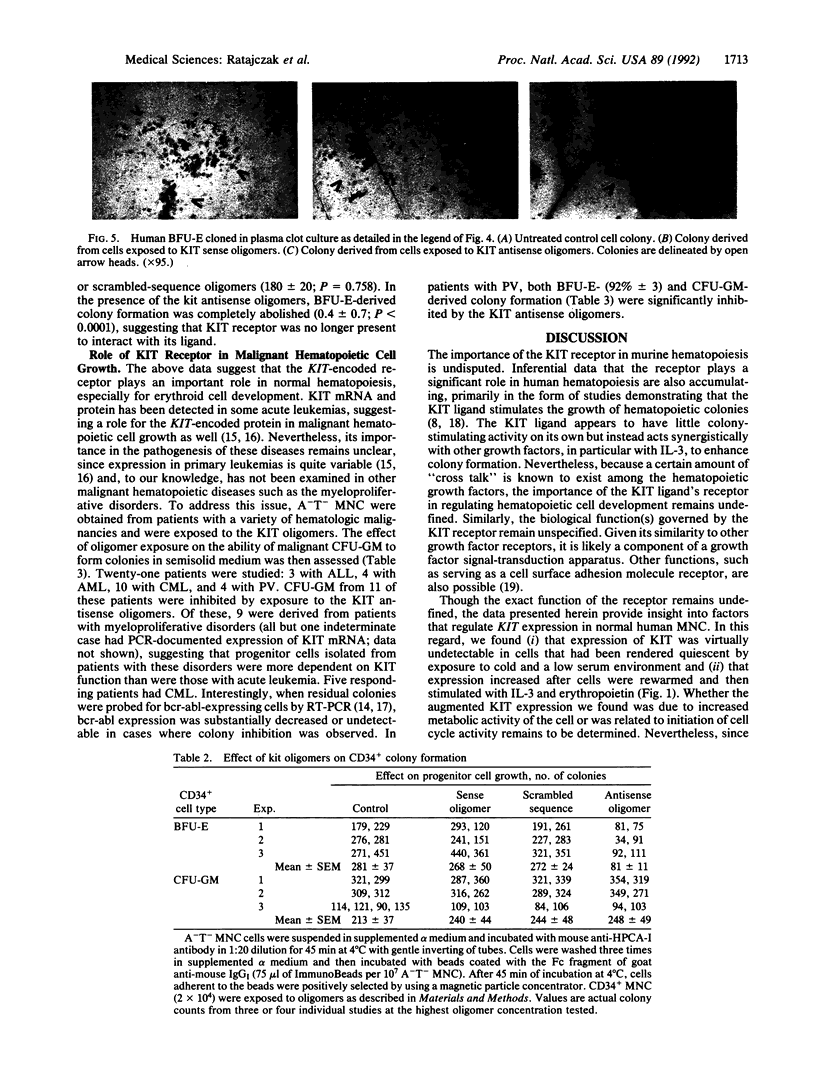
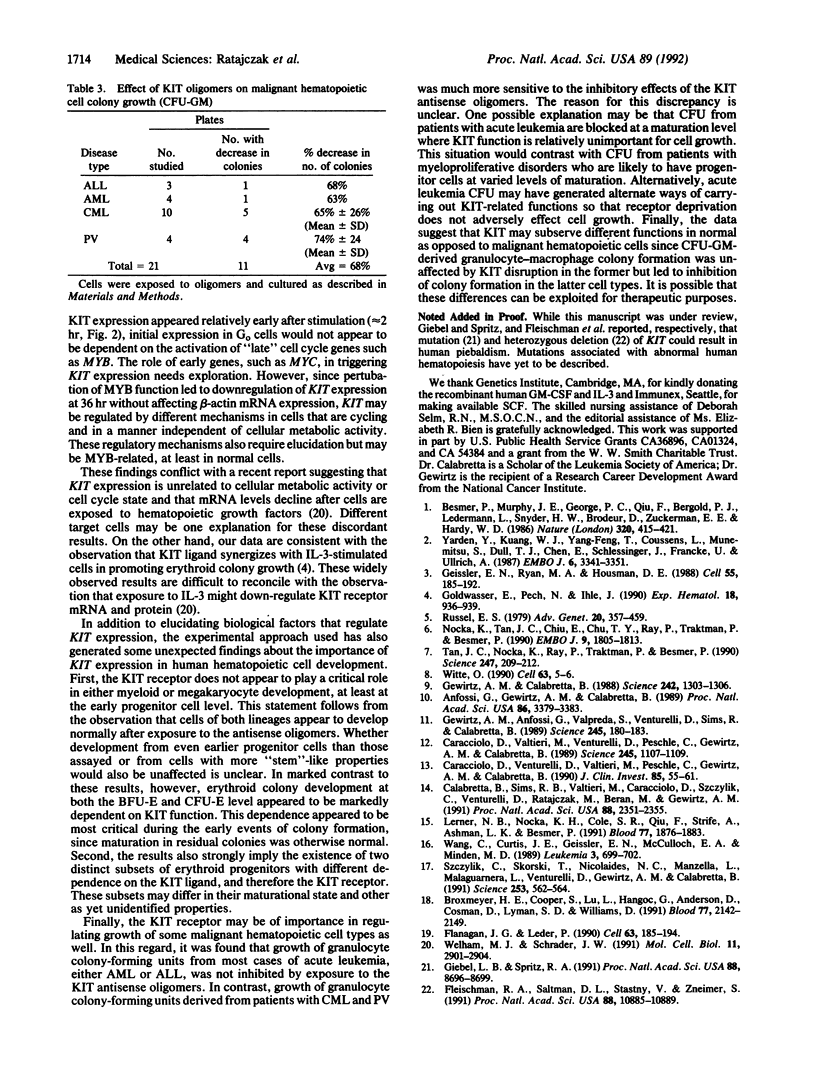
The role of the KIT protooncogene in human hematopoiesis is uncertain. Therefore, we examined KIT mRNA expression in normal human bone marrow mononuclear cells (MNC) and used antisense oligodeoxynucleotides (oligomers) to disrupt KIT function. KIT mRNA was detected with certainty only in growth factor-stimulated MNC. Expression was essentially abrogated by making MNC quiescent or by inhibiting myb gene function. Oligomers blocked KIT mRNA expression in a dose-response and sequence-specific manner, thereby allowing functional examination of the KIT receptor. In experiments with either partially purified or CD34(+)-enriched MNC, neither granulocyte nor megakaryocyte colony formation was inhibited by oligomer exposure. In contrast, KIT antisense oligomers inhibited interleukin 3/erythropoietin-driven erythroid colony formation approximately 70% and "stem cell factor"/erythropoietin-driven colony formation 100%. The presence of erythroid progenitor cell subsets with differential requirements for KIT function is therefore suggested. Growth of hematopoietic colonies from chronic myeloid leukemia and polycythemia vera patients was also inhibited, while acute leukemia colony growth appeared less sensitive to KIT deprivation. These results suggest that KIT plays a predominant role in normal erythropoiesis but may be important in regulating some types of malignant hematopoietic cell growth as well. They also suggest that KIT expression is linked to cell metabolic activity and that its expression may be regulated by or coregulated with MYB.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfossi G., Gewirtz A. M., Calabretta B. An oligomer complementary to c-myb-encoded mRNA inhibits proliferation of human myeloid leukemia cell lines. Proc Natl Acad Sci U S A. 1989 May;86(9):3379–3383. doi: 10.1073/pnas.86.9.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Cooper S., Lu L., Hangoc G., Anderson D., Cosman D., Lyman S. D., Williams D. E. Effect of murine mast cell growth factor (c-kit proto-oncogene ligand) on colony formation by human marrow hematopoietic progenitor cells. Blood. 1991 May 15;77(10):2142–2149. [PubMed] [Google Scholar]
- Calabretta B., Sims R. B., Valtieri M., Caracciolo D., Szczylik C., Venturelli D., Ratajczak M., Beran M., Gewirtz A. M. Normal and leukemic hematopoietic cells manifest differential sensitivity to inhibitory effects of c-myb antisense oligodeoxynucleotides: an in vitro study relevant to bone marrow purging. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2351–2355. doi: 10.1073/pnas.88.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo D., Valtieri M., Venturelli D., Peschle C., Gewirtz A. M., Calabretta B. Lineage-specific requirement of c-abl function in normal hematopoiesis. Science. 1989 Sep 8;245(4922):1107–1110. doi: 10.1126/science.2672339. [DOI] [PubMed] [Google Scholar]
- Caracciolo D., Venturelli D., Valtieri M., Peschle C., Gewirtz A. M., Calabretta B. Stage-related proliferative activity determines c-myb functional requirements during normal human hematopoiesis. J Clin Invest. 1990 Jan;85(1):55–61. doi: 10.1172/JCI114433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Fleischman R. A., Saltman D. L., Stastny V., Zneimer S. Deletion of the c-kit protooncogene in the human developmental defect piebald trait. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10885–10889. doi: 10.1073/pnas.88.23.10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Gewirtz A. M., Anfossi G., Venturelli D., Valpreda S., Sims R., Calabretta B. G1/S transition in normal human T-lymphocytes requires the nuclear protein encoded by c-myb. Science. 1989 Jul 14;245(4914):180–183. doi: 10.1126/science.2665077. [DOI] [PubMed] [Google Scholar]
- Gewirtz A. M., Calabretta B. A c-myb antisense oligodeoxynucleotide inhibits normal human hematopoiesis in vitro. Science. 1988 Dec 2;242(4883):1303–1306. doi: 10.1126/science.2461588. [DOI] [PubMed] [Google Scholar]
- Giebel L. B., Spritz R. A. Mutation of the KIT (mast/stem cell growth factor receptor) protooncogene in human piebaldism. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8696–8699. doi: 10.1073/pnas.88.19.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwasser E., Pech N., Ihle J. Interleukin 3 can compensate for the deficiency in erythroid burst-forming cells in anemic mice of genotype W/Wv. Exp Hematol. 1990 Sep;18(8):936–939. [PubMed] [Google Scholar]
- Lerner N. B., Nocka K. H., Cole S. R., Qiu F. H., Strife A., Ashman L. K., Besmer P. Monoclonal antibody YB5.B8 identifies the human c-kit protein product. Blood. 1991 May 1;77(9):1876–1883. [PubMed] [Google Scholar]
- Nocka K., Tan J. C., Chiu E., Chu T. Y., Ray P., Traktman P., Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990 Jun;9(6):1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Szczylik C., Skorski T., Nicolaides N. C., Manzella L., Malaguarnera L., Venturelli D., Gewirtz A. M., Calabretta B. Selective inhibition of leukemia cell proliferation by BCR-ABL antisense oligodeoxynucleotides. Science. 1991 Aug 2;253(5019):562–565. doi: 10.1126/science.1857987. [DOI] [PubMed] [Google Scholar]
- Tan J. C., Nocka K., Ray P., Traktman P., Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990 Jan 12;247(4939):209–212. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- Wang C., Curtis J. E., Geissler E. N., McCulloch E. A., Minden M. D. The expression of the proto-oncogene C-kit in the blast cells of acute myeloblastic leukemia. Leukemia. 1989 Oct;3(10):699–702. [PubMed] [Google Scholar]
- Welham M. J., Schrader J. W. Modulation of c-kit mRNA and protein by hemopoietic growth factors. Mol Cell Biol. 1991 May;11(5):2901–2904. doi: 10.1128/mcb.11.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N. Steel locus defines new multipotent growth factor. Cell. 1990 Oct 5;63(1):5–6. doi: 10.1016/0092-8674(90)90280-r. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]