Abstract
Objective Assess the association between fine motor (FM) and visual–motor integration (VMI) skills and academic achievement in pediatric acute lymphoblastic leukemia (ALL) survivors. Methods In this 28-site cross-sectional study of 256 children in first remission, a mean of 8.9 ± 2.2 years after treatment for standard-risk precursor-B ALL, validated measures of FM, VMI, reading, math, and intelligence were administered at mean follow-up age of 12.8 ± 2.5 years. Results VMI was significantly associated with written math calculation ability (p < .0069) after adjusting for intelligence (p < .0001). VMI was more strongly associated with math in those with lower intelligence (p = .0141). Word decoding was also significantly associated with VMI but with no effect modification by intelligence. FM skills were not associated with either reading or math achievement. Conclusion These findings suggest that VMI is associated with aspects of math and reading achievement in leukemia survivors. These skills may be amenable to intervention.
Keywords: academic achievement, childhood acute lymphoblastic leukemia, fine motor skills, visual–motor integration
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy and has a peak incidence between 1 and 4 years of age (Howlader et al., 2014). Nearly 90% of children diagnosed with ALL will be cured (Howlader et al., 2014), but survivors are at increased risk of neurocognitive sequelae of treatment across a range of domains, including attention, information processing, and intellectual functioning (Campbell et al., 2007). These cognitive processing difficulties are consistently reported in survivors even after cranial radiation was eliminated from most therapeutic protocols (Campbell et al., 2007; Iyer, Balsamo, Bracken, & Kadan-Lottick, 2015). Impairments contribute to diminished academic achievement and higher utilization of special education services (Harshman et al., 2012; Kaemingk, Carey, Moore, Herzer, & Hutter, 2004; Mitby et al., 2003). Factors such as female gender (Waber, Tarbell, Kahn, Gelber, & Sallan, 1992), younger age at diagnosis (Copeland, Moore, Francis, Jaffe, & Culbert, 1996), and greater time elapsed since treatment (Moore, 2005) have been associated with worse neurocognitive outcomes in some, but not all, studies.
Reduced fine motor functioning is consistently found in children treated for ALL (Espy et al., 2001; Hockenberry et al., 2007; Jansen et al., 2008; Kingma et al., 2001; Vainionpaa, Kovala, Tolonen, & Lanning, 1995). Treatment protocols for ALL include drugs such as vincristine that cause diminished motor function as a result of peripheral neuropathy both during (Casey, Jellife, Le Quesne, & Millett, 1973) and after the conclusion of therapy (DeAngelo, 2009; Hartman, van den Bos, Stijnen, & Pieters, 2006; Lehtinen et al., 2002). Treatment with central nervous system-directed chemotherapy, such as intrathecal methotrexate, has also been demonstrated to cause motor and sensory problems in children with ALL (Harila-Saari, Huuskonen, Tolonen, Vainionpaa, & Lanning, 2001; Harila-Saari, Vainionpaa, Kovala, Tolonen, & Lanning, 1998; Vainionpaa, Kovala, Tolonen, & Lanning, 1997). Fine motor skills are also integral to visual–motor integration (VMI; Beery & Beery, 2004), the ability to coordinate motor and visual perception. This neurocognitive function is also consistently affected in survivors of ALL with other cognitive deficits (Copeland et al., 1996; Espy et al., 2001; Hockenberry et al., 2007).
Among young, typically developing children, fine motor and VMI skills are associated with the emergence of early math and reading skills (Cameron et al., 2012; Grissmer, Grimm, Aiyer, Murrah, & Steele, 2010; Kaemingk et al., 2004; Son & Meisels, 2006). In spina bifida patients with known motor deficits, fine motor skills, visual-spatial skills and visual–motor coordination were predictors of math ability at age 3 and 5 years (Barnes et al., 2011). Fine motor functioning was uniquely associated with counting knowledge, oral counting, and arithmetic ability among typically developing children in this study (Barnes et al., 2011).
It is hypothesized that fine motor and finger skills play a critical role in learning to count and calculate (Butterworth, 1999, 2005). Butterworth (1999, 2005) suggests that children learning to count and execute simple arithmetic problems use their fingers as tangible representations of number. Neuroimaging findings support the connection between counting and mental math calculations and finger movements (Andres, Seron, & Olivier, 2007; Zago et al., 2001). Additionally, it has been suggested that finger counting among young children may reduce working memory demands and thus facilitate the acquisition of basic arithmetic skills (Alibali & DiRusso, 1999). Conversely, without fine motor facility, a strong foundation of arithmetic and problem solving skills may not develop efficiently (Barnes & Raghubar, 2014).
There is also a relationship, though less direct, between VMI and word reading. For example, VMI is strongly associated with handwriting ability (Daly, Kelley, & Krauss, 2003) for which there are heavy demands in the elementary school classroom. Interventions to improve letter formation in first graders have simultaneously improved single word decoding skills (Berninger et al., 2006). Writing, speaking, and reading all require coordinated fine motor movements of the hand, mouth, and eye, respectively (Grissmer et al., 2010). More broadly, a body of research explicates the links between motor and cognitive skills (Diamond, 2000) as evidenced by functional imaging studies that show simultaneous activation in prefrontal and cerebellar areas for cognitive tasks.
Whether fine motor and VMI significantly contribute to the diminished reading (Harshman et al., 2012; Mulhern, Fairclough, & Ochs, 1991; Peterson et al., 2008) and math (Espy et al., 2001; Harshman et al., 2012; Mulhern et al., 1991; Peterson et al., 2008) achievement in ALL survivors has not been demonstrated. Because the peak incidence of ALL occurs between the ages of 1 and 4 years (Howlader et al., 2014), the effects of reduced fine motor function may particularly impact these young children who are learning rudimentary counting, arithmetic, and decoding skills. To our knowledge, there are no available studies of reading outcomes and only one small study of math and cognition in 15 patients (Kaemingk et al., 2004). Furthermore, this small study did not adjust for intelligence, which is strongly associated with achievement and could be a confounder (Barnhardt, Borsting, Deland, Pham, & Vu, 2005; Cameron et al., 2012; Fayol, Barrouillet, & Marinthe, 1998; Kulp, 1999).
Specific studies in ALL patients are needed because this young population is at risk for acquiring a range of neurocognitive deficits, and the relative contribution of fine motor and VMI skills in this setting may be different from the general population (Kaemingk et al., 2004). Fine motor difficulties are amenable to intervention (Dankert, Davies, & Gavin, 2003) and could be an appropriate target for remediation.
In this current study of a large, homogenous sample of children previously treated for ALL with chemotherapy-only regimens, our aim was to determine if fine motor and VMI skills were associated with math calculation and word decoding, while controlling for intelligence. We also sought to identify other patient and treatment factors that potentially contribute to any association.
Methods
Participants
We conducted a cross-sectional study at limited institutions of patients with ALL previously enrolled and randomized in legacy therapy protocols (i.e., closed to enrollment), CCG 1922 and 1952, which were open from March 1993 to August 1995 and May 1996 to February 2000, respectively (Bostrom et al., 2003; Stork et al., 2010). Patients were eligible for participation in the current neurocognitive follow-up study if they were diagnosed and enrolled on one of these protocols for standard risk precursor B-ALL at 1 of the 28 sites. The National Cancer Institute Criteria (Smith et al., 1996) defines standard risk precursor B-ALL as peripheral white blood count <50,000 and age between 1.0 and 9.99 years. Patients were also off-therapy and in first remission. Participating institutions were chosen based on their interest and availability of a licensed psychologist, as well as the support staff necessary to implement the study. An effort was made to include both community and tertiary care programs from all major regions of the nation to maximize ethnic and geographic diversity. All patients received central nervous system-directed chemotherapy with intrathecal medications; however, no patient had a history of central nervous system leukemia. Thus, no child received cranial radiation. Additional eligibility requirements included completion of all therapy at least 1 year previously, age at evaluation of 6–16.99 years, no history of preexisting developmental disorders (e.g., trisomy 21, developmental delay), no history of very low birth weight (<1,500 g), which is associated with nonverbal and math disabilities (Harnadek & Rourke, 1994; Taylor, Hack, Klein, & Schatschneider, 1995; Worling, Humphries, & Tannock, 1999), and no pre-cancer history of having been retained in school or requiring special education services. The age restriction corresponded to the validated age range of the standardized neuropsychological instruments used in the evaluation. In addition, individuals were excluded if they had been nonrandomly assigned to more intensive therapy because of unfavorable cytogenetic findings or a slow response after induction. The methodology has been previously reported (Kadan-Lottick, Brouwers, Breiger, Kaleita, Dziura, Liu, et al., 2009; Kadan-Lottick, Brouwers, Breiger, Kaleita, Dziura, Northrup, et al., 2009; Kunin-Batson, Kadan-Lottick, & Neglia, 2014; Walsh et al., 2015). There was no difference in vincristine dosing between protocols.
A total of 746 patients were enrolled on the two therapeutic studies at the participating sites and were potentially eligible. Of these, 263 patients consented and completed the evaluation and 256 had complete data for tests of fine motor and VMI skills. Of the remaining 483 patients, 219 were lost to follow-up and could not be traced, 236 refused participation, and 28 were never offered participation owing to accidental oversight by study staff. There were no differences between participants and nonparticipants with respect to age at diagnosis, elapsed time since treatment, or gender (Kadan-Lottick, Brouwers, Breiger, Kaleita, Dziura, Liu et al., 2009; Kadan-Lottick, Brouwers, Breiger, Kaleita, Dziura, Northrup, et al., 2009). Also see Supplementary Materials.
Procedures
The institutional review board of each participating center as well as the Yale University Human Investigation Committee approved the current study. Informed consent and assent were obtained for all participants in accordance with the Declaration of Helsinki. Participants underwent a comprehensive, half-day, neurocognitive assessment supervised by a licensed psychologist. This evaluation was paid by research funds, at no cost to the patient. Parents of participants completed a demographic and medical history survey regarding their education and income. This questionnaire also confirmed that the child was developing normally before the ALL diagnosis as an additional check of eligibility for this study.
Measures
As part of the test battery, participants completed the Numerical Operations and Word Reading subtests from the Wechsler Individual Achievement Test—Second Edition—Abbreviated (WIAT-II-A) (Wechsler, 2005). The Numerical Operations subtest is untimed and requires the child to complete written math calculations of increasing complexity. Beginning items assess skills such as number discrimination and rote counting, while later items assess basic addition and subtraction, multidigit addition and subtraction, multiplication and division. The most advanced tasks include geometry, algebraic equations, and trigonometry. The Word Reading subtest is an untimed decoding task composed of words of graduated difficulty and decreased frequency in the English language. The WIAT-II-A is a widely used standardized assessment of achievement with excellent test–retest reliability (0.98) and validity (Wechsler, 2005).
Fine motor skills were assessed with the Lafayette Grooved Pegboard (Lafayette Instrument Company, 2002), an apparatus that contains 25 holes with randomly positioned slots. Pegs must be rotated to match the hole before they can be inserted. For children aged ≥9 years, the efficiency of speed and finger dexterity is measured in the time required to place all pegs into the pegboard. For children aged <9 years, only the first two rows require placement of pegs. It is a commonly used measure of motor functioning with sufficient reliability and validity for the testing of manual dexterity (Rourke, Yanni, MacDonald, & Young, 1973; Solan, Mozlin, & Rumpf, 1985). The dominant hand score was used in the analyses.
The Beery-Buktenica Developmental Test of Visual-Motor Integration, Fifth Edition (Beery & Beery, 2004) was used to assess VMI performance. This test measures the ability to copy figures of graduated complexity requiring coordination of visual perception and finger and hand movements. The Beery VMI was standardized on a national sample of 1,737 children, age 2–18 years, and 1,021 adults, age 19–100 years. This test has proven test–retest reliability (0.90) and validity (Beery & Beery, 2004).
The standard scaled score from the Full Scale Intelligence Quotient (FSIQ) of the Wechsler Intelligence Scales for Children—Fourth Edition (WISC-IV) was used as an indication of intelligence or overall cognitive ability (Crocker, Riley, & Mattson, 2015). This composite of subtests assesses verbal reasoning skills, nonverbal reasoning skills, working memory and processing speed (Wechsler, 2003). The WISC-IV used a standardization sample of 2,200 children and has internal consistency reliability coefficients that range from 0.88 to 0.97. For all measures, age-adjusted standard scores with a population mean of 100 (SD = 15) were used for analyses.
Data Analysis
The primary outcomes of interest were the WIAT-II-A Word Reading and Numerical Operations subscales as continuous variables. Univariate and multivariate linear regressions were used to analyze the association of fine motor and VMI skills (as continuous variables) with word decoding and math calculation. Female gender (Waber et al., 1992), younger age at diagnosis (Copeland et al., 1996), and greater time elapsed since treatment (Moore, 2005) have been associated with worse neurocognitive outcomes; hence, these variable were included as covariates in the multivariate model. FSIQ was also used as a covariate. In selecting the FSIQ as compared with other estimates of global cognitive function, a comprehensive assessment of global intellectual ability is used. Because this composite score also reflects motor, working memory, and processing speed demands, use of this covariate yields the unique contribution of visual-motor abilities on written math calculation and word decoding. Protocol assignment, intelligence, and age at diagnosis were examined as potential effect modifiers. All statistical assessments were performed using SAS software, version 9.3 (SAS Institute, Cary NC).
Results
Participants
The participants had a mean age of 3.9 (SD = 1.8, range = 1.0–9.8) years at diagnosis of ALL and were 12.8 (SD = 2.5, range = 7.0–16.9) years at evaluation. The average time elapsed since diagnosis was 8.9 (SD = 2.2, range = 4.3–13.7) years. Eighty-one percent were White and 53% were males (Table I). Mean scores for VMI, fine motor functioning, intelligence, math calculation, and word reading fell in the average range (Table II).
Table I.
Demographic and Clinical Characteristics of Participants
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Age at diagnosis (years) | 3.9 (1.8) |
| Age at evaluation (years) | 12.8 (2.5) |
| Time elapsed since diagnosis (years) | 8.9 (2.2) |
| Sex | |
| Female | 120 (46.9) |
| Male | 136 (53.1) |
| Race and ethnicity | |
| White, non-Hispanic | 207 (80.9) |
| Hispanic | 19 (7.4) |
| Black, non-Hispanic | 6 (2.3) |
| Asian | 5 (2.0) |
| Other | 19 (7.5) |
| Maternal education | |
| Grade school | 24 (9.4) |
| High school | 54 (21.1) |
| Some college or training | 83 (32.4) |
| College or higher | 84 (32.8) |
| Unknown | 11 (4.3) |
| Household Income | |
| Less than $50,000 | 74 (28.9) |
| $50,000–$79,900 | 71 (27.7) |
| $80,000 or more | 90 (35.2) |
| Unknown | 21 (8.2) |
Table II.
Neuropsychological Assessment of Participants
| Domain assessed | Measure | Mean (SD) | Percent performing below averagea | p value of expected to actual percentage of below average performers |
|---|---|---|---|---|
| Visual–motor integration | VMI | 92.8 (13.2) | 23.7 | <.0007b,c |
| Fine motor | Lafayette Grooved Pegboard | 102.1 (10.9) | 7.9 | <.0001b,d |
| Intelligence | Full Scale Intelligence Quotient, WISC-IV | 100.0 (12.1) | 8.7 | <.05b,d |
| Math calculation | Numerical operations, WIAT-II-A | 100.7 (15.6) | 14.6 | .57 |
| Word reading | Word reading, WIAT-II-A | 101.2 (11.5) | 7.1 | <.0001b,d |
Note. VMI = Beery-Buktenica Developmental Test of Visual-motor Integration; WISC-IV = Wechsler Intelligence Scales for Children—Fourth Edition; WIAT-II-A = Wechsler Individual Achievement Test—Second Edition—Abbreviated.
For all tests, the normative mean = 100, SD = 15.
aScore > 1 SD below the mean.
bStatistically significant.
cMore than expected.
dFewer than expected.
Numerical Operations
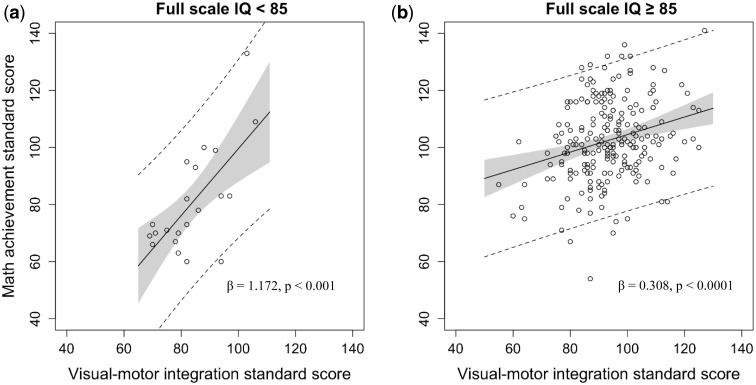
In univariate analysis, significant predictors of math calculation ability included VMI, fine motor function, and FSIQ (Table III). The residual plot and the Q-Q plot of the residuals were calculated for the multivariate models and data were found to be normally distributed and the assumption for homoscedasticity was met. In the multivariate model (Table IV), VMI (β = 1.185, p < .0069) and FSIQ (β = 1.672, p < .0001) remained significantly associated with math calculation. FSIQ was found to moderate the association between VMI and math calculation. For children with lower FSIQ, performance on the task of VMI was more strongly associated with math calculation ability (Figure 1). There were no significant interactions between VMI or fine motor functioning with gender, age at diagnosis, or time elapsed since diagnosis. Neither protocol nor age at diagnosis was a significant moderator.
Table III.
Numerical Operations and Word Reading: Univariate Associations of Patient Factors and Neuropsychological Assessments
| Variable | Numerical operations |
Word reading |
||
|---|---|---|---|---|
| β (SE) | p-value | β (SE) | p-value | |
| Visual–motor integration | 0.367 (0.073) | <.0001* | 0.254 (0.053) | <.0001* |
| Fine motor | 0.235 (0.086) | .0070* | 0.120 (0.064) | .0623 |
| FSIQ | 0.546 (0.070) | <.0001* | 0.596 (0.067) | <.0001* |
| Male gender | −1.507 (1.964) | .44 | 0.122 (1.741) | .86 |
| Age at diagnosis (years) | 1.034 (0.549) | .0609 | 1.237 (0.499) | .58 |
| Time elapsed since diagnosis (years) | −3.02 (0.449) | .50 | −0.181 (0.415) | .48 |
Note. SE = standard error; FSIQ = Full Scale Intelligence Quotient.
*Statistically significant.
Table IV.
Numerical Operations: Multivariate Association (β) of Patient Factors and Neuropsychological Assessments
| Variable | β (SE) | p-value |
|---|---|---|
| Intercept | −76.732 (4.831) | .0544 |
| Visual–motor integration | 1.185 (0.434) | .0069* |
| Fine motor function | −0.0455 (0.0776) | .56 |
| FSIQ | 1.672 (0.398) | <.0001* |
| Male gender | 0.149 (1.630) | .93 |
| Age at diagnosis (years) | 0.858 (0.465) | .0693 |
| Time elapsed since diagnosis (years) | −0.089 (0.387) | .82 |
| Interaction between visual–motor integration and FSIQ | −0.0104 (0.0042) | .0141* |
| Interaction between visual–motor integration and age at diagnosis | −0.06302 (0.03578) | .0795 |
Note. SE = standard error; FSIQ = Full Scale Intelligence Quotient.
*Statistically significant.
Figure 1.
The effect of intelligence on the association between visual–motor integration and math calculation ability. To assist with the visual representation of the interaction, the full scale IQ (FSIQ) variable was dichotomized into two groups: (a) patients with below average FSIQ and (b) patients with at least average FSIQ. The gray shading represents the 95% confidence interval. The dashed lines depict the 95% prediction interval.
Word Reading
Table III displays the results of the univariate analysis. VMI and intelligence, but not fine motor functioning, were significantly associated with word reading. In the multivariate model (Table V), VMI (β = 0.639, p = .0408) and FSIQ (β = 1.158, p < .0001) remained significantly associated with word reading. There was no significant interaction between VMI and FSIQ. Additionally, there were no significant interactions between visual-motor or fine motor functioning, gender, age at diagnosis, and time elapsed since diagnosis. Neither protocol nor age at diagnosis was a significant moderator of the association.
Table V.
Word Reading: Multivariate Association (β) of Patient Factors and Neuropsychological Assessments
| Variable | β (SE) | p-value |
|---|---|---|
| Intercept | −3.879 (28.353) | .99 |
| Visual–motor integration | 0.639 (0.310) | .0408* |
| Fine motor function | −0.102 (0.055) | .0667 |
| FSIQ | 1.158 (0.284) | <.0001* |
| Male gender | 0.567 (1.163) | .63 |
| Age at diagnosis (years) | −0.298 (0.333) | .37 |
| Time elapsed since diagnosis (years) | −0.439 (0.272) | .11 |
| Interaction between Visual–motor integration and FSIQ | −0.0059 (0.0030) | .0531 |
| Interaction between visual–motor integration and age at diagnosis | −0.03876 (0.02543) | .1289 |
Note. SE = standard error; FSIQ = Full Scale Intelligence Quotient.
*Statistically significant.
Conclusion
This large cross-sectional study of 256 children treated for ALL with modern therapy demonstrated that VMI was significantly associated with math calculation ability after adjusting for intelligence. For children with a lower FSIQ, VMI ability was more strongly associated with math calculation. Word reading was also associated with VMI. Fine motor functioning alone was not associated with math calculation or word reading.
Contrary to our proposed reasoning, we did not find fine motor skills alone to be significantly associated with math calculation or word reading. In contrast, among typical kindergarten students, fine motor skills, independent of a visual-spatial component, contribute to academic achievement (Cameron et al., 2012). It may be that the severity and frequency of fine motor deficits in these ALL survivors does not reach a significant threshold. Perhaps only with the added spatial processing demands a meaningful association with math calculation ability was found. Visual-spatial skills are thought to be associated with a child’s ability to spatially represent numerical meaning (Geary, 1993) and is associated with counting ability and processing of number magnitude (Dehaene, Spelke, Pinel, Stanescu, & Tsivkin, 1999; Kyttala, Aunio, Lehto, Van Luit, & Hautamaki, 2003).
The association between VMI ability and math calculation has not been previously demonstrated in children treated for ALL or other types of cancer, though it is well-documented among typical children and those with developmental motor impairments (Barnes et al., 2011; Son & Meisels, 2006). Our study differs from a small study of 15 ALL patients and controls by Kaemingk et al. (2004) that identified psychomotor speed, but not VMI ability, as associated with arithmetic and applied math achievement. In addition to having a smaller sample size, Kaemingk et al. (2004) did not adjust for intelligence.
Further, we found that for leukemia survivors with below average intellectual abilities, the association of VMI on math was higher than in those children with average or higher IQ. Given the strong association between IQ and general cognitive abilities as well as cognitive reserve, children with higher IQ may have the benefit to be able to compensate for their deficits in lower order skills. This may be likened to findings that IQ explains a greater proportion of variance on performance of executive function tasks than does a diagnosis of Attention-Deficit/Hyperactivity Disorder (ADHD) (Mahone et al., 2002). At an above average IQ, the effects of ADHD on performance are less pronounced, suggesting that children may compensate for deficits (Mahone et al., 2002).
We posit another possible explanation for the observed association between VMI and academic achievement. It is suggested that visual-motor skills are acutely sensitive to cerebral dysfunction and suggest impairment even in the absence of clinical observations or gross neurological deficits (Buizer, De Sonneville, van den Heuvel-Eibrink, Njiokiktjien, & Veerman, 2005; Frank, Foley, & Kuchuk, 1997; Heitger et al., 2004). As evidence, among childhood ALL survivors who may not show any significant neurological issues, deficits in VMI can be apparent (Buizer et al., 2005; Espy et al., 2001; Jansen et al., 2008). This discussion is speculative in nature but may be an avenue for study.
Visual-motor deficiencies may first present years after treatment and continue to progress over time (Espy et al., 2001), and thus, surveillance is particularly important. Because this cross-sectional study does not provide evidence that written diminished visual-motor skills cause poorer written math calculation, these weaknesses may better serve as a general marker for children at risk for reduced academic progress. This may be particularly important for leukemia survivors who may not evidence gross impairment in written math calculation but whose performance reflects a possible decline in function. As this sample is better educated and with higher household income than the general population, normative means may be an underestimate of the expected level of function for unaffected matched controls (Kadan-Lottick, Ness, Bhatia, & Gurney, 2003). Early identification of diminished VMI ability may allow educators to target interventions at children at greater risk for manifesting problems with written math calculation skills. Improving number formation and spatial planning, including use of the number line, may be used as components of a more comprehensive program to augment calculation ability (Coles, Kable, & Taddeo, 2009; Gersten et al., 2009; Moore, Hockenberry, Anhalt, McCarthy, & Krull, 2012).
We found that word reading was not significantly associated with fine motor skills, and the association of VMI and word reading was smaller than with written math calculation. Reading impairment, as compared with problems with math, is less commonly observed among ALL survivors (Brown et al., 1996; Brown, Sawyer, Antoniou, Toogood, & Rice, 1999; Mulhern et al., 1991). Moreover, associations of motor-related skills to word reading are typically of smaller magnitude relative to other cognitive processes (Kudo, Lussier, & Swanson, 2015) so a reduced effect size in the population is consistent with these findings.
This study has some potential limitations with which the findings should be considered. This was a cross-sectional study that cannot determine a causal relationship between motor and cognitive abilities and academic achievement. Therapy exposures may have concurrently resulted in impaired VMI and math calculation abilities, which are both at least in part subserved by areas within the left perisylvian cortex (Dehaene et al., 1999). Additionally, there is a preponderance of data that indicates many cognitive processes contribute to success in calculation, including, but not limited to executive processes (Bull, Espy, & Wiebe, 2008; Hassinger-Das, Jordan, Glutting, Irwin, & Dyson, 2014), working memory (Bull et al., 2008; Geary, Hoard, Byrd-Craven, Nugent, & Numtee, 2007), and sustained attention (Buizer, de Sonneville, van den Heuvel-Eibrink, & Veerman, 2006; Hassinger-Das et al., 2014). While examining a broader range of cognitive variables may add to our understanding of achievement in leukemia survivors, we chose to examine a specific area, for example, fine motor functioning, for which there exists potential interventions and where there is interest in modifying vincristine dosing. In this study, we excluded patients that reported a pre-cancer diagnosis of a learning or developmental disability. This allowed us to attribute better any post-cancer deficit to treatment-related effects; however, given the young median age at which leukemia is diagnosed, emerging and as yet undiagnosed learning or attention problems could not be accounted for. Additionally, we did not collect data to assess the impact of school absences on academic achievement. However, as these survivors did not show a greater than expected percentage of below average performance in either calculation or decoding, the effect of absences is likely to be minimal. Finally, although this is a large-scale study of a group of homogeneously treated leukemia patients, participation in this study was partially dependent on the availability of psychology personnel to implement the protocol. While every effort was made to include community and tertiary care programs from all major regions of the nation to maximize ethnic and geographic diversity, a selection bias may still exist. However, we would not expect these factors to affect the toxic effects of chemotherapy or the relationship between VMI and academic achievement.
In conclusion, the results of this study support an association between VMI and math calculation and word reading among long-term pediatric survivors of ALL. Routine screening assessments are important to identify any deficits or emerging weaknesses in VMI. As such, the appropriate educational supports can be implemented in a timely manner. Consideration of VMI weaknesses as a marker for subtle neurocognitive dysfunction may also be considered.
Supplementary Data
Supplementary data can be found at: http://www.jpepsy.oxfordjournals.org/.
Funding
This research was supported by research funding from the American Cancer Society (J.P.N.). K.-L. was supported in part by CTSA Grant Number KL2 RR024138 from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. K.-L. is a Hyundai Hope on Wheels Scholar. Additional support was obtained from Children’s Oncology Group Chair’s Grant U10 CA98543, Statistics and Data Center Grant U10CA98413, and CCOP Grant U10 CA95861.
Conflicts of interest: None declared.
References
- Alibali M. W., DiRusso A. A. (1999). The function of gesture in learning to count: More than keeping track. Cognitive Development, 14, 37–56. doi: 10.1016/S0885-2014%2899%2980017-3 [Google Scholar]
- Andres M., Seron X., Olivier E. (2007). Contribution of hand motor circuits to counting. Journal of Cognitive Neuroscience, 19, 563–576. doi: 10.1162/jocn.2007.19.4.563 [DOI] [PubMed] [Google Scholar]
- Barnes M. A., Raghubar K. P. (2014). Mathematics development and difficulties: The role of visual-spatial perception and other cognitive skills. Pediatric Blood and Cancer, 61, 1729–1733. doi: 10.1002/pbc.24909 [DOI] [PubMed] [Google Scholar]
- Barnes M. A., Stubbs A., Raghubar K. P., Agostino A., Taylor H., Landry S., Fletcher J. M., Smith-Chant B. (2011). Mathematical skills in 3- and 5-year-olds with spina bifida and their typically developing peers: A longitudinal approach. Journal of the International Neuropsychological Society, 17, 431–444. doi: 10.1017/S1355617711000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhardt C., Borsting E., Deland P., Pham N., Vu T. (2005). Relationship between visual-motor integration and spatial organization of written language and math. Optometry and Vision Science, 82, 138–143. [DOI] [PubMed] [Google Scholar]
- Beery K., Beery N. (2004). The Beery-Buktenica developmental test of visual-motor integration: Administration, scoring and teaching manual. (5th ed.). Minneapolis MN: NCS Pearson. [Google Scholar]
- Berninger V. W., Rutberg J. E., Abbott R. D., Garcia N., Anderson-Youngstrom M., Brooks A., Fulton C. (2006). Tier 1 and Tier 2 early intervention for handwriting and composing. Journal of School Psychology, 44, 3–30. doi: 10.1016/j.jsp.2005.12.003 [Google Scholar]
- Bostrom B. C., Sensel M. R., Sather H. N., Gaynon P. S., La M. K., Johnston K., Erdmann G. R., Gold S., Heerema N. A., Hutchinson R. J., Provisor A. J., Trigg M. E.; Children's Cancer, G. (2003). Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: A report from the Children's Cancer Group. Blood, 101, 3809–3817. doi: 10.1182/blood-2002-08-2454 [DOI] [PubMed] [Google Scholar]
- Brown R. T., Sawyer M. B., Antoniou G., Toogood I., Rice M., Thompson N., Madan-Swain A. (1996). A 3-year follow-up of the intellectual and academic functioning of children receiving central nervous system prophylactic chemotherapy for leukemia. Journal of Developmental and Behavioral Pediatrics, 17, 373–446. doi: 10.1097/00004703-199612000-00004 8960568 [DOI] [PubMed] [Google Scholar]
- Brown R. T., Sawyer M. G., Antoniou G., Toogood I., Rice M. (1999). Longitudinal follow-up of the intellectual and academic functioning of children receiving central nervous system-prophylactic chemotherapy for leukemia: A four-year final report. Journal of Developmental and Behavioral Pediatrics, 20, 73–377. doi: 10.1097/00004703-199910000-00013 10533997 [DOI] [PubMed] [Google Scholar]
- Buizer A. I., De Sonneville L. M., van den Heuvel-Eibrink M. M., Njiokiktjien C., Veerman A. J. (2005). Visuomotor control in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Journal of the International Neuropsychological Society, 11, 554–565. doi: 10.1017/S1355617705050666 [DOI] [PubMed] [Google Scholar]
- Buizer A. I., de Sonneville L. M., van den Heuvel-Eibrink M. M., Veerman A. J. (2006). Behavioral and educational limitations after chemotherapy for childhood acute lymphoblastic leukemia or Wilms tumor. Cancer, 106, 2067–2075. doi: 10.1002/cncr.21820 [DOI] [PubMed] [Google Scholar]
- Bull R., Espy K. A., Wiebe S. A. (2008). Short-term memory, working memory, and executive functioning in preschoolers: Longitudinal predictors of mathematical achievement at age 7 years. Developmental Neuropsychology, 33, 205–208. doi: 10.1080/87565640801982312 18473197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. (1999). The mathematical brain. London: Macmillan. [Google Scholar]
- Butterworth B. (2005). The development of arithmetical abilities. Journal of Child Psychology and Psychiatry, 46, 3–18. doi: 10.1111/j.1469-7610.2004.00374.x 15660640 [DOI] [PubMed] [Google Scholar]
- Cameron C. E., Brock L. L., Murrah W. M., Bell L. H., Worzalla S. L., Grissmer D., Morrison F. J. (2012). Fine motor skills and executive function both contribute to kindergarten achievement. Child Development, 83, 1229–1244. doi: 10.1111/j.1467-8624.2012.01768.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. K., Scaduto M., Sharp W., Dufton L., Van Slyke D., Whitlock J. A., Compas B. (2007). A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatric Blood and Cancer, 49, 65–73. doi: 10.1002/pbc.20860 [DOI] [PubMed] [Google Scholar]
- Casey E. B., Jellife A. M., Le Quesne P. M., Millett Y. L. (1973). Vincristine neuropathy. Clinical and electrophysiological observations. Brain, 96, 69–86. [DOI] [PubMed] [Google Scholar]
- Coles C. D., Kable J. A., Taddeo E. (2009). Math performance and behavior problems in children affected by prenatal alcohol exposure: Intervention and follow-up. Journal of Developmental and Behavioral Pediatrics, 30, 7–15. doi: 10.1097/DBP.0b013e3181966780 [DOI] [PubMed] [Google Scholar]
- Lafayette Instrument Company. (2002). Grooved pegboard test user instructions. Lafayette, IN: Lafayette Instrument Company. [Google Scholar]
- Copeland D. R., Moore B. D., III, Francis D. J., Jaffe N., Culbert S. J. (1996). Neuropsychologic effects of chemotherapy on children with cancer: A longitudinal study. Journal of Clinical Oncology, 14, 2826–2835. [DOI] [PubMed] [Google Scholar]
- Crocker N., Riley E. P., Mattson S. N. (2015). Visual-spatial abilities relate to mathematics achievement in children with heavy prenatal alcohol exposure. Neuropsychology, 29, 108–116. doi: 10.1037/neu0000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C. J., Kelley G. T., Krauss A. (2003). Relationship between visual-motor integration and handwriting skills of children in kindergarten: A modified replication study. American Journal of Occupational Therapy, 57, 459–462. doi: 10.5014/ajot.57.4.459 12911088 [DOI] [PubMed] [Google Scholar]
- Dankert H. L., Davies P. L., Gavin W. J. (2003). Occupational therapy effects on visual-motor skills in preschool children. The American Journal of Occupational Therapy, 57, 542–549. [DOI] [PubMed] [Google Scholar]
- DeAngelo D. J. (2009). Nelarabine for the treatment of patients with relapsed or refractory T-cell acute lymphoblastic leukemia or lymphoblastic lymphoma. Hematology/Oncology Clinics of North America, 23, 1121–1135, vii–viii. doi: 10.1016/j.hoc.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Dehaene S., Spelke E., Pinel P., Stanescu R., Tsivkin S. (1999). Sources of mathematical thinking: Behavioral and brain-imaging evidence. Science, 284, 970–974. doi: 10.1126/science.284.5416.970 10320379 [DOI] [PubMed] [Google Scholar]
- Diamond A. (2000). Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development, 71, 44–56. [DOI] [PubMed] [Google Scholar]
- Espy K. A., Moore I. M., Kaufmann P. M., Kramer J. H., Matthay K., Hutter J. J. (2001). Chemotherapeutic CNS prophylaxis and neuropsychologic change in children with acute lymphoblastic leukemia: A prospective study. Journal of Pediatric Psychology, 26, 1–9. [DOI] [PubMed] [Google Scholar]
- Fayol M., Barrouillet P., Marinthe C. (1998). Predicting arithmetical achievement from neuro-psychological performance: A longitudinal study. Cognition, 68, B63–B70. doi: 10.1016/S0010-0277%2898%2900046-8 9818514 [DOI] [PubMed] [Google Scholar]
- Frank E. G., Foley G. M., Kuchuk A. (1997). Cognitive functioning in school-age children with human immunodeficiency virus. Perceptual and Motor Skills, 85, 267–272. doi: 10.2466/pms.1997.85.1.267 [DOI] [PubMed] [Google Scholar]
- Geary D. C. (1993). Mathematical disabilities: Cognitive, neuropsychological, and genetic components. Psychological Bulletin, 114, 345–362. doi: 10.1037/0033-2909.114.2.345 8416036 [DOI] [PubMed] [Google Scholar]
- Geary D. C., Hoard M. K., Byrd-Craven J., Nugent L., Numtee C. (2007). Cognitive mechanisms underlying achievement deficits in children with mathematical learning disability. Child Development, 78, 1343–1359. doi: 10.1111/j.1467-8624.2007.01069.x 17650142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersten R., Chard D., Jayanthi M., Baker S., Morphy P., Flojo J. (2009). Mathematics instruction for students with learning disabilities: A meta-analysis of instructional components. Review of Educational Research, 79, 1202–1242. [Google Scholar]
- Grissmer D., Grimm K. J., Aiyer S. M., Murrah W. M., Steele J. S. (2010). Fine motor skills and early comprehension of the world: Two new school readiness indicators. Developmental Psychology, 46, 1008–1017. doi: 10.1037/a0020104 [DOI] [PubMed] [Google Scholar]
- Harila-Saari A. H., Huuskonen U. E., Tolonen U., Vainionpaa L. K., Lanning B. M. (2001). Motor nervous pathway function is impaired after treatment of childhood acute lymphoblastic leukemia: A study with motor evoked potentials. Medical and Pediatric Oncology, 36, 345–351. doi: 10.1002/mpo.1084 [DOI] [PubMed] [Google Scholar]
- Harila-Saari A. H., Vainionpaa L. K., Kovala T. T., Tolonen E. U., Lanning B. M. (1998). Nerve lesions after therapy for childhood acute lymphoblastic leukemia. Cancer, 82, 200–207. [DOI] [PubMed] [Google Scholar]
- Harnadek M. C., Rourke B. P. (1994). Principal identifying features of the syndrome of nonverbal learning disabilities in children. Journal of Learning Disabilities, 27, 144–154. [DOI] [PubMed] [Google Scholar]
- Harshman L. A., Barron S., Button A. M., Smith B. J., Link B. K., Lynch C. F., Denburg N. L. (2012). Population-based exploration of academic achievement outcomes in pediatric acute lymphoblastic leukemia survivors. Journal of Pediatric Psychology, 37, 458–466. doi: 10.1093/jpepsy/jsr119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman A., van den Bos C., Stijnen T., Pieters R. (2006). Decrease in motor performance in children with cancer is independent of the cumulative dose of vincristine. Cancer, 106, 1395–1401. doi: 10.1002/cncr.21706 [DOI] [PubMed] [Google Scholar]
- Hassinger-Das B., Jordan N. C., Glutting J., Irwin C., Dyson N. (2014). Domain-general mediators of the relation between kindergarten number sense and first-grade mathematics achievement. Journal of Experimental Child Psychology, 118, 78–92. doi: 10.1016/j.jecp.2013.09.008 24237789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitger M. H., Anderson T. J., Jones R. D., Dalrymple-Alford J. C., Frampton C. M., Ardagh M. W. (2004). Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain, 127(Pt 3), 575–590. doi: 10.1093/brain/awh066 [DOI] [PubMed] [Google Scholar]
- Hockenberry M., Krull K., Moore K., Gregurich M. A., Casey M. E., Kaemingk K. (2007). Longitudinal evaluation of fine motor skills in children with leukemia. Journal of Pediatric Hematology/Oncology, 29, 535–539. doi: 10.1097/MPH.0b013e3180f61b92 [DOI] [PubMed] [Google Scholar]
- Howlader N. A., Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feur EF, Cronin KA. (eds). (2014). SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute. [Google Scholar]
- Iyer N. S., Balsamo L. M., Bracken M. B., Kadan-Lottick N. S. (2015). Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: A review and meta-analysis. Blood, 126, 346–353. doi: 10.1182/blood-2015-02-627414 [DOI] [PubMed] [Google Scholar]
- Jansen N. C., Kingma A., Schuitema A., Bouma A., Veerman A. J., Kamps W. A. (2008). Neuropsychological outcome in chemotherapy-only-treated children with acute lymphoblastic leukemia. Journal of Clinical Oncology, 26, 3025–3030. doi: 10.1200/JCO.2007.12.4149 [DOI] [PubMed] [Google Scholar]
- Kadan-Lottick N. S., Brouwers P., Breiger D., Kaleita T., Dziura J., Liu H., Chen L., Nicoletti M., Stork L., Bostrom B., Neglia J. P. (2009). A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood, 114, 1746–1752. doi: 10.1182/blood-2008-12-186502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadan-Lottick N. S., Brouwers P., Breiger D., Kaleita T., Dziura J., Northrup V., Chen L., Nicoletti M., Bostrom B., Stork L., Neglia J. P. (2009). Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. Journal of Clinical Oncology, 27, 5986–5992. doi: 10.1200/JCO.2009.23.5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadan-Lottick N. S., Ness K. K., Bhatia S., Gurney J. G. (2003). Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA, 290, 2008–2014. doi: 10.1001/jama.290.15.2008 [DOI] [PubMed] [Google Scholar]
- Kaemingk K. L., Carey M. E., Moore I. M., Herzer M., Hutter J. J. (2004). Math weaknesses in survivors of acute lymphoblastic leukemia compared to healthy children. Child Neuropsychology, 10, 14–23. doi: 10.1076/chin.10.1.14.26240 [DOI] [PubMed] [Google Scholar]
- Kingma A., van Dommelen R. I., Mooyaart E. L., Wilmink J. T., Deelman B. G., Kamps W. A. (2001). Slight cognitive impairment and magnetic resonance imaging abnormalities but normal school levels in children treated for acute lymphoblastic leukemia with chemotherapy only. The Journal of Pediatrics, 139, 413–420. doi: 10.1067/mpd.2001.117066 [DOI] [PubMed] [Google Scholar]
- Kudo M. F., Lussier C. M., Swanson H. L. (2015). Reading disabilities in children: A selective meta-analysis of the cognitive literature. Research in Developmental Disabilities, 40C, 51–62. doi: 10.1016/j.ridd.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Kulp T. M. (1999). Relationship between visual motor integration skill and academic performance in kindergarten through third grade. Optometry and Vision Science, 76, 159–163. [DOI] [PubMed] [Google Scholar]
- Kunin-Batson A., Kadan-Lottick N., Neglia J. P. (2014). The contribution of neurocognitive functioning to quality of life after childhood acute lymphoblastic leukemia. Psychooncology, 23, 692–699. doi: 10.1002/pon.3470 [DOI] [PubMed] [Google Scholar]
- Kyttala M., Aunio P., Lehto J. E., Van Luit J., Hautamaki J. (2003). Visuospatial working memory and early numeracy. Educational and Child Psychology, 20, 65–76. [Google Scholar]
- Lehtinen S. S., Huuskonen U. E., Harila-Saari A. H., Tolonen U., Vainionpaa L. K., Lanning B. M. (2002). Motor nervous system impairment persists in long-term survivors of childhood acute lymphoblastic leukemia. Cancer, 94, 2466–2473. doi: 10.1002/cncr.10503 [DOI] [PubMed] [Google Scholar]
- Mahone E. M., Hagelthorn K. M., Cutting L. E., Schuerholz L. J., Pelletier S. F., Rawlins C., Singer H. S., Denckla M. B. (2002). Effects of IQ on executive function measures in children with ADHD. Child Neuropsychology, 8, 52–65. doi: 10.1076/chin.8.1.52.8719 [DOI] [PubMed] [Google Scholar]
- Mitby P. A., Robison L. L., Whitton J. A., Zevon M. A., Gibbs I. C., Tersak J. M., Meadows A. T., Stovall M., Zeltzer L. K., Mertens A. C.; Childhood Cancer Survivor Study Steering Commitee. (2003). Utilization of special education services and educational attainment among long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer, 97, 1115–1126. doi: 10.1002/cncr.11117 [DOI] [PubMed] [Google Scholar]
- Moore B. D. (2005). Neurocognitive outcomes in survivors of childhood cancer. Journal of Pediatric Psychology, 30, 51–63. [DOI] [PubMed] [Google Scholar]
- Moore I. M., Hockenberry M. J., Anhalt C., McCarthy K., Krull K. R. (2012). Mathematics intervention for prevention of neurocognitive deficits in childhood leukemia. Pediatr Blood Cancer, 59, 278–284. doi: 10.1002/pbc.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern R. K., Fairclough D., Ochs J. (1991). A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18-Gy, 24-Gy, or no cranial irradiation. Journal of Clinical Oncology, 9, 1348–1356. [DOI] [PubMed] [Google Scholar]
- Peterson C. C., Johnson C. E., Ramirez L. Y., Huestis S., Pai A. L., Demaree H. A., Drotar D. (2008). A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer, 51, 99–104. doi: 10.1002/pbc.21544 [DOI] [PubMed] [Google Scholar]
- Rourke B. P., Yanni D. W., MacDonald G. W., Young G. C. (1973). Neuropsychological significance of lateralized deficits on the Grooved Pegboard Test for older children with learning disabilities. Journal of Consulting and Clinical Psychology, 41, 128–134. doi: 10.1037/h0035613 4726695 [DOI] [PubMed] [Google Scholar]
- Smith M., Arthur D., Camitta B., Carroll A. J., Crist W., Gaynon P., Gelber R., Heerema N., Korn E. L., Link M., Murphy S., Pui C. H., Pullen J., Reamon G., Sallan S. E., Sather H., Shuster J., Simon R., Trigg M., Tubergen D., Uckun F., Ungerleider R. (1996). Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. Journal of Clinical Oncology, 14, 18–24. [DOI] [PubMed] [Google Scholar]
- Solan H. A., Mozlin R., Rumpf D. A. (1985). The relationship of perceptual-motor development to learning readiness in kindergarten: A multivariate analysis. Journal of Learning Disabilities, 18, 337–344. doi: 10.1177/002221948501800606 4020288 [DOI] [PubMed] [Google Scholar]
- Son S.-H., Meisels S. J. (2006). The relationship of young children's motor skills to later reading and math achievement. Merrill-Palmer Quarterly, 52, 755–778. [Google Scholar]
- Stork L. C., Matloub Y., Broxson E., La M., Yanofsky R., Sather H., Hutchinson R., Heerema N. A., Sorrell A. D., Masterson M., Bleyer A., Gaynon P. S. (2010). Oral 6-mercaptopurine versus oral 6-thioguanine and veno-occlusive disease in children with standard-risk acute lymphoblastic leukemia: Report of the Children's Oncology Group CCG-1952 clinical trial. Blood, 115, 2740–2748. doi: 10.1182/blood-2009-07-230656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. G., Hack M., Klein N., Schatschneider C. (1995). Achievement in children with birth weights less than 750 grams with normal cognitive abilities: Evidence for specific learning disabilities. Journal of Pediatric Psychology, 20, 703–719. [DOI] [PubMed] [Google Scholar]
- Vainionpaa L., Kovala T., Tolonen U., Lanning M. (1995). Vincristine therapy for children with acute lymphoblastic leukemia impairs conduction in the entire peripheral nerve. Pediatric Neurology, 13, 314–318. [DOI] [PubMed] [Google Scholar]
- Vainionpaa L., Kovala T., Tolonen U., Lanning M. (1997). Chemotherapy for acute lymphoblastic leukemia may cause subtle changes of the spinal cord detectable by somatosensory evoked potentials. Medical and Pediatric Oncology, 28, 41–47. [DOI] [PubMed] [Google Scholar]
- Waber D. P., Tarbell N. J., Kahn C. M., Gelber R. D., Sallan S. E. (1992). The relationship of sex and treatment modality to neuropsychologic outcome in childhood acute lymphoblastic leukemia. Journal of Clinical Oncology, 10, 810–817. [DOI] [PubMed] [Google Scholar]
- Walsh K. S., Paltin I., Gioia G. A., Isquith P., Kadan-Lottick N. S., Neglia J. P., Brouwers P. (2015). Everyday executive function in standard-risk acute lymphoblastic leukemia survivors. Child Neuropsychology, 21, 78–89. doi: 10.1080/09297049.2013.876491 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (2003). Weschler intelligence scale for children (4th ed.). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D. (2005). Wechsler Individual Achievement Test 2nd Edition (WIAT II). London: The Psychological Corporation. [Google Scholar]
- Worling D. E., Humphries T., Tannock R. (1999). Spatial and emotional aspects of language inferencing in nonverbal learning disabilities. Brain and Language, 70, 220–239. doi: 10.1006/brln.1999.2156 [DOI] [PubMed] [Google Scholar]
- Zago L., Pesenti M., Mellet E., Crivello F., Mazoyer B., Tzourio-Mazoyer N. (2001). Neural correlates of simple and complex mental calculation. Neuroimage, 13, 314–327. doi: 10.1006/nimg.2000.0697 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.