Summary
Background
Antiretroviral therapy (ART) and retention in care are essential for the prevention of mother-to-child HIV transmission (PMTCT). We aimed to assess the effect of a family-focused, integrated PMTCT care package.
Methods
In this parallel, cluster-randomised controlled trial, we pair-matched 12 primary and secondary level health-care facilities located in rural north-central Nigeria. Clinic pairs were randomly assigned to intervention or standard of care (control) by computer-generated sequence. HIV-infected women (and their infants) presenting for antenatal care or delivery were included if they had unknown HIV status at presentation (there was no age limit for the study, but the youngest participant was 16 years old); history of antiretroviral prophylaxis or treatment, but not receiving these at presentation; or known HIV status but had never received treatment. Standard of care included health information, opt-out HIV testing, infant feeding counselling, referral for CD4 cell counts and treatment, home-based services, antiretroviral prophylaxis, and early infant diagnosis. The intervention package added task shifting, point-of-care CD4 testing, integrated mother and infant service provision, and male partner and community engagement. The primary outcomes were the proportion of eligible women who initiated ART and the proportion of women and their infants retained in care at 6 weeks and 12 weeks post partum (assessed by generalised linear mixed effects model with random effects for matched clinic pairs). The trial is registered with ClinicalTrials.gov, number NCT01805752.
Findings
Between April 1, 2013, and March 31, 2014, we enrolled 369 eligible women (172 intervention, 197 control), similar across groups for marital status, duration of HIV diagnosis, and distance to facility. Median CD4 count was 424 cells per μL (IQR 268–606) in the intervention group and 314 cells per μL (245–406) in the control group (p<0·0001). Of the 369 women included in the study, 363 (98%) had WHO clinical stage 1 disease, 364 (99%) had high functional status, and 353 (96%) delivered vaginally. Mothers in the intervention group were more likely to initiate ART (166 [97%] vs 77 [39%]; adjusted relative risk 3·3, 95% CI 1·4–7·8). Mother and infant pairs in the intervention group were more likely to be retained in care at 6 weeks (125 [83%] of 150 vs 15 [9%] of 170; adjusted relative risk 9·1, 5·2–15·9) and 12 weeks (112 [75%] of 150 vs 11 [7%] of 168 pairs; 10·3, 5·4–19·7) post partum.
Interpretation
This integrated, family-focused PMTCT service package improved maternal ART initiation and mother and infant retention in care. An effective approach to improve the quality of PMTCT service delivery will positively affect global goals for the elimination of mother-to-child HIV transmission.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development and US National Institutes of Health.
Introduction
Activities that result in successful prevention of mother-to-child HIV transmission (PMTCT) have transformed the delivery of HIV services for infants and mothers. The most crucial intervention along the PMTCT continuum of care is antiretroviral therapy (ART). If given promptly, consistently, and correctly, ART is highly effective in decreasing vertical HIV transmission.1 However, gaps along the care continuum continue to constrain the ability of PMTCT programmes to stem the tide of perinatal HIV infections, especially in resource-limited, rural settings.2 Specifically, early ART initiation and retention in care, two crucial elements for the prevention of mother-to-infant HIV transmission and improvement of survival of the mother and infant pair,3 are increasingly problematic for many PMTCT programmes in sub-Saharan Africa.4
Nigeria is a major contributor to the global gap in elimination of mother-to-child HIV transmission, accounting for the largest proportion of new HIV infections in children worldwide.5,6 Among the many barriers to effective delivery of PMTCT services in Nigeria are the shortage of trained, skilled health-care providers, especially in rural areas;7 delays in care associated with a dearth of reliable and affordable laboratory infrastructure;8 customs that limit a woman’s autonomy to make independent health-care decisions, including the absence of men participating in PMTCT services;9,10 and poorly integrated maternal and child health and HIV services.11 To address these impediments to effective PMTCT care and the elimination of mother-to-child HIV transmission, we used a systematic, multipronged approach. We present findings from an innovative trial in rural Nigeria that includes task shifting, point-of-care testing, integration of services for HIV-infected women and their exposed infants, and involvement of male partners and peer mentors as a package of services for PMTCT programmes in resource-limited settings.
Methods
Study design and participants
The design of this parallel, cluster-randomised controlled trial has been described previously.12 Briefly, the study took place at 12 sites located in the rural Niger state of north-central Nigeria, one of two states with clinical sites supported by Friends in Global Health, Vanderbilt University’s implementation partner for the US President’s Plan for AIDS Relief (PEPFAR). The intervention facilities included a comprehensive health centre in Agwara, rural hospitals in Kaffin Koro, Agaie, and Auna, a National Council of Women’s Societies clinic in Farin Doki, and a basic health clinic in Wuse. Matched standard-of-care (control) sites included maternal and child health clinics in Paiko and Chanchaga; basic health clinics in Ijah, Garam, and Izom; and a primary health clinic in Gauraka.
We included HIV-infected women (age was not included in the elegibility criteria, but the youngest participant was 16 years old) and their infants, presenting for antenatal care or delivery who met one of the following inclusion criteria: unknown HIV status at time of presentation; history of antiretroviral prophylaxis or treatment, but not receiving prophylaxis or treatment at the time of presentation; or known HIV status but had never received treatment. HIV-infected women who were on antiretroviral prophylaxis or treatment at the time of presentation to antenatal care were excluded from the study.
Ethical approval was obtained from the Nigeria National Human Research Ethics Committee and the Institutional Review Board of Vanderbilt University, USA. Consent was given orally.
Randomisation and masking
Sites were matched with each other and then randomly assigned by computer-generated sequence within matched pairs to deliver integrated PMTCT (n=6) or the standard of care (n=6). Matching was based on monthly antenatal clinic volume, number of HIV-infected patients seen during the past 6 months, urbanisation of the area, and site accessibility. For the matching procedure, a distance matrix was generated by the study biostatistician (MB) that described the pairwise similarity between all sites based on the characteristics mentioned and weighting these charact eristics based on their perceived matching importance (the weight we assigned to each variable used in the matching, generated by a distance matrix). Optimum non-bipartite matching was used to minimise the average reweighted Mahalanobis distance between pairs.13 The web application used to do non-bipartite matching is available online. Enrolment was done by clinic staff, with active oversight by study research staff, and were involved in the rest of the trial. There was no masking of participants, staff, or investigators.
Procedures
The procedures used across intervention and control groups have been described previously.12 Briefly, for the control group, we referred HIV-positive women to nearby secondary level, comprehensive clinics supported by Friends in Global Health for clinical and laboratory assessment and ART initiation. Pregnant women with advanced WHO clinical stage disease (WHO stage 3 or 4) or advanced immunosuppression (CD4 count <350 cells per μL) were initiated on ART for their own health, or placed on ART as prophylaxis if they did not qualify for ART for their own health (option B). Breastfeeding mothers were continued on ART until 1 week after breastfeeding ceased and stayed under the care of the referral centre. HIV-exposed infants were started immediately after birth on once daily nevirapine 10 mg in babies weighing less than 2·5 kg or once daily nevirapine 15 mg in babies weighing 2·5 kg or more for 6 weeks, as per Nigerian guidelines.14 Mothers were asked to bring their infants back to the clinic at 6 weeks for follow-up, continuation of nevirapine (if mother was not compliant with ART and was breastfeeding), immunisations, initiation of co-trimoxazole prophylaxis, and early infant diagnosis testing.
The intervention sites received an integrated package of PMTCT services in maternal and child health settings that included point-of-care CD4 cell count or percentage testing, transition of decentralised PMTCT tasks to trained midwives (task shifting), integrated mother and infant care services, active influential family member (male partner) participation, and community involvement.
For same-day point-of-care CD4 cell count or percentage testing the CyFlow miniPOC system (PartecGmbH, Görlitz, Germany) was installed in each intervention site. This system is a user-friendly instrument well suited for resource-limited settings. Clinic staff were trained in sample collection and internal quality control.
A three-pronged strategy (training, on-site mentoring, and continuous quality assurance) was used for the task-shifting intervention. A minimum of three staff members (two nurses or midwives, one community health worker, and one pharmacist or pharmacy technician was included when available) from each intervention site had 5 day training with adopted curriculums.15 A medical officer provided regular on-site mentoring and consultation for complex cases. Our task-shifting intervention was not substantially staff-intensive because no new staff were hired (all participating staff were already working in the sites, but the scope of their responsibilities was expanded), and training was done for all participating staff at the same time.
We integrated mother and infant services in the postpartum period by comanaging mother and infant pairs in the maternal and child health clinic and scheduling mother and infant visits to occur on the same visit date to reduce visit burden. Infants who tested positive for HIV were initiated on ART at the maternal and child health clinic and monitored throughout the study.
Men were encouraged to take part in their wives’ antenatal care via personalised invitation letters handed out to all women who tested positive for HIV and presented for antenatal care without their partner. During the antenatal visit, clinicians educated male partners on the importance of PMTCT, tested the partners, and encouraged them to support their family. Man-friendly health settings were created in intervention facilities by training health-care staff and amending standard operational procedures to include men in all prenatal and postnatal appointments. Health facilities were altered to allow privacy for couple counselling and testing. A male counsellor was made available to see men who preferred to receive counselling from a man.
The community component included recruitment and training (by six peer mentors per intervention clinic) of spouses of HIV-positive women enrolled in the study as community mobilisation champions so that they could educate others and share their own experience. The champion role included organising outreach activities to raise awareness of involvement of men in PMTCT; educating and encouraging men to accompany their spouses to PMTCT clinics; educating men about safe sex practices; distributing condoms; linking men to key referral services such as couples counselling and testing, services for sexually transmitted infections, and family planning; and soliciting the support of community leaders and influential family members through one-on-one interactions and community forums. Peer male champions were paid a small monthly stipend (NGN2000, equivalent to US$10) to support their transpor costs.
Participants in both intervention and control sites received group health education, opt-out HIV testing, same-day HIV test results, counselling on infant feeding, home-based care services, infant prophylaxis, early infant diagnosis, and linkage to family spacing services, if desired. No new interventions were started during the study.
We tracked demographic, PMTCT, and HIV care and treatment data that were routinely collected by our PMTCT programme and documented in the WHO HIV care/ART card or management of patients and monitoring forms for all women included in the study. Specific clinical and laboratory data from these forms were entered by research assistants into CAREWare (JPROG, New Orleans, LA, USA), an electronic medical record system adapted at supported sites and REDCap, a secure, web-based electronic data capture instrument hosted at Vanderbilt University.16
Outcomes
The primary outcomes were to compare between the intervention and control groups both the proportion of eligible women who initiated antiretroviral medications for PMTCT and the proportion of HIV-infected women and their infants retained in care at 6 weeks and 12 weeks post partum. We also compared HIV incidence in infants at 6 weeks and 12 weeks across control and intervention groups; however, this was a secondary outcome because the study was not powered to measure this aim.
ART initiation was defined as having occurred if there was a record that ART was prescribed in patients’ folders and the first medication pick-up was completed during pregnancy or up to 1 day post partum. Mother and infant retention at 6 weeks was defined as mother initiating ART, mother having a follow-up visit 4–8 weeks after delivery, and child having blood drawn for HIV DNA PCR testing 4–14 weeks after birth. Mother and infant retention at 12 weeks was similarly defined, the only difference being that the follow-up visit window was 10–14 weeks after delivery.
Only DNA PCR results at or after 4 weeks post partum were used to establish HIV incidence. The time of infection was set as the midpoint between the last HIV-negative and the first HIV-positive result, or the midpoint between birth and the first HIV-positive result, if the infant did not have a previous HIV-negative test. Date of death was recorded according to clinic records and maternal or family member report. Event time for stillborn infants was set immediately post partum (1 day).
Statistical analysis
With 12 clinics recruiting roughly 25 eligible patients each (n=300), we projected 90% power to detect a difference between 40% for six control clinics and 65% for six matched intervention clinics (coefficient of variation equal to 0·12 and probability of type I error equal to 0·05).12 An intention-to-treat approach was used for all analyses. To compare the proportion of eligible women who initiated antiretroviral treatments for PMTCT between the intervention and control groups, we used a generalised linear mixed effects model with a log link function, study site and matched pairs were included as nested random effects, and a fixed effect was used for randomisation to the intervention group. Covariates included in the mixed effects model that were specified a priori as being important to ART initiation and retention were mother’s age, years of education, travel time to the facility, employment status, ethnicity, and time of HIV diagnosis (before or during antenatal care vs delivery). Similar models were used to establish the effect of the intervention on retention of mother and infant pairs on ART at 6 weeks and 12 weeks. For the primary retention analyses, mother and infant pairs were excluded if either died, transferred to another site, or relocated within 14 weeks of birth. The adjusted models were also estimated with post-hoc adjustment variables to assess the robustness of our conclusions to patient-level differences in CD4 cell count and gestational age across groups. These post-hoc models needed multiple imputation to account for substantial missing CD4 cell count records.
The cumulative incidence of HIV infection for infants during their first 12 weeks was computed treating death as a competing risk. Infants were censored at their last clinic visit or at 12 weeks post partum. We used non-parametric methods to estimate the cumulative incidence function. Confidence intervals were calculated with the delta method, and Gray’s method was used to test for differences across study group.17 The probability of death and the probability of the composite endpoint of HIV infection or death for infants were computed with Kaplan-Meier techniques, censoring infants at the date of last clinic visit or at 12 weeks post partum. Separate Cox regression models were used to assess the effect of randomisation to intervention on infant HIV infection, death, and the composite endpoint of HIV infection or death. Models were adjusted for the same covariates listed previously (a priori and post hoc) and stratified by site; robust covariance estimation was used to account for clustering of twins (n=22) within mothers. Sensitivity analyses were done to investigate the effect of poor retention on the combined endpoint of HIV infection or death; infants of mothers who did not receive ART treatment were assigned a probability of infection equal to 0·035 for power calculations (or 0·07, which was closer to the observed cumulative incidence of HIV infection in control-group participants) and infants of mothers who were not retained in care at 6 weeks were assigned a probability of infection equal to 0·017 (or 0·035). HIV status for eligible infants was randomly imputed with the Bernoulli distribution, the interval time was assigned as 84 days, and Cox regression models were done. This step was repeated ten times and estimates from the multiple regressions were combined with multiple imputation techniques. R-software (version 3.0.2) was used for data analyses).
The trial is registered with ClinicalTrials.gov, number NCT01805752.
Roles of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the manuscript for publication. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
Within the 12 rural sites randomly assigned to deliver either the intervention package or the standard of care, 372 HIV-infected women gave consent and were enrolled into the study between April 1, 2013, and March 31, 2014 between 25 and 30 women were screened at each of the 12 sites, 172 (46%) of whom were eligible in the intervention group and 197 (53%) in the control group (figure 1). Most participants were married, homemakers, and enrolled in Sabon Wuse hospital (intervention) or Garam Hospital (control; table 1). Almost all enrolled women were newly diagnosed as HIV-infected while attending antenatal care. At enrolment, gestational age was later for participants in the intervention group than for those in the control group. Participants in the intervention group were slightly younger than those in the control group. More than a quarter of all participants had no formal education; this proportion was higher in the intervention than in the control group. Participants were similar across groups for time of HIV diagnosis, distance to facility, and marital status. Participants did not differ across groups by bodyweight, functional status, WHO clinical stage, and mode of delivery. Most participants had WHO clinical stage 1 disease and a high functional status (working or ambulatory). Median CD4 cell count at enrolment was higher for mothers in the intervention group than for those in the control group. Most births occurred at term (37–42 weeks; table 2) at home and by vaginal delivery. Four mothers died during the study; however, maternal deaths were not associated with HIV-related illness (table 1).
Figure 1. Trial profile.
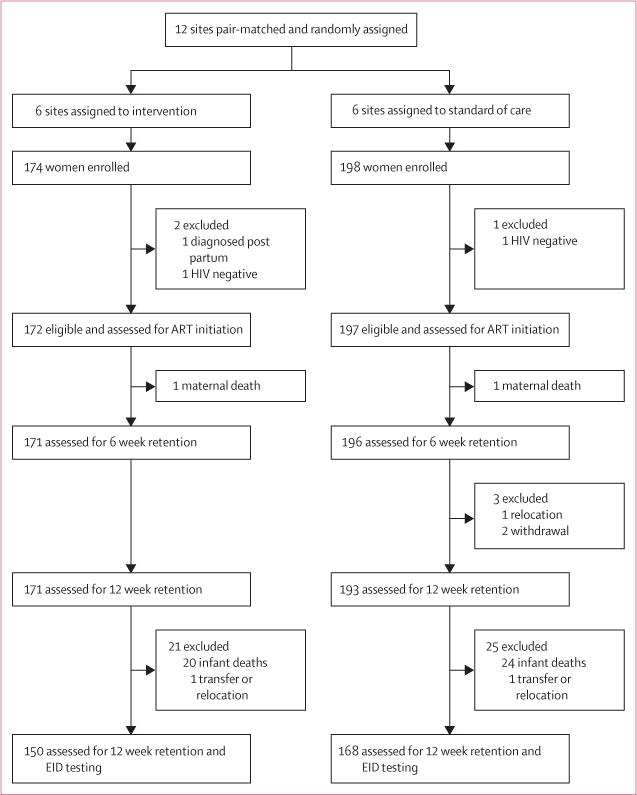
EID=early infant diagnosis.
Table 1.
Baseline characteristics
| Intervention (n=172) | Control (n=197) | Combined (n=369) | |
|---|---|---|---|
| Facility | |||
| Agwara | 30 (17%) | ·· | 30 (8%) |
| Kafin-Koro | 25 (15%) | ·· | 25 (7%) |
| Agaie | 32 (19%) | ·· | 32 (9%) |
| Farin-Doki | 25 (15%) | ·· | 25 (7%) |
| Auna | 11 (6%) | ·· | 11 (3%) |
| Sabon-Wuse | 49 (28%) | ·· | 49 (13%) |
| Chanchaga | ·· | 44 (22%) | 44 (12%) |
| Garam | ·· | 49 (25%) | 49 (13%) |
| Gauraka | ·· | 21 (11%) | 21 (6%) |
| Ijah | ·· | 36 (18%) | 36 (10%) |
| Izom | ·· | 23 (12%) | 23 (6%) |
| Paiko | ·· | 24 (12%) | 24 (7%) |
|
| |||
| Distance to facility (km) | 10 (3–17) | 7 (7–17) | 10 (5–17) |
|
| |||
| Age (years) | 26 (23–30) | 28 (25–31) | 28 (24–30) |
|
| |||
| Parity | 2 (1–4) | 1 (0–2) | 2 (1–3) |
|
| |||
| Gestational age at enrolment during antenatal care (weeks) | 24 (16–29) | 20 (12–24) | 20 (14–28) |
|
| |||
| Marital status | |||
| Missing | 0 | 2 (1%) | 2 (1%) |
| Single | 1 (1%) | 3 (2%) | 4 (1%) |
| Married | 170 (99%) | 187 (96%) | 357 (97%) |
| Separated or divorced | 1 (1%) | 2 (1%) | 3 (1%) |
| Widowed | 0 | 3 (2%) | 3 (1%) |
|
| |||
| Schooling | |||
| Started primary school | 13 (8%) | 18 (9%) | 31 (8%) |
| Completed primary school | 17 (10%) | 70 (36%) | 87 (24%) |
| Secondary school | 35 (20%) | 52 (26%) | 87 (24%) |
| Post-secondary school | 5 (3%) | 7 (4%) | 12 (3%) |
| Qur’anic* | 31 (18%) | 17 (9%) | 48 (13%) |
| None | 71 (41%) | 32 (16%) | 103 (28%) |
| Other | 0 | 1 (1%) | 1 (<1%) |
|
| |||
| Employment | |||
| Employed | 5 (3%) | 11 (6%) | 16 (4%) |
| Unemployed | 40 (23%) | 80 (41%) | 120 (33%) |
| Student | 1 (1%) | 3 (2%) | 4 (1%) |
| Housewife | 117 (68%) | 83 (42%) | 200 (54%) |
| Other | 9 (5%) | 20 (10%) | 29 (8%) |
|
| |||
| Time of HIV diagnosis | |||
| Before antenatal care | 0 | 1 (1%) | 1 (<1%) |
| During antenatal care | 165 (96%) | 194 (98%) | 359 (97%) |
| Delivery | 7 (4%) | 2 (1%) | 9 (2%) |
|
| |||
| Height (m) | 1·57 (1·50–1·62) | 1·54 (1·50–1·60) | 1·55 (1·50–1·61) |
|
| |||
| Weight (kg) | 58 (52–64) | 60 (53–65) | 58 (52–65) |
|
| |||
| Functional status | |||
| Working | 172 (100%) | 192 (97%) | 364 (99%) |
| Ambulatory | 0 | 1 (1%) | 1 (<1%) |
| Bedridden | 0 | 4 (2%) | 4 (1%) |
|
| |||
| WHO clinical stage of disease | |||
| 1 | 167 (97%) | 196 (99%) | 363 (98%) |
| 2 | 4 (2%) | 1 (1%) | 5 (1%) |
| 3 | 1 (1%) | 0 | 1 (<1%) |
|
| |||
| CD4 cell count (cells per μL) | 424 (268–606) | 314 (245–406) | 356 (256–494) |
| Missing | 5 (3%) | 62 (31%) | 67 (18%) |
|
| |||
| Type of delivery | |||
| Vaginal | 166 (97%) | 187 (95%) | 353 (96%) |
| Elective caesarean section | 3 (2%) | 7 (4%) | 10 (3%) |
| Emergency caesarean section | 2 (1%) | 3 (2%) | 5 (1%) |
| Other | 1 (1%) | 0 | 1 (<1%) |
|
| |||
| Location of delivery | |||
| Health facility | 60 (35%) | 47 (24%) | 107 (29%) |
| Home with traditional birth attendant | 46 (27%) | 77 (39%) | 123 (33%) |
| Home with no traditional birth attendant | 66 (38%) | 73 (37%) | 139 (38%) |
|
| |||
| Number of babies | |||
| Single | 167 (97%) | 191 (97%) | 358 (97%) |
| Twins | 5 (3%) | 6 (3%) | 11 (3%) |
|
| |||
| Maternal death | 1 (1%) | 3 (2%) | 4 (1%) |
|
| |||
| Cause of maternal death | |||
| Non-HIV-associated illness | 1 (100%) | 0 | 1 (25%) |
| Other | 0 | 1 (33%) | 1 (25%) |
| Unknown | 0 | 2 (67%) | 2 (50%) |
|
| |||
| Patient accompanied by partner at least once | 130 (76%) | 65 (33%) | 195 (53%) |
|
| |||
| Partner HIV testing status | |||
| Missing | 1 (1%) | 22 (11%) | 23 (6%) |
| Self-report | 33 (19%) | 26 (15%) | 59 (17%) |
| Tested during antenatal care† | 70 (41%) | 24 (14%) | 94 (27%) |
| Source of HIV status not documented | 32 (19%) | 5 (3%) | 37 (11%) |
| No testing or self-report | 36 (21%) | 120 (69%) | 156 (45%) |
|
| |||
| HIV status of partner if tested during antenatal care | |||
| Positive | 29 (41%) | 15 (62%) | 44 (47%) |
| Negative | 41 (59%) | 9 (38%) | 50 (53%) |
Data are n (%) or median (IQR). We used χ² tests to compare the distribution of study characteristics for participants by group and a Wilcoxon rank sum test for continuous variables by group: distance to facility (p=0·86), age (p=0·008), parity, gestational age, schooling, employment, CD4 cell count, patient being accompanied by the partner at least once, and partner HIV testing status (p<0·0001 for all), marital status (p=0·29), time of HIV diagnosis and functional status (p=0·11), height (p=0·003), weight (p=0·10), disease stage (p=0·18), delivery type (p=0·50) and location (p=0·018), number of babies (p=0·99), and partner HIV status if tested during antenatal care (p=0·12). These tests ignore the effects of clustering in matched clinics.
System of Islamic elementary education that is focused on learning to read and write in Arabic text.
Corresponds to study period.
Table 2.
Infant characteristics and outcomes
| Intervention (n=177) | Control (n=203) | Combined (n=380) | |
|---|---|---|---|
| Girls | 84 (47%) | 104 (51%) | 188 (49%) |
|
| |||
| Gestational age at delivery (weeks) | 38 (36–39) | 38 (38–40) | 38 (36–39) |
|
| |||
| Birthweight (kg) | 3·0 (2·6–3·2) | 2·8 (2·5–3·1) | 2·9 (2·5–3·1) |
| Missing | 14 (8%) | 19 (9%) | 33 (9%) |
|
| |||
| Birth length (m) | 0·49 (0·47–0·51) | 0·49 (0·48–0·50) | 0·49 (0·48–0·50) |
| Missing | 14 (8%) | 28 (14%) | 42 (11%) |
|
| |||
| Infant antiretroviral regimen at birth | |||
| Daily nevirapine | 159 (90%) | 151 (74%) | 310 (82%) |
| None | 18 (10%) | 52 (26%) | 70 (18%) |
|
| |||
| Breastfeeding status | |||
| Missing | 35 (20%) | 72 (35%) | 107 (28%) |
| Ever breastfed* | 140 (99%) | 131 (100%) | 271 (99%) |
| Never breastfed* | 2 (1%) | 0 | 2 (1%) |
|
| |||
| Infant death | 28 (16%) | 42 (21%) | 70 (18%) |
|
| |||
| Cause of infant death† | |||
| Missing | 0 | 1 (2%) | 1 (1%) |
| Stillbirth or miscarriage | 11 (39%) | 18 (44%) | 29 (41%) |
| Birth complication | 1 (4%) | 4 (10%) | 5 (7%) |
| HIV-associated illness (eg, antiretroviral drug side-effect or opportunistic infection) | 1 (4%) | 1 (2%) | 2 (3%) |
| Non-HIV-associated illness | 0 | 1 (2%) | 1 (1%) |
| Accidental | 1 (4%) | 1 (2%) | 2 (3%) |
| Unknown | 13 (46%) | 15 (37%) | 28 (41%) |
| Other | 1 (4%) | 1 (2%) | 2 (3%) |
|
| |||
| Cumulative infant HIV incidence (%)‡ | |||
| 6 weeks | 1·8% (0·6–5·4) | 6·0% (2·5–14·0) | 3·2% (1·6–6·2) |
| 12 weeks | 2·4% (0·9–6·3) | 7·3% (3·3–15·6) | 4·0% (2·2–7·3) |
|
| |||
| Infant mortality (%)‡ | |||
| 6 weeks | 13·7% (8·5–18·7) | 19·6% (12·1–26·4) | 15·9% (11·7–19·9) |
| 12 weeks | 15·0% (9·5–20·1) | 25·1% (16·2–33·1) | 18·5% (13·9–22·8) |
Data are n (%), median (IQR), or % (95% CI).
Denominator for percentages is number of infants for whom data were available.
Denominator for percentages is number of deceased infants.
All infants are included in this analysis. Infants are censored at loss to follow-up (off-study date).
The proportion of male partners who accompanied (at least once) their partners over the course of the study was greater in the intervention than in the control group. Similarly, the proportion of male partners who were tested for HIV at intervention site antenatal clinics was greater than those who tested at control sites. Of the male partners tested during antenatal care, a smaller proportion of those were HIV-positive when tested at the intervention sites than were those tested at the control sites. However, HIV status was not available as a result of no HIV testing during antenatal care or self-report for 45% of male partners (table 1).
166 (97%) women initiated ART in the intervention group compared with only 77 (39%) in the control group. Among the women who initiated ART, the median duration of treatment before delivery was 10 weeks (IQR 3–19) for those in the intervention group and 21 weeks (10–32) for those in the control group. After adjustment for maternal age, education, travel time to facility, employment, maternal ethnicity, and time of HIV diagnosis, mothers in the intervention group were more likely to initiate ART than mothers in the control group (adjusted relative risk [RR] 3·3, 95% CI 1·4–7·8; table 3).
Table 3.
Mixed effects Poisson regression for treatment initiation and retention
| Unadjusted analysis | Adjusted analysis | Post-hoc analysis adjusted | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | p value | RR (95% CI) | p value | RR (95% CI) | p value | |
| ART initiation | 3·4 (1·4–8·2) | 0·006 | 3·3 (1·4–7·8) | 0·005 | 3·4 (1·4–8·2) | 0·005 |
| Mother retention at 6 weeks | 4·3 (1·6–11·3) | 0·004 | 4·1 (1·6–10·7) | 0·004 | 4·2 (1·6–11·2) | 0·004 |
| Mother and infant retention at 6 weeks | 9·4 (5·5–16·1) | <0·0001 | 9·1 (5·2–15·9) | <0·0001 | 9·3 (5·2–16·4) | <0·0001 |
| Mother retention at 12 weeks | 4·6 (1·9–11·5) | 0·001 | 4·3 (1·7–10·6) | 0·002 | 4·4 (1·8–11·0) | 0·001 |
| Mother and infant retention at 12 weeks | 11·4 (6·1–21·2) | <0·0001 | 10·3 (5·4–19·7) | <0·0001 | 10·8 (5·6–20·8) | <0·0001 |
Control group as reference. Study site and matched pair are included as nested random effects. Adjustment variables include maternal age, years of education, travel time to facility, employment status, ethnicity of mother, and time of HIV diagnosis (before antenatal care or during antenatal care vs delivery). Post-hoc adjustment includes the aforementioned plus gestational age and CD4 cell count with multiple imputation (because CD4 cell count had substantial missing data). The scale parameter for all Poisson mixed models was between 0·50 and 0·65, suggesting an absence of model overdispersal. If a mother gave birth to two children then testing status was confirmed to be the same for both infants and the mother and infant status was only counted once. RR=relative risk.
380 neonates were born to the 369 study participants, and no significant differences were noted in birthweight and birth length between study groups (table 2). Almost all infants for whom breastfeeding data were available were ever breastfed (table 2). Fewer babies born to mothers in the intervention group did not receive any nevirapine in the post-partum period compared with babies born to women in the control group (table 2). Only nine (5%) infants born to mothers in the intervention group were lost to follow-up before study termination compared with 101 (50%) of infants born to mothers in the control group. 70 infants (including fetal death) died across both groups during the study (table 2), and the cause of fetal or infant death was unknown in 28 (41%) cases.
The proportion of mother and infant pairs retained in the intervention group at 6 weeks post partum was 0·83 (125 of 150 pairs) and at 12 weeks post partum was 0·75 (112 of 150 pairs). By contrast, the proportion of mother and infant pairs retained in the control group at 6 weeks post partum was 0·09 (15 of 170 pairs) and at 12 weeks was 0·07 (11 of 168 pairs). After adjustment for maternal age, education, travel time, employment, ethnicity, and time of HIV diagnosis, mother and infant pairs in the intervention group were roughly ten times more likely to be retained in care at 6 weeks (adjusted RR 9·1, 95% CI 5·2–15·9) and 12 weeks (10·3, 5·4–19·7) post partum than were pairs in the control group (table 3).
Three infants in the intervention group and five infants in the control group were infected with HIV at 6 weeks, and four infants in the intervention group and six infants in the control group were infected at 12 weeks. At the 12 week visit, the cumulative incidence of HIV infection in retained infants in the intervention group was 2·4% versus 7·3% in the control group (table 2, figure 2). After adjustment for maternal age, education, travel time to facility, employment, maternal ethnicity, and time of HIV diagnosis, infants born to mothers in the intervention group were 74% less likely to acquire HIV infection than those in the control group at 12 weeks post partum (adjusted hazard ratio [aHR] 0·26, 95% CI 0·08–0·85). If maternal CD4 cell count and gestational age are included as post-hoc adjustment variables, then the effect of intervention on HIV infection is inconclusive (aHR 0·36, 0·09–1·46; p=0·15). Similarly, in sensitivity analyses in which infants who were lost to follow-up were assumed to have been infected at rates specified in the Methods section, the effect of intervention on HIV infection became inconclusive (appendix). At 12 weeks post partum, infants born to mothers in the intervention group were also 18% less likely to have died than were those born to mothers in the control group, but this difference was not significant (aHR 0·82, 0·47–1·43; p=0·49; figure 2). Similar results were noted for the composite outcome of infant HIV infection or death at 12 weeks post partum (0·72, 0·42–1·22; p=0·22).
Figure 2.
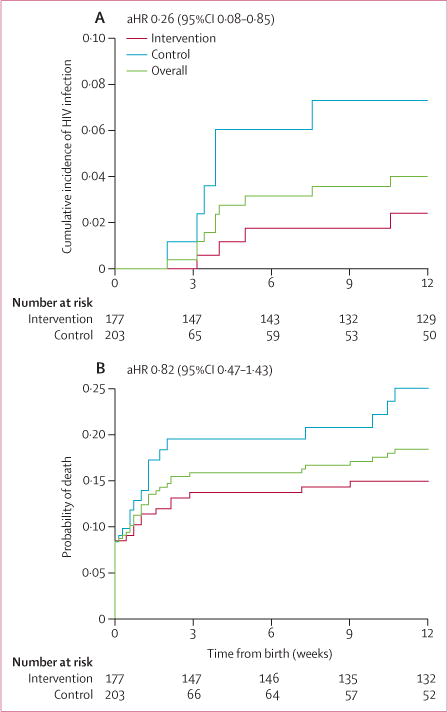
Cumulative incidence of (A) infant HIV infection and (B) infant mortality during the first 12 weeks post partum by trial group
Discussion
This parallel, cluster-randomised trial from rural Nigeria showed a large effect of a creative package of PMTCT services on three important outcomes: maternal ART initiation, early maternal and infant retention in care, and HIV infection in infants at 12 weeks. Mothers in the intervention group were three times as likely to initiate ART as women in the control group, and mother and infant pairs had a roughly ten times higher rate of retention at 6 weeks and 12 weeks post partum than did pairs in the control group. More importantly, our intervention significantly reduced incident HIV infection in infants, with those in the intervention group being 74% less likely to be diagnosed with HIV at 12 weeks than those in the control group. Reduced HIV infection was not a primary outcome in our study because we did not expect such a large difference between the two groups and expected to be underpowered for this definitive endpoint.
The potential for our intervention to have a major effect on public health is better appreciated within the context of Nigeria’s sizeable burden of vertical HIV transmission and continued reports of suboptimum PMTCT outcomes. In 2010, only 8% of eligible HIV-infected women were offered antiretroviral drugs to prevent vertical HIV transmission.18,19 With a national HIV prevalence rate of 4·1% in antenatal attendees (>200 000 HIV-infected pregnant women),20,21 Nigeria alone contributed 29% of the global gap in reaching 90% of pregnant women in need of PMTCT services.19,21 Services for HIV-exposed or HIV-infected children have been even more dismal. In 2010, only 6% of HIV-exposed infants in Nigeria received any form of HIV prophylaxis or treatment, falling far short of the 90% national target.19 Interventions that effectively stem the tide of vertical HIV transmission in Nigeria will probably have a substantial effect on global goals for the elimination of mother-to-child HIV transmission.
Individual components of the package of services provided in this study (point-of-care CD4 testing, task shifting, male involvement, and integration of mother and child services) have previously been effective in increasing uptake of HIV services and improving antiretroviral treatment outcomes across different locations.22–26 Our study shows that putting these components into use as a package of services in rural settings is feasible. Our findings also suggest that lower-cadre health workers can be effectively used to bridge the gap in human resource capacity and provide HIV care and treatment in PMTCT programmes in analogous environments, an important finding given that most service providers in Nigeria are concentrated in urban health centres.7 Although our report does not allow us to tease out which components of the intervention had the largest effect, our findings suggest that in combination they can greatly improve the PMTCT cascade.
Early initiation of ART has proven benefits, including improved maternal and infant survival, accelerated immune recovery, and reduced transmission of HIV.27,28 The advent and gradual scale-up of option B+ (lifelong combination ART for all HIV-infected pregnant and breastfeeding women, irrespective of clinical stage or CD4 cell count) across Africa, although not yet adopted by Nigeria, is an opportunity to explore new strategies for improving uptake of ART and long-term retention of mother and infant pairs. Although option B+ will increase the pool of patients eligible for treatment, it will also make CD4 cell count testing redundant for decision making about initiation of ART by pregnant women. However, CD4 cell count testing will probably continue to be useful as a valuable method for monitoring response to treatment, especially in resource-limited settings. Our findings suggest that other user-friendly point-of-care diagnostics (eg, viral load testing) that improve the efficiency of referrals can help to improve treatment initiation and retention in PMTCT programmes.
Cultural norms in Nigeria, (particularly gender disparities) have a major role in women’s self-autonomy and ability to make independent decisions about their health care, especially when it relates to reproductive health.9,10 Our study location (northern Nigeria) has historically low rates of male involvement in maternity care.9 Our study suggests that a male partner intervention that is part of a package of PMTCT services and includes a combination of individual, facility-based, and community-based approaches (invitation letters delivered by wives, peer male champions, and male-friendly settings) is acceptable and feasible. The use of invitation letters and tracing to recruit male partners for couple HIV testing and counselling has been effective in option B+ settings,29 and could prove feasible in our environment when option B+ becomes national policy. Our study also shows that male partners can provide assistance in the use of culturally sensitive and sustainable PMTCT care delivered by lower-cadre providers. The effect of active male engagement on ART uptake in our study is consistent with other reports.25 Our study has limitations: our protocol did not include measurement of outcomes in male partners, an oversight that would have otherwise added value to our under standing of the role of men in PMTCT. Our analyses also deemed women who transferred clinics without notification as failed retention, a reasonable assumption, since relocations and clinic transfers (silent transfers) are a common cause of non-mortality losses in HIV programmes.30 We were also unable to show whether our intervention affected viral load suppression, because viral load testing was not done in our setting. We also had to assess retention at an earlier timepoint than the national cutoff point for loss to follow-up (mother and baby >90 days late for an appointment at 6 months post partum) because of time constraints associated with the grant period. Because many infants might still have been breastfeeding at the time of the 6 week and 12 week DNA PCR testing, our results might overestimate the true HIV-free survival rate in this cohort. We only knew the HIV status of infants who were retained, and this differed by intervention group. Infants who were not retained might have been more likely to be HIV-infected, and therefore the relative risk effect of the intervention on infant HIV infection might actually be greater than that reported here. Although study sites were randomly assigned, participants in the control and intervention groups had differences that could have affected our study outcomes. The finding that women in the intervention group were less well educated and came to antenatal care later in gestation might be biased towards the null hypothesis, whereas the higher CD4 cell counts in women in the intervention group might be a bias away from the null hypothesis. The observed cluster size was not uniform, and our regression analysis makes the most efficient use of the data by giving more weight to clusters that provide more information because of a larger sample size; thus, smaller sites have less influence on the results. Because the intervention group had more staffing and ongoing quality assurance activities, the completeness or correctness of data might be better at the intervention sites. Differences in risk of infant death between study groups could affect differences in rates of uptake of early infant diagnosis in an intention-to-treat analysis. However, risk of infant death was not significantly different between study groups. The strengths of our study include the cluster-randomised design, a large cohort drawn across 12 busy PMTCT sites, a mixed-methods approach to monitoring the inter vention, and a prospective design that favours causal inference.
In summary, we have shown that a culturally sensitive bundle of PMTCT services improves treatment uptake and mother and infant retention in care, decreases infant HIV infection, is feasible in rural venues, and has the potential to enable Nigeria’s attainment of elimination of mother-to-child HIV transmission goals. National policies that address inclusion of lower-cadre service providers, engage men in the community, integrate maternal and child health and HIV services, and support provision of point-of-care diagnostics in resource-constrained settings will help to stem vertical HIV transmission, save lives, and optimise maternal and infant outcomes. As previously advocated by others,31 a robust implementation research framework will generate the evidence basis for adopting best practices needed to overcome the challenges impeding effective implementation of PMTCT programmes in real-world settings.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, Web of Science, Ovid MEDLINE, the Cochrane Register of Clinical Trials, and the Cochrane Database of Systematic Reviews for articles on task shifting, male involvement in prevention of mother-to-child HIV transmission (PMTCT), integration of mother and infant care, and point-of-care CD4 cell count testing that were published between Jan 1, 1995, and Dec 31, 2015. We included published randomised controlled trials, cluster-randomised trials, controlled before-and-after studies, population-based retrospective cohort studies, and systematic reviews that emerged with the broad MeSH terms “HIV infections/prevention & control”, “Africa south of the Sahara”, “health services accessibility”, and “point-of-care systems”. We narrowed the search by selecting for the following terms in identified articles: “task shifting”, “male participation”, “antiretroviral treatment initiation”, “retention in care”, “CD4 point-of-care testing”, and “PMTCT integration”. The search was done by a research assistant. Investigators selected eligible studies for review, taking into consideration the methodological quality of identified papers. Known barriers to effective PMTCT scale-up in Nigeria and similar settings in sub-Saharan Africa include shortages of skilled health-care providers who are trained in HIV prevention, especially in rural settings; absences of reliable and affordable laboratory infrastructure, particularly point-of-care diagnostics; cultural norms that inhibit participation of men in antenatal care; and fragmented maternal and child health services. Evidence-based solutions to these challenges are known; examples include task shifting, community-based and facility-based male engagement initiatives, couple counselling and testing, point-of-care CD4 cell count testing, and post-partum integration of services for HIV-infected mothers and their babies. A systematic approach that tackles these problems in a holistic manner has, however, not been rigorously assessed.
Added value of this study
This parallel, cluster-randomised trial shows that a package of individual PMTCT interventions that have independently been effective in increasing uptake of HIV services and improving antiretroviral treatment outcomes (task shifting, point-of-care CD4 cell count testing, integrated mother and infant service provision, and male partner and community engagement) increases maternal antiretroviral treatment uptake and mother and infant retention in care, decreases infant HIV infection, and is feasible in rural venues.
Implications of all the available evidence
Packaging of individually effective PMTCT interventions can have positive and measurable effects on important PMTCT outcomes. In view of Nigeria’s sizeable contribution to the global burden of mother-to-child HIV transmission, an effective approach to improve the quality of PMTCT service delivery will positively affect global goals for the elimination of this transmission.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R01HD075075), National Institutes of Health-funded Tennessee CFAR (P30 AI110527), and salary support (K01MH107255-01) for CMA. The findings and conclusions here are those of the authors and do not necessarily represent the official position of the National Institutes of Health. We thank the following Friends in Global Health Nigeria staff: Mukhtar Muhammad, Saidu Ishaq, Awwal Gambo, and Ibrahim Sodangi.
Footnotes
For the web application to do non-bipartite matching see http://data.vanderbilt.edu/rapache/matching
For the R-software analysis scripts see http://biostat.mc.vanderbilt.edu/ArchivedAnalyses
See Online for appendix
Contributors
MHA, CMA, MB, MK, MLL, CWW, BES, and SHV designed the study. MHA, UIG, and OO enrolled participants to the study. MHA, CMA, MB, CWW, UIG, OO, and SHV contributed to the coordination and oversight of the study. MB and BES did the data analysis. All authors participated in data interpretation and writing of the manuscript. All authors approved the final version of the manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2011;7:CD003510. doi: 10.1002/14651858.CD003510.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Aizire J, Fowler MG, Coovadia HM. Operational issues and barriers to implementation of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Sub-Saharan Africa. Curr HIV Res. 2013;11:144–59. doi: 10.2174/1570162x11311020007. [DOI] [PubMed] [Google Scholar]
- 3.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23:41–49. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlap J, Foderingham N, Bussell S, Wester CW, Audet CM, Aliyu MH. Male involvement for the prevention of mother-to-child HIV transmission: A brief review of initiatives in East, West, and Central Africa. Curr HIV/AIDS Rep. 2014;11:109–18. doi: 10.1007/s11904-014-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. The Gap Report. 2014 http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf (accessed Feb 13, 2015).
- 6.Anigilaje EA, Dabit OJ, Olutola A, Ageda B, Aderibigbe SA. HIV-free survival according to the early infant-feeding practices; a retrospective study in an anti-retroviral therapy programme in Makurdi, Nigeria. BMC Infect Dis. 2015;15:132. doi: 10.1186/s12879-015-0871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ugo A, Hatt L, Arur A, et al. Nigeria HIV/AIDS service provision assessment 2008. Bethesda, MD: Health Systems 20/20 Project, Abt Associates Inc; 2008. [Google Scholar]
- 8.Abimiku AG, Institute of Human Virology, University of Maryland School of Medicine PEPFAR Program (AIDS Care Treatment in Nigeria [ACTION]) Building laboratory infrastructure to support scale-up of HIV/AIDS treatment, care, and prevention: in-country experience. Am J Clin Pathol. 2009;131:875–86. doi: 10.1309/AJCPELMG6GX6RQSM. [DOI] [PubMed] [Google Scholar]
- 9.Iliyasu Z, Abubakar IS, Galadanci HS, Aliyu MH. Birth preparedness, complication readiness and fathers’ participation in maternity care in a northern Nigerian community. Afr J Reprod Health. 2010;14:21–32. [PubMed] [Google Scholar]
- 10.Isiugo-Abanihe UC. Sociocultural aspects of HIV/AIDS infection in Nigeria. Afr J Med Med Sci. 2006;35:45–55. [PubMed] [Google Scholar]
- 11.Aliyu MH, Varkey P, Salihu HM, Iliyasu Z, Abubakar IS. The HIV/AIDS epidemic in Nigeria: progress, problems and prospects. Afr J Med Med Sci. 2010;39:233–39. [PubMed] [Google Scholar]
- 12.Aliyu MH, Blevins M, Audet C, et al. Optimizing PMTCT service delivery in rural North-Central Nigeria: protocol and design for a cluster randomized study. Contemp Clin Trials. 2013;36:187–97. doi: 10.1016/j.cct.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greevy R, Lu B, Silber JH, Rosenbaum P. Optimal multivariate matching before randomization. Biostatistics. 2004;5:263–75. doi: 10.1093/biostatistics/5.2.263. [DOI] [PubMed] [Google Scholar]
- 14.Federal Ministry of Health Nigeria. National Guidelines for Prevention of mother-to-child HIV transmission (PMTCT) (4th) http://www.emtct-iatt.org/wp-content/uploads/2013/04/Nigeria_National-PMTCT-Guidelines_2010.pdf (accessed Feb 13, 2016).
- 15.WHO. Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice. Geneva: Emergency Plan for AIDS Relief (PEPFAR); 2006. Integrated Management of Pregnancy and Childbirth (IMPAC) [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 18.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. http://www.unaids.org/globalreport/Global_report.htm (accessed Oct 8, 2015).
- 19.WHO, UNAIDS, UNICEF. Global HIV/AIDS response: epidemic update and health services progress toward universal access. Progress report 2011. http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf (accessed Oct 8, 2015)
- 20.Bashorun A, Nguku P, Kawu I, et al. A description of HIV prevalence trends in Nigeria from 2001 to 2010: what is the progress, where is the problem? Pan Afr Med J. 2014;18:3. doi: 10.11694/pamj.supp.2014.18.1.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Federal Ministry of Health, Nigeria. National AIDS Control Agency (NACA) National HIV/AIDS Prevention Plan, 2014–2015. http://sbccvch.naca.gov.ng/sites/default/files/National%20HIV%20PrevPlan%202014-2015%281%29.pdf (accessed Feb 13, 2015)
- 22.Morris MB, Chapula BT, Chi BH, et al. Use of task-shifting to rapidly scale-up HIV treatment services: experiences from Lusaka, Zambia. BMC Health Serv Res. 2009;9:5. doi: 10.1186/1472-6963-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen R, Lynch S, Bygrave H, et al. Antiretroviral treatment outcomes from a nurse-driven, community-supported HIV/AIDS treatment programme in rural Lesotho: observational cohort assessment at two years. J Int AIDS Soc. 2009;12:23. doi: 10.1186/1758-2652-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–26. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Killam WP, Tambatamba BC, Chintu N, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS. 2010;24:85–91. doi: 10.1097/QAD.0b013e32833298be. [DOI] [PubMed] [Google Scholar]
- 26.Jani IV, Sitoe NE, Chongo PL, et al. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011;25:807–812. doi: 10.1097/QAD.0b013e328344f424. [DOI] [PubMed] [Google Scholar]
- 27.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. HPTN 052-ACTG Study Team. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–90. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg NE, Mtande TK, Saidi F, et al. Recruiting male partners for couple HIV testing and counselling in Malawi’s option B+ programme: an unblinded randomised controlled trial. Lancet HIV. 2015;2:e483–91. doi: 10.1016/S2352-3018(15)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalal RP, Macphail C, Mqhayi M, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–07. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 31.Sturke R, Harmston C, Simonds RJ, et al. A multi-disciplinary approach to implementation science: the NIH-PEPFAR PMTCT implementation science alliance. J Acquir Immune Defic Syndr. 2014;67:S163–67. doi: 10.1097/QAI.0000000000000323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


