Abstract
Objective
Look-alike, sound-alike (LASA) drug name substitution errors in children may pose potentially severe consequences. Our objective was to determine the degree of potential harm pediatricians ascribe to specific ambulatory LASA drug substitution errors.
Methods
We developed a unified list of LASA pairs from published sources, removing selected drugs on the basis of preparation type (eg, injectable drugs). Using a modified Delphi method over 3 rounds, 38 practicing pediatricians estimated degree of potential harm that might occur should a patient receive the delivered drug in error and the degree of potential harm that might occur from not receiving the intended drug.
Results
We identified 3550 published LASA drug pairs. A total of 1834 pairs were retained for the Delphi surveys, and 608 drug pairs were retained for round 3. Final scoring demonstrated that participants were able to identify pairs where the substitutions represented high risk of harm for receiving the delivered drug in error (eg, did not receive methylphenidate/received methadone), high risk of harm for not receiving the intended drug (eg, did not receive furosemide/received fosinopril), and pairs where the potential harm was high from not receiving the intended drug and from erroneously receiving the delivered drug (eg, did not receive albuterol/received labetalol).
Conclusions
Pediatricians have identified LASA drug substitutions that pose a high potential risk of harm to children. These results will allow future efforts to prioritize pediatric LASA errors that can be screened prospectively in outpatient pharmacies.
Keywords: children, medication error, patient safety, prescription error
Look-alike, sound-alike medication errors occur when the names of 2 drugs have orthographic similarity (eg, nitroglycerin/nitrofurantoin) or phonetic similarity (eg, albuterol/atenolol), forming a look-alike, sound-alike pair.1–5 In previous research, we estimated how often look-alike, sound-alike (LASA) medication errors occur in ambulatory pediatric prescriptions using 11 selected LASA pairs from a list published by the Institute for Safe Medication Practices.2 That study demonstrated that prescription dispensing patterns can be used to screen for potential LASA errors in pediatrics, but the frequencies of these errors appear to be much lower than other types of pediatricmedication errors, occurring in fewer than 1 per 1000 prescriptions dispensed.2 The relatively low frequency of these errors and the large number of medications that are part of LASA pairs suggest that the problem of LASA substitution errors may be best addressed using automated processes.
Although only a few studies have evaluated LASA errors in children,2,6,7 we are not aware of investigation into the clinical value of these substitution errors. Establishing the clinical value of potential harm from any specific LASA substitution error is required in order to prioritize drug pairs in processes created to prevent these errors. Because drug error prevention may be best addressed by automated approaches (eg, computerized decision support that includes electronic alerts), processes designed to prevent these errors should prioritize errors that pose the most severe harm. Focusing on high potential harm errors allows systems to minimize adverse effects on provider or pharmacy work flow and minimize alert fatigue, whereby providers and pharmacists ignore decision support because of alert volume.1,8–11 The expanding use of electronic health records has the potential to decrease LASA errors because of improvements in legibility and improved transmission of prescriptions, but computerized systems can introduce new ways to produce LASA errors, particularly when medication selection is done using an alphabetized menu, termed a menu selection error.1,12–14
The objective of this study was to identify LASA substitution errors that providers thought posed a high degree of potential harm to children. As a preliminary evaluation of the frequency of these errors, we also utilized 10 years of prescription data to estimate the frequency of LASA errors in outpatient pediatric prescriptions. Ultimately, future efforts will combine the estimation of harm with additional analyses on the frequency of LASA substitution errors to permit investigators to prioritize pairs for inclusion in future efforts to prevent these errors in real time in clinical settings.
Methods
Overview of Approach
In part because of a relative lack of data on medication errors in pediatric outpatients,15 we focused our investigation on drug pairs that are prescribed in outpatient pediatric practice. We also set a goal of identifying potential high-harm pairs that could be translated into future clinical interventions to reduce LASA errors. The concern of alert fatigue was a paramount concern in our approach, informing our decisions about pairs to exclude.
Establishing List of LASA Pairs
We identified 2 published lists of LASA pairs. One list was published by the Institute for Safe Medication Practices (668 pairs),16 and the other was published by MED-MARX (3156 pairs), for a total of 3824 published pairs.17 However, there were 274 pairs that appeared on both lists, leaving 3550 published LASA pairs. Those published pairs included reciprocals of the same pairs (drug A for drug B, and drug B for drug A in any pair), meaning that there were actually 1775 discrete pairs of 2 drugs that exhibited either look-alike or sound-alike name confusion. Our goal was to evaluate the opinion-generated estimate of the potential harm presented by these substitution errors in outpatient practice. Two investigators (WTB and SSG) independently reviewed the pairs in order to identify pairs to retain or remove from the list. After independent review, the 2 investigators met to come to consensus about pairs that should be retained. We removed pairs where at least one of the preparations in the pair was an intravenous, intramuscular, or other injectable product (including vaccines). In order to reduce the list to a manageable set for evaluation by panelists, we also removed pairs where both drugs were vitamins or nutritional supplements. We removed pairs where a topical, otic, ophthalmic, or nasal preparation was paired with an oral drug of the same name under the assumption that a different route of administration would be more likely to alert the parent or pharmacist to the LASA error and prevent the error before dispensing. Because listing an indication on a prescription is one method that pharmacists might use to detect a LASA error,18 we elected to remove pairs where both drugs were of the same class. For example, many of the statins and many of the cephalosporin antibiotics are part of LASA pairs with other statins or other cephalosporins, respectively. Although these drugs may present potentially harmful substitutions, they generally would be indicated for the same condition, therefore making it very difficult for an automated process to detect a substitution error without introducing alert fatigue. We did not remove pairs that contained 2 drugs of the same drug class if the 2 drugs had potentially harmful differences in potency (eg, benzodiazepines, where “10 mg” of one drug may be of equal potency to “1 mg” of another). Finally, we removed pairs where one of the drug formulations was a long-acting version of the same drug. After exclusions, the merged list contained 917 discrete pairs (or 1834 error combinations with reciprocals).
Development of Survey Instrument
Development of the survey instrument was conducted within the Division of General Pediatrics at the Medical University of South Carolina. We began with focus groups of 2 to 3 general pediatric faculty members to discuss how to conceptualize the Delphi approach and refine the terminology to be used in the survey. The pediatricians involved in these focus group discussions did not ultimately participate in the Delphi study. In those focus groups sessions, we came to consensus on the terminology to be used, including the approach to estimating “degree of potential harm” and the physical layout of the survey instrument, as well as determining what would be a reasonable time to complete the surveys. We utilized this process to refine the terminology and language of the questions to ensure that the participants were estimating the degree of potential harm and not the estimated probability that harm might occur.
Once the terminology and approach for the survey were developed, we conducted cognitive pretesting via in-person interviews with 5 practicing pediatricians who had not participated in the previous focus groups. In those sessions, we asked them to provide feedback on the clarity of the questions, specifically on the language of estimating degree of harm and not the probability of harm, and the ability of pediatricians to differentiate potential harm among the different LASA substitution errors. Cognitive pretesting revealed that the participants thought they could estimate degree of potential harm but not the probability of harm. We further refined the questions and the visual presentation of the survey on the basis of the input from these interviews. Through this process, we developed the consensus that each LASA substitution is actually a combination of 2 errors—the patient receiving the delivered drug in error, and the patient not receiving the intended drug. Each of those errors may have different degrees of estimated potential harm. Therefore, each LASA pair was broken into 2 questions, asking the participant to estimate the degree of potential harm represented by receiving the delivered drug in error as well as the degree of potential harm represented by not receiving the intended drug.
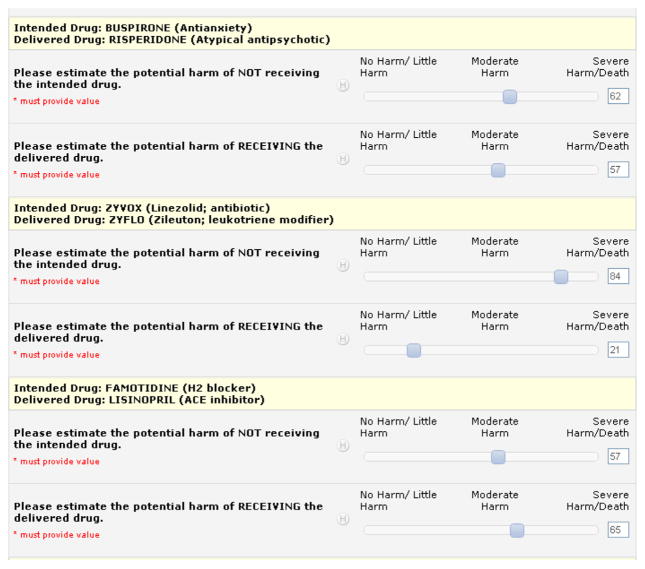
We utilized the REDCap online survey tool, and each question was answered on a continuous Likert-type scale whereby the participant moved a cursor to a point from 0 to 100 that represented his or her scoring for each error (Fig. 1). In cognitive pretesting, we identified 5 anchor terms for the continuous scale: “no harm,” “little harm,” “moderate harm,” “severe harm,” and “death,” consistent with the terminology used in the MEDMARX drug error literature. 17 Because REDCap only allows 3 anchors on a scale, the terms at the ends of the scale were placed together, but still showing their relative position left to right, as shown in Figure 1: “no harm/little harm,” “moderate harm,” and “severe harm/death.” Cognitive pretesting revealed that the participants thought they could better estimate degree of potential harm but not the probability of harm.
Figure 1.
Screen capture of REDCap survey instrument.
As shown in Figure 1, each pair was presented as the intended drug and the delivered drug, along with a brief description of the drug class in case a participant was unfamiliar with any drug. When the drug in a LASA pair was a brand name, the generic name was also provided. Through the process of survey development, testing, and piloting, it became apparent that we needed to provide participants with several assumptions. First, they were to estimate the degree of potential harm that might be experienced by the average patient who had no acute or chronic medical conditions other than the one for which the patient was prescribed the intended drug. They were to assume that drug allergies were not a concern. They were to assume that the dose of the prescription did not change, only the drug dispensed. They were instructed to assume only a 1-month error, meaning that the substitution error would not recur at the subsequent dispensing of the drug. The instructions reminded participants to not estimate the chance that harm would occur. Instead, they were to estimate the degree of potential harm that might occur should the patient experience adverse effects from the drug substitution.
Through the piloting process, we determined that completing a survey with 50 LASA pairs took approximately 20 minutes. Therefore, we developed 37 versions of the survey for rounds 1 and 2 and recruited 37 pediatricians to participate in the surveys.
Recruitment of Participants
All participants were general pediatricians. The authors recruited community practitioners from the South Carolina Pediatric Practice Research Network, a network of 14 community practices with >70 pediatric providers in South Carolina. In addition, we recruited from the membership of the Academic Pediatric Association, primarily composed of academic general pediatricians. We recruited membership of the different organizations via e-mail, informing potential participants of the study and describing the commitment and the type of assessment they would be asked to complete. Interested parties replied to the principal investigator or the research coordinator, and subsequent follow-up was via e-mail.
Delphi Process
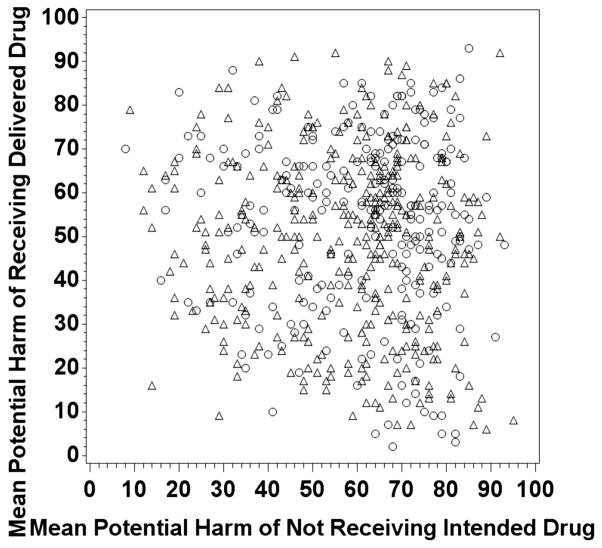
In the first 2 rounds of the Delphi survey, each participant evaluated 2 separate groups of 50 pairs. Therefore, every pair was scored by at least by 2 participants, and every participant scored 100 pairs total between rounds 1 and 2. Feedback was not provided to the participants between the rounds. After rounds 1 and 2, we completed cluster analysis of the scores to identify drugs to eliminate for the next round of the survey, seeking to retain pairs with higher estimates of potential harm. Scores were not merged from the 2 initial evaluations, such that a pair that was scored highly by either of the 2 participants was retained for round 3. Cluster analysis revealed that a score of 82 of 100 was a natural break point for LASA pairs to be retained, so pairs were retained if either rater (round 1 or round 2) rated either part of a LASA error (not getting intended drug or getting delivered drug in error) as greater than 82. The cut point did not represent a clinical point but rather a statistical cut point. Adding additional clusters would have moved the cut point closer to 50 of 100 and would have greatly expanded the number of drug pairs, reducing our opportunity to focus on pairs with the highest estimated potential harm. Round 3 contained 608 potential LASA error pairs, sent out to 36 participants in differing combinations such that each LASA pair was scored by 3 participants. The round 3 scores were then averaged to get the final scatterplot shown in Figure 2. The average of the round 3 scoring for each pair is represented by a single point on Figure 2. The point’s location on the x-axis is determined by the answer to the question assessing the degree of potential harm that might occur from not receiving the intended drug, while the position on the y-axis is determined by the answer to the question assessing the degree of potential harm that might occur from receiving the delivered drug in error.
Figure 2.
Scatterplot of round 3 mean scores for 608 look-alike, sound-alike (LASA) drug substitution errors. Each point on the plot above represents the mean score for each LASA pair, measuring the opinion-generated estimate of harm for not receiving intended drug (x-axis) and the opinion-generated estimate of harm for receiving the delivered drug in error (y-axis). Circles represent LASA pair errors where no patient received both drugs within 6 months of each other, and triangles represent pairs where at least 1 patient received both drugs within 6 months of each other, based on 10 years of pharmacy claims data studied.
Initial Assessment of LASA Error Frequency
We conducted a preliminary assessment of the frequency at which patients may have experienced potential LASA errors among the 608 pairs in round 3. We used a broad definition of potential LASA error such that any patient who received both drugs of a LASA pair within any 6-month period represented a potential LASA error.2 Such an event could be detected at the point of prescribing or dispensing utilizing automated systems that would trigger an electronic alert when 2 drugs in a LASA pair were prescribed or dispensed within 6 months of one another.2 This approach produces a very inclusive error estimate, likely representing the upper bound of the true frequency of LASA substitution error. For these analyses, we utilized 2000–2009 South Carolina Medicaid paid claims data for patients < 21 years old, obtained from the South Carolina Office of Research and Statistics. We utilized encrypted patient identifiers to link enrollees to pharmacy and diagnostic data. When identifying drugs prescribed, we utilized files available from the US Food and Drug Administration to match National Drug Code numbers to generic names, formulations (concentrations, etc), and brand names of the generic drugs. Because LASA pairs may contain both generic and brand names, we accounted for any within-pharmacy substitutions of generic drug for a brand name listed on the prescription or a branded generic substitution for any generic drug name on the prescription by including generic and brand versions the drugs in each LASA pair in our frequency analyses. A data manager (ME) experienced with South Carolina Medicaid data reviewed the files and removed duplicate entries. The institutional review board of the Medical University of South Carolina and South Carolina Office of Research and Statistics approved the study.
Results
One participant who completed round 1 did not participate in subsequent rounds, resulting in 38 total participants over the course of the surveys. Twenty participants (53%) were women. The racial breakdown was 34 white participants (89.5%), 3 (7.9%) Asian, and 1 (2.6%) black. No participant was Hispanic. Seven participants (18.4%) were community practitioners, and 31 (81.6%) were at academic medical centers. Participants hailed from 9 different states.
Rounds 1 and 2 identified 608 potential LASA error pairs to retain for round 3. Round 3 final rankings (Fig. 2) demonstrated that participants were able to identify pairs where the substitutions represented high risk of potential harm for receiving the delivered drug in error (eg, did not receive methylphenidate/received methadone), high risk of harm for not receiving the intended drug (eg, did not receive furosemide/received fosinopril), and pairs where the potential harm was high from not receiving the intended drug and from erroneously receiving the delivered drug (eg, did not receive albuterol/received labetalol).
Table 1 represents the top decile (n = 60 pairs) of the rank-ordered list of pairs based on the scores for the opinion-generated estimate of potential harm that might result from receiving the delivered drug in error. The full rank-ordered list is provided in Online Appendix Table 1. Table 2 contains the top decile of the rank-ordered list of pairs on the basis of the scores for the opinion-generated estimate of potential harm that might result from not receiving the intended drug. The full rank-ordered list is provided in Online Appendix Table 2. LASA pairs that were in the top decile of both measures of harm (receiving the delivered drug in error or not receiving the intended drug) appear in bold type in Tables 1 and 2, and in Online Appendix Tables 1 and 2. Consistent with the MEDMARX presentation of LASA pairs, brand names appear in italics in Tables 1 and 2, and in Online Appendix Tables 1 and 2.
Table 1.
List of Highest Decile of LASA Errors, Ranked by Potential Harm of Receiving the Delivered Drug in Error
| Generic Name of Delivered Drug | Published Name of Delivered Drug* | Published Name of Intended Drug† | Generic Name of Intended Drug | Average Harm Score‡ | No. of Subjects§ |
|---|---|---|---|---|---|
| Potassium | K Dur | Kayexalate | Sodium polystyrene sulfonate | 93 | 0 |
| Cyclosporine | Cyclosporine | Cyclophosphamide | Cyclophosphamide | 92 | 5 |
| Digoxin | Lanoxin | Levothyroxine | Levothyroxine | 92 | 15 |
| Warfarin | Coumadin | Cardura | Doxazosin | 91 | 5 |
| Warfarin | Jantoven | Januvia | Sitagliptin | 90 | 1 |
| Warfarin | Warfarin | Levaquin | Levofloxacin | 90 | 52 |
| Warfarin | Coumadin | Mephyton | Phytonadione | 89 | 5 |
| Warfarin | Coumadin | Avandia | Rosiglitazone | 88 | 2 |
| Warfarin | Jantoven | Janumet | Sitagliptin + Metformin | 88 | 0 |
| Azathioprine | Azathioprine | Azithromycin | Azithromycin | 87 | 63 |
| Temozolomide | Temodar | Tambocor | Flecainide | 86 | 0 |
| Cyclophosphamide | Cyclophosphamide | Cyclosporine | Cyclosporine | 85 | 5 |
| Disopyramide | Disopyramide | Desipramine | Desipramine | 85 | 0 |
| Lithium | Eskalith | Effexor | Venlafaxine | 85 | 99 |
| Letrozole | Femara | Famvir | Famciclovir | 85 | 0 |
| Labetalol | Labetalol | Albuterol | Albuterol | 85 | 40 |
| Lithium | Lithium | Lanthanum | Lanthanum | 85 | 0 |
| Amiodarone | Amiodarone | Amlodipine | Amlodipine | 84 | 3 |
| Glipizide | Glucotrol | Detrol | Tolterodine | 84 | 1 |
| Nitroglycerin | Nitro Bid | Macrobid | Nitrofurantoin | 84 | 5 |
| Flecainide | Tambocor | Tamoxifen | Tamoxifen | 84 | 0 |
| Alprazolam | Xanax | Atarax | Hydroxyzine | 84 | 430 |
| Hydromorphone | Hydromorphone | Haloperidol | Haloperidol | 83 | 0 |
| Levorphanol | Levorphanol | Levothyroxine | Levothyroxine | 83 | 0 |
| Capecitabine | Xeloda | Xenical | Orlistat | 83 | 0 |
| Anastrazole | Arimidex | Aromasin | Exemestane | 82 | 0 |
| Clomipramine | Clomipramine | Clozapine | Clozapine | 82 | 1 |
| Clomipramine | Clomipramine | Clomid | Clomiphene | 82 | 0 |
| Cyclosporine | Cyclosporine | Cycloserine | Cycloserine | 82 | 0 |
| Diazoxide | Diazoxide | Diamox | Acetazolamide | 82 | 0 |
| Fludrocortisone | Florinef | Fioricet | Butalbital + acetaminophen + caffeine | 82 | 0 |
| Methadone | Methadone | Methylphenidate | Methylphenidate | 82 | 9 |
| Morphine | Morphine | Magnesium Sulfate | Magnesium Sulfate | 82 | 0 |
| Nifedipine | Nifedipine | Nortriptyline | Nortriptyline | 82 | 1 |
| Digoxin | Lanoxin | Lotensin | Benazepril | 81 | 18 |
| Tamoxifen | Nolvadex | Flomax | Tamsulosin | 81 | 0 |
| Amlodipine | Amlodipine | Amiodarone | Amiodarone | 80 | 3 |
| Metoprolol | Lopressor | Lyrica | Pregabalin | 80 | 5 |
| Amiodarone | Pacerone | Pancrease | Pancrelipase | 80 | 3 |
| Midodrine | ProAmatine | Bromocriptine | Bromocriptine | 80 | 0 |
| Tacrolimus | Tacrolimus | Tamsulosin | Tamsulosin | 80 | 0 |
| Digoxin | Lanoxin | Lasix | Furosemide | 79 | 447 |
| Chlorambucil | Leukeran | Leucovorin | Leucovorin | 79 | 0 |
| Hydrocodone + Acetaminophen | Lortab | Loratadine | Loratadine | 79 | 8259 |
| Loxapine | Loxitane | Soriatane | Acitretin | 79 | 0 |
| Methotrexate | Methotrexate | Minoxidil | Minoxidil | 79 | 0 |
| Procainamide | Procanbid | Probenecid | Probenecid | 79 | 0 |
| Reserpine | Reserpine | Ropinirole | Ropinirole | 79 | 0 |
| Rosiglitazone + Metformin | Avandamet | Anzemet | Dolasetron | 78 | 0 |
| Chlorpromazine | Chlorpromazine | Clomipramine | Clomipramine | 78 | 2 |
| Clozapine | Clozaril | Pletal | Cilostazol | 78 | 0 |
| Digoxin | Lanoxin | Levsin | Hyoscyamine | 78 | 24 |
| Methadone | Methadone | Mephyton | Phytonadione | 78 | 1 |
| Methimazole | Methimazole | Metaxalone | Metaxalone | 78 | 6 |
| Nitroglycerin | Nitroglycerin | Nitrofurantoin | Nitrofurantoin | 78 | 5 |
| Sitagliptin + Metformin | Janumet | Jantoven | Warfarin | 77 | 0 |
| Theophylline | Slow Bid | Slow K | Potassium | 77 | 0 |
| Hydrocodone + Acetaminophen | Vicodin | Vytorin | Ezetimibe + simvastatin | 77 | 20 |
| Digoxin | Lanoxin | Lomotil | Diphenoxylate + atropine | 76 | 7 |
| Digoxin | Lanoxin | Levaquin | Levofloxacin | 76 | 24 |
LASA indicates look alike, sound alike.
Bold type indicates that LASA pair is in highest decile for potential harm for receiving delivered drug in error and highest decile for potential harm of not receiving intended drug. Brand names appear in italic type.
Delivered drug indicates drug received in error.
Intended drug indicates drug patient was supposed to receive.
Average harm score represents the average of round 3 evaluations of the opinion-generated estimate of the harm that might occur from this substitution, where patient received delivered drug instead of intended drug.
Number of subjects in 2000–2009 data who received both drugs in respective LASA pair within 6-month period, regardless of whether this was intended or in error. Each occurrence represents potential LASA substitution error.
Table 2.
List of Highest Decile of LASA Errors, Ranked by Potential Harm of Not Receiving One’s Intended Drug
| Generic Name of Intended Drug | Published Name of Intended Drug* | Published Name of Delivered Drug† | Generic Name of Delivered Drug | Average Harm Score‡ | No. of Subjects§ |
|---|---|---|---|---|---|
| Warfarin | Warfarin | Levaquin | Levofloxacin | 95 | 52 |
| Moricizine | Ethmozine | Ethambutol | Ethambutol | 93 | 0 |
| Cyclophosphamide | Cyclophosphamide | Cyclosporine | Cyclosporine | 92 | 5 |
| Tacrolimus | Prograf | Prozac | Fluoxetine | 92 | 8 |
| Dantrolene | Dantrium | Danocrine | Danazol | 91 | 0 |
| Amiodarone | Cordarone | Cardene | Nicardipine | 89 | 0 |
| Warfarin | Coumadin | Avandia | Rosiglitazone | 89 | 2 |
| Methotrexate | Folex | Foltx | Vitamin B combination | 89 | 1 |
| Ritonavir | Norvir | Norvasc | Amlodipine | 88 | 1 |
| Oxcarbazepine | Oxcarbazepine | Oxaprozin | Oxaprozin | 88 | 11 |
| Warfarin | Coumadin | Cardura | Doxazosin | 87 | 5 |
| Chlorambucil | Leukeran | Leucovorin | Leucovorin | 87 | 0 |
| Phenytoin Sodium | Phenytoin Sodium | Nystatin | Nystatin | 87 | 72 |
| Topiramate | Topamax | Tofranil | Imipramine | 87 | 59 |
| Azathioprine | Azathioprine | Azithromycin | Azithromycin | 86 | 63 |
| Warfarin | Coumadin | Phytonadione | Phytonadione | 86 | 5 |
| Cyclosporine | Neoral | Inderal | Propranolol | 86 | 9 |
| Carbamazepine | Carbamazepine | Carisoprodol | Carisoprodol | 85 | 17 |
| Sodium Polystyrene Sulfonate | Kayexalate | K Dur | Potassium | 85 | 0 |
| Levetiracetam | Levetiracetam | Levocarnitine | Levocarnitine | 85 | 123 |
| Clopidogrel | Plavix | Paxil | Paroxetine | 85 | 6 |
| Flecainide | Tambocor | Tamoxifen | Tamoxifen | 85 | 0 |
| Linezolid | Zyvox | Zyflo | Zileuton | 85 | 0 |
| Lamivudine | Epivir | Elavil | Amitriptyline | 84 | 1 |
| Isosorbide | Isordil | Inderal | Propranolol | 84 | 2 |
| Levetiracetam | Keppra | Keflex | Cephalexin | 84 | 419 |
| Lamotrigine | Lamotrigine | Lamivudine | Lamivudine | 84 | 1 |
| Procainamide | Procainamide | Probenecid | Probenecid | 84 | 0 |
| Quinine | Quinine | Quinidine | Quinidine | 84 | 0 |
| Oxcarbazepine | Trileptal | Tegretol | Carbamazepine | 84 | 259 |
| Digoxin | Digoxin | Levothyroxine | Levothyroxine | 83 | 8 |
| Letrozole | Femara | Famvir | Famciclovir | 83 | 0 |
| Furosemide | Furosemide | Fosinopril | Fosinopril | 83 | 5 |
| Tiagabine | Gabitril | Glucotrol | Glipizide | 83 | 0 |
| Isosorbide | Imdur | Inderal | Propranolol | 83 | 2 |
| Warfarin | Jantoven | Janumet | Sitagliptin + metformin | 83 | 0 |
| Lopinavir + Ritonavir | Kaletra | Levitra | Vardenafil | 83 | 0 |
| Flecainide | Tambocor | Temodar | Temozolomide | 83 | 0 |
| Dofetilide | Tikosyn | Ticlopidine | Ticlopidine | 83 | 0 |
| Clozapine | Clozapine | Clomipramine | Clomipramine | 82 | 1 |
| Digoxin | Lanoxin | Levothyroxine | Levothyroxine | 82 | 15 |
| Procarbazine | Matulane | Materna | Prenatal Multivitamin | 82 | 0 |
| Nevirapine | Nevirapine | Nefazodone | Nefazodone | 82 | 0 |
| Penicillin | Penicillin | Penicillamine | Penicillamine | 82 | 0 |
| Phenytoin Sodium | Phenytoin Sodium | Feldene | Piroxicam | 82 | 3 |
| Thalidomide | Thalomid | Thiamine | Thiamine | 82 | 0 |
| Vancomycin | Vancomycin | Valacyclovir | Valacyclovir | 82 | 1 |
| Amiodarone | Amiodarone | Amantadine | Amantadine | 81 | 0 |
| Hydroxyurea | Hydroxyurea | Hydroxychloroquine | Hydroxychloroquine | 81 | 0 |
| Lopinavir + Ritonavir | Kaletra | Keppra | Levetiracetam | 81 | 0 |
| Lamotrigine | Lamotrigine | Levothyroxine | Levothyroxine | 81 | 58 |
| Leucovorin | Leucovorin | Leukeran | Chlorambucil | 81 | 0 |
| Chlordiazepoxide + Amitriptyline | Limbitrol | Librium | Chlordiazepoxide | 81 | 2 |
| Lithium | Lithium | Chlordiazepoxide | Chlordiazepoxide | 81 | 38 |
| Topiramate | Topamax | Protonix | Pantoprazole | 81 | 48 |
| Linezolid | Zyvox | Zithromax | Azithromycin | 81 | 93 |
| Carbamazepine | Carbatrol | Labetalol | Labetalol | 80 | 4 |
| Cyclosporine | Cyclosporine | Cyclophosphamide | Cyclophosphamide | 80 | 5 |
| Phenytoin | Dilantin | Dilaudid | Hydromorphone | 80 | 3 |
LASA indicates look alike, sound alike.
Bold type indicates that the LASA pair is in the highest decile for potential harm for receiving the delivered drug in error and the highest decile for potential harm of not receiving the intended drug. Brand names appear in italic type.
Intended drug indicates drug patient was supposed to receive.
Delivered drug indicates drug received in error.
Average harm score represents the average of round 3 evaluations of the opinion-generated estimate of the harm that might occur from patient not receiving intended drug.
Number of subjects in 2000–2009 data who received both drugs in respective LASA pair within 6-month period, regardless of whether this was intended or in error. Each occurrence represents potential LASA substitution error.
The estimated frequency of each LASA drug substitution error is denoted by the number of subjects who received both drugs in a pair within 6 months of each other. There were 207 LASA pairs (34%) for which no patient received both drugs within a 6-month period. For an additional 298 pairs (49%), the cumulative total of subjects who received both drugs in a pair was 3610, amounting to <1 potential LASA error per day over the 10-year data span. By contrast, among the remaining 103 (17%) of 608 pairs, there were 97,163 subjects who received both drugs, a total that would amount to at least 27 potential LASA errors per day over the course of 10 years.
Discussion
Using a modified Delphi process, we were able to rank order LASA pediatric drug substitution errors on the basis of pediatrician estimates of the potential harm that might occur should a patient receive one of these substitutions. In addition, through our initial evaluation of the frequency of these substitutions using claims data, we can refine a target list of LASA errors to be prioritized for future efforts.
Given the number of drugs in published LASA pairs, it would not be reasonable at either the provider point of producing a prescription or the pharmacy point of dispensing a prescription for a provider or pharmacist to review every prescription that might be involved in a LASA error. Therefore, electronic approaches to identify prescriptions as potential substitution errors likely represent the best way to operationalize this aspect of patient safety. As described in previous research, dispensing patterns can be utilized to identify patients who routinely receive one drug in a LASA pair who then are either prescribed (through electronic health records) or dispensed (at a pharmacy) the second drug in a LASA pair.2,7 Such events could trigger an electronic alert in an electronic health record or pharmacy’s system, prompting review by either the provider or pharmacist. The most significant challenge in implementing an alert system revolves around ensuring that the screening burden to the provider or pharmacist is not so excessive that the alerts are ignored, a phenomenon known as alert fatigue.8,11 We believe that this study helps to identify LASA errors that represent the highest potential harm to children according to the opinions of practicing clinicians and therefore assists with LASA pair prioritization for development of interventions to reduce these errors.
In deciding which LASA errors to prioritize, it is preferable that frequently occurring but low potential harm substitutions should not be placed into an alert system, as they would provide too many alerts. Many low potential harm substitutions were eliminated after rounds 1 and 2 of our Delphi process (not shown) and are not represented in the Online Appendixes. However, this study provided an unexpected finding—that many of the high potential harm LASA errors appear to occur infrequently. We identified high potential harm errors that either do not appear to occur (34% of round 3 pairs) or that occur very infrequently (additional 49% of round 3 pairs) in children on the basis of the 10 years of claims data evaluated for the second part of the study. This means that for 83% of the LASA pairs evaluated, one could make the argument that provider or pharmacy alert systems could be set for these LASA pairs such that any patient who receives both drugs within a 6-month period could trigger an electronic alert without excessively disrupting work flow. This appears to be a novel finding, as previous LASA studies in children did not evaluate specific pair frequencies and so did not offer insight into the feasibility of LASA error prevention via electronic alert systems. 2,4–6 For the remaining 103 LASA pairs (17%) evaluated in this study, the path toward their prevention is less clear in that automated alerts would occur frequently if the same prevention approach was utilized, perhaps overwhelming the system. For these 103 pairs, further investigation into the best trade-off between the clinical value of LASA substitution errors and frequency of these errors will be needed in order to implement automated screening for those LASA errors.
There are several limitations to this study. Perhaps the most significant limitation is that we eliminated many nonambulatory drugs used in pediatrics that appear on published LASA drug pair lists. However, our primary goal was to evaluate drugs used typically in ambulatory settings; there are without a doubt important inpatient LASA errors that can be targeted for reduction. In addition, we eliminated several classes of drugs, including immunizations, pairs where both drugs were vitamins or nutritional supplements, and LASA pairs where both drugs were from the same drug class, such as cephalosporins. All of these decisions were made in order to refine the published LASA pairs into a workable list for the Delphi assessment and ultimately for inclusion in an automated process to identify LASA substitution errors. In addition, specific to the elimination of pairs in the same drug class, we thought that an automated process including these pairs may actually introduce alert fatigue and detract from an important method that pharmacists might use to identify LASA substitution errors, matching the drug being dispensed with the indication as provided on the prescription or by the parent. A study published after we began this investigation demonstrated the effectiveness of indication alerts in reducing LASA errors, while also demonstrating that the most common LASA error type in the era of electronic health records may be errant selection of the wrong form of a drug from a pull-down list.18 Another limitation is that the published lists of LASA pairs are not pediatric specific, meaning that pediatricians may be less clinically familiar with many of the drugs in the pairs evaluated in this study. For example, many drugs used to treat parkinsonism appear in LASA pair lists, but we presume that most pediatricians have little familiarity with these drugs and therefore may be unfamiliar with their potential harm. Participants were not allowed to respond that they “did not know” the potential degree of potential harm, so some may have been forced to estimate harm for a pair with which they had little familiarity. The fact that REDCap allows only 3 descriptor positions on the Likert scale is potentially limiting, but we were able to preserve the relative rank of each substitution. Because we did not ask the participants to estimate the probability of a patient experiencing harm, just the degree of potential harm the patients might experience, we do not have a complete picture of the clinical importance of these substitution errors. In addition, each pair in round 3 was evaluated by only 3 individuals instead of 10 or more, which might have provided a more robust mean assessment of the potential harm. Finally, these data are drawn from one state’s prescription data for public insurance, so it is possible that prescribing patterns would be different in other states or for other insured child populations.
Conclusions
Pediatricians established the opinion-generated clinical value of over 600 pediatric LASA drug substitution errors. This study is novel in that it ascribes clinical value to pediatric-specific LASA drug substitution errors. These data, combined with future efforts to determine the potential frequency of pediatric LASA drug substitutions, will allow the development of a prioritized list of high potential harm LASA substitution errors for future prevention efforts.
Supplementary Material
What’s New.
This study establishes the opinion-generated clinical value of pediatric drug substitution errors among a group of drugs confused by name and prioritizes drug names for inclusion in future efforts to curb substitution errors that affect children.
Acknowledgments
Supported in part by a grant from the Agency for Healthcare Research and Quality (R03 HS018841; W. T. Basco Jr, PI). Additional research support was provided by awards UL1RR029882 and UL1TR000062 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The authors would like to thank the pediatricians who participated in this Delphi study.
Footnotes
The authors declare that they have no conflict of interest.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.acap.2015.06.014.
References
- 1.Cohen MR. Medication Errors. 2. Washington, DC: American Pharmacists Association; 2007. [Google Scholar]
- 2.Basco WT, Jr, Ebeling M, Hulsey TC, et al. Using pharmacy data to screen for look-alike, sound-alike substitution errors in pediatric prescriptions. Acad Pediatr. 2010;10:233–237. doi: 10.1016/j.acap.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Lambert BL, Lin SJ, Chang KY, et al. Similarity as a risk factor in drug-name confusion errors: the look-alike (orthographic) and sound-alike (phonetic) model. Med Care. 1999;37:1214–1225. doi: 10.1097/00005650-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lambert BL, Chang KY, Lin SJ. Effect of orthographic and phonological similarity on false recognition of drug names. Soc Sci Med. 2001;52:1843–1857. doi: 10.1016/s0277-9536(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 5.Phatak HM, Cady PS, Heyneman CA, et al. Retrospective detection of potential medication errors involving drugs with similar names. J Am Pharm Assoc. 2005;45:616–621. doi: 10.1331/1544345055001247. [DOI] [PubMed] [Google Scholar]
- 6.Shaw KN, Lillis KA, Ruddy RM, et al. Reported medication events in a paediatric emergency research network: sharing to improve patient safety. Emerg Med J. 2013;30:815–819. doi: 10.1136/emermed-2012-201642. [DOI] [PubMed] [Google Scholar]
- 7.Phatak HM, Cady PS, Heyneman CA, et al. Utilization of the Idaho Medicaid claims database to analyze potential look-alike/sound-alike medication errors. J Am Pharm Assoc. 2001;41:324. [Google Scholar]
- 8.Embi PJ, Leonard AC. Evaluating alert fatigue over time to EHR-based clinical trial alerts: findings from a randomized controlled study. J Am Med Inform Assoc. 2012;19(e1):e145–e148. doi: 10.1136/amiajnl-2011-000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carspecken CW, Sharek PJ, Longhurst C, et al. A clinical case of electronic health record drug alert fatigue: consequences for patient outcome. Pediatrics. 2013;131:e1970–e1973. doi: 10.1542/peds.2012-3252. [DOI] [PubMed] [Google Scholar]
- 10.Joint Commission on the Accreditation of Healthcare Organizations (JCAHO) [Accessed July 21, 2015];Safely implementing health information and converging technologies. 2008 Available at: http://www.jointcommission.org/sentinel_event_alert_issue_42_safely_implementing_health_information_and_converging_technologies/ [PubMed]
- 11.Perna G. Clinical alerts that cried wolf. As clinical alerts pose physician workflow problems, healthcare IT leaders look for answers. Healthc Inform. 2012;29:18, 20. [PubMed] [Google Scholar]
- 12.Walsh KE, Adams WG, Bauchner H, et al. Medication errors related to computerized order entry for children. Pediatrics. 2006;118:1872–1879. doi: 10.1542/peds.2006-0810. [DOI] [PubMed] [Google Scholar]
- 13.Shamliyan TA, Duval S, Du J, et al. Just what the doctor ordered. Review of the evidence of the impact of computerized physician order entry system on medication errors. Health Serv Res. 2008;43(1 pt 1):32–53. doi: 10.1111/j.1475-6773.2007.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naunton M, Gardiner HR, Kyle G. Look-alike, sound-alike medication errors: a novel case concerning a Slow-Na, Slow-K prescribing error. Int Med Case Rep J. 2015;8:51–53. doi: 10.2147/IMCRJ.S78637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinke ML, Bundy DG, Velasquez CA, et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134:338–360. doi: 10.1542/peds.2013-3531. [DOI] [PubMed] [Google Scholar]
- 16.Institute for Safe Medication Practices. [Accessed June 23,2015];List of confused drug names. Available at: http://www.ismp.org.
- 17.US Pharmacopeia. [Accessed June 23, 2015];8th annual MEDMARX report indicates look-alike/ sound-alike drugs lead to thousands of medication errors nationwide. 2008 Available at: http://www.drugs.com/news/u-s-pharmacopeia-8th-annual-medmarx-report-indicates-look-alike-sound-alike-lead-thousands-errors-7666.html.
- 18.Galanter WL, Bryson ML, Falck S, et al. Indication alerts intercept drug name confusion errors during computerized entry of medication orders. PLoS One. 2014;9:e101977. doi: 10.1371/journal.pone.0101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.