Abstract
Context:
For individuals with patellofemoral pain (PFP), altered muscle activity and pain are common during functional tasks. Clinicians often seek interventions to improve muscle activity and reduce impairments. One intervention that has not been examined in great detail is electrical stimulation.
Objective:
To determine whether a single patterned electrical neuromuscular stimulation (PENS) treatment would alter muscle activity and pain in individuals with PFP during 2 functional tasks, a single-legged squat and a lateral step down.
Design:
Cohort study.
Setting:
Sports medicine research laboratory.
Patients of Other Participants:
A total of 22 individuals with PFP (15 women, 7 men; age = 26.0 ± 7.9 years, height = 173.8 ± 8.1 cm, mass = 75.1 ± 17.9 kg).
Intervention(s):
Participants were randomized into 2 intervention groups: a 15-minute PENS treatment that produced a strong motor response or a 15-minute 1-mA subsensory (sham) treatment.
Main Outcome Measure(s):
Before and immediately after the intervention, we assessed normalized electromyography amplitude, percentage of activation time across functional tasks, and onset of activation for the vastus medialis oblique, vastus lateralis, gluteus medius, adductor longus, biceps femoris, and medial gastrocnemius muscles during a single-legged squat and a lateral step down. Scores on the visual analog scale for pain were recorded before and after the intervention.
Results:
After a single treatment of PENS, the percentage of gluteus medius activation increased (0.024) during the lateral step down. Visual analog scores decreased during both the single-legged squat (PENS: preintervention = 2.7 ± 1.9, postintervention = 0.9 ± 0.7; sham: preintervention = 3.2 ± 1.6, postintervention = 2.8 ± 1.9; group × time interaction: P = .041) and lateral step down (PENS: preintervention = 3.4 ± 2.4, postintervention = 1.1 ± 0.8; sham: preintervention = 3.9 ± 1.7, postintervention = 3.3 ± 2.0; group × time interaction: P = .023). No changes in electromyography or pain measures were noted in the sham group.
Conclusions:
The PFP participants who received PENS had immediate improvement in gluteus medius activation and a reduction in pain during functional tasks.
Key Words: anterior knee pain, myoelectric stimulation, therapeutic modalities
Key Points
Patterned electrical neuromuscular stimulation produced an immediate decrease in pain that was greater than the minimal clinically important difference in both a single-legged squat task and a lateral step-down task.
Gluteus medius percentage of activation increased by 100% during the lateral step-down task after a single 15-minute patterned electrical neuromuscular stimulation treatment.
Increased quadriceps activation and decreased pain during the lateral step down were strongly correlated.
Patellofemoral pain (PFP) is a common knee condition that often requires medical treatment.1,2 It accounted for more than 25% of all knee-related injury diagnoses in sports medicine facilities2 and 16% of all knee pain in patients at a running clinic.3 However, the true prevalence of those experiencing PFP within the general population is unknown.4,5 Individuals with PFP commonly have pain during a variety of activities, such as running, jumping, and squatting, and with any pressure on the patella.6,7 These limitations often result in decreased function secondary to pain and can have a profound effect on quality of life.8
The exact cause of PFP is unknown, so treating this chronic condition can be a challenge for clinicians. Knowing the specific cause of PFP would enable clinicians to prescribe treatment plans and identify risk factors.1 Many proposed causes have been examined, such as anatomical,9,10 soft tissue restriction,9 decreased lower extremity strength,11,12 and muscle-activation patterns as measured by electromyography (EMG).13,14 Electromyography has received a great deal of attention recently, with researchers identifying altered firing patterns of the vastus medialis oblique (VMO), vastus lateralis (VL), gluteus medius (GM), and adductor longus (AL) during functional tasks.13–16 When compared with a healthy control group, individuals with PFP demonstrated a delay in the time of activation and decreased duration of activation and EMG amplitude in various lower extremity muscles during functional tasks.15,17,18 Interventions to address the altered EMG patterns have produced favorable results, such as decreased pain and improved self-reported function.17,19
Clinicians have intervened with respect to many factors suggested to be associated with PFP, including soft tissue restriction, muscle weakness, and patellar maltracking. However, addressing these potential causes has produced mixed results.1,9,11 Common treatments are quadriceps strengthening, GM strengthening,20,21 patellar taping,22 and foot orthotics.10 Although multiple treatment options exist, the recurrence rate of PFP has been reported23 to be as high as 91%. This high rate of recurrence has led researchers to shift their focus to other potential interventions to improve pain and function. The GM has been targeted recently due to its role in hip abduction and external rotation.20,21 The GM is believed to maintain optimal kinematic positions during functional tasks and to minimize excessive forces placed on the patellofemoral joint.24,25 Strengthening the GM has increased hip-abduction torque but has not produced much improvement in the performance of biomechanical tasks.20,26 This result raises the possibility that other factors underlying neuromuscular control may be responsible for the inconsistent improvements during functional tasks for those with PFP.27
One possible factor that has not been addressed as a way to improve functional movements and decrease pain is an intervention that attempts to correct the abnormal muscle-firing pattern. Electrical stimulation has been used to create an electrically induced muscular contraction but often only with the goal of pain modulation and strength gains. Patterned electrical neuromuscular stimulation (PENS) is a novel form of electrical stimulation that creates a precise pattern of muscular contractions that replicates EMG activity derived from a healthy individual's firing patterns during functional activities.28,29 Using a triphasic pattern, PENS mimics voluntary movement patterns: rhythmic stimulation to the agonist muscle, the antagonist muscle, and the agonist muscle again.28 This rhythmic contraction has been theorized to replicate spinal alterations that occur during movement from the stimulation of muscle stretch receptors and motor neurons.30 Patterned electrical neuromuscular stimulation has been used in previous studies to improve functional tasks such as vertical jump height, but the literature31 on this new device is limited. We do not know whether using PENS would be advantageous in individuals with abnormal EMG patterns to reestablish optimal patterns. Therefore, the purpose of our research was to determine whether a single PENS treatment could alter EMG activity during functional movements in individuals with PFP.
METHODS
Design
This study was a double-blinded, randomized laboratory investigation. The dependent variables were EMG activity of 6 lower extremity muscles and pain during 2 functional tasks. Independent variables were treatment group (PENS and sham) and time (preintervention and postintervention).
Participants
Volunteers were recruited from the university and local community and via referral from local physicians' offices. A convenience sample of 22 participants (15 women, 7 men; age = 26.0 ± 7.9 years, height = 173.8 ± 8.1 cm, and mass = 75.1 ± 17.9 kg) with PFP were enrolled within a larger single-intervention study that examined strength, EMG, and kinematics during functional tasks (Table 1). Participants were enrolled in the study if they were between 15 and 65 years old, had atraumatic knee pain lasting longer than 3 months, and had pain with more than 2 of the following activities: jumping, kneeling, prolonged sitting, quadriceps contraction, running, squatting, or stair climbing or when pressure was placed on the patella.7,32 Recruits were required to score less than 85 of 100 on the Anterior Knee Pain Scale.24 Exclusion criteria were previous knee surgery, ligamentous instability, meniscal injury, or other sources of anterior knee pain, such as patellar tendinitis, bursitis, or patella subluxation.17 A single researcher (N.R.G.) conducted the physical examinations to identify possible exclusion criteria before participants enrolled in the study. We also excluded participants if they had contraindications for electrical stimulation, including an implanted biomedical device, neuropathy, muscular abnormality, or hypersensitivity or active infection where the electrodes would be placed.33 Participants who presented with bilateral PFP selected the most affected knee for testing. The study received approval from the institutional review board, and all participants provided written informed consent before enrollment.
Table 1. .
Anthropometric Characteristics
| Characteristic |
Group |
P Value |
|
| Patterned Electrical Neuromuscular Stimulation (n = 11) |
Sham (n = 11) |
||
| Sex | 8 women, 3 men | 7 women, 4 men | |
| Mean ± SD |
|||
| Age, y | 25.1 ± 7.3 | 26.4 ± 8.7 | .69 |
| Height, cm | 170.9 ± 7.9 | 176.7 ± 7.5 | .10 |
| Mass, kg | 68.6 ± 9.2 | 81.5 ± 22.3 | .09 |
| Anterior Knee Pain Scale score (maximum = 100) | 70.4 ± 11.4 | 72.5 ± 9.2 | .64 |
| Visual analog scale score (range, 0–10) | 1.9 ± 1.4 | 1.9 ± 0.9 | .89 |
Testing Procedures
Volunteers reported to the laboratory to be screened for inclusion. Those who met the inclusion criteria and had none of the exclusion criteria were enrolled in the study. They were allowed to warm up for 5 minutes on a treadmill at a self-selected pace and were provided 5 minutes to perform any lower extremity stretching they desired.
We then prepared the skin for EMG electrode placement, which included shaving, debriding, and cleaning with isopropyl alcohol.34 Surface EMG electrodes were used to measure the muscle activity of the VMO, VL, GM, AL, biceps femoris (BF), and medial gastrocnemius (MG).15,34 Parallel bar electrodes were placed over the muscle bellies of the VMO, VL, GM, BF, and MG as described by Hermens et al34 and over the AL as described by Aminaka et al.15 Using an oscilloscope, we visually confirmed appropriate electrode placement during both quiet sitting and a maximum voluntary isometric contraction (MVIC) with manual muscle testing. A 16-channel Trigno wireless EMG system with surface EMG electrode sensors (Delsys, Inc, Natick, MA) was used to measure muscle activity at baseline and during the functional tasks via MotionMonitor software (Innovative Sports Training, Inc, Chicago, IL). Data were collected at a 2000-Hz sampling rate, with a 10- to 500-Hz band-pass filter, a 60-Hz notch filter, and a common-mode rejection rate of 80 db.
After placement of the EMG electrodes, the participant performed MVICs for hip abduction, hip adduction, hip external rotation, hip extension, knee extension, and ankle plantar flexion. We positioned a handheld dynamometer (model ACP MMT; Accelerated Care Plus Corporation, Reno, NV) on the distal segment of each limb and outlined the location with a permanent marker. This outlined location was used both to standardize testing location and to measure the distance of the external moment arm to the proximal joint center. Manual muscle testing with the “make” method was conducted for all MVICs except for hip abduction.35 Hip-abduction MVIC was performed in a side-lying position, with the leg abducted 10° and supported by a pillow between the participant's legs.12 Stabilization straps were placed over the lateral trunk and just proximal to the lateral joint line, the latter with enough slack to accommodate the handheld dynamometer between the strap and the distal aspect of the leg.12 Participants were given verbal instructions and allowed 1 practice trial. Three 5-second MVIC trials were conducted with a 15-second rest between trials; the handheld dynamometer and EMG data were recorded simultaneously.12 Participants were provided verbal encouragement during the testing. Additional trials were conducted if the dynamometer force measures varied by more than 10%.13
After all the MVIC trials, participants stood with feet shoulder-width apart for a quiet-standing measurement that was used to normalize both the percentage-of-activation and onset-of-activation analyses.18 Participants were then instructed on how to perform 2 functional tasks, a single-legged squat and a lateral step down. They were given verbal instructions for both tasks and were provided no more than 3 practice trials of each. To perform the single-legged squat, the participant was instructed to stand on the injured leg and squat so that the knee was flexed to more than 60° and then return to the starting position.13 He or she was also instructed to maintain the nonstanding limb at 90° of knee flexion for the duration of the task. The time to perform the task was standardized with a metronome to create a 4-second time period: 2 seconds to lower and 2 seconds to return to the starting position.13 The task was valid if the participant performed the entire task at the designated speed without losing balance.36 Five valid trials were collected, with a 1-minute rest period provided between trials. For the lateral step-down task, the participant stood on a step that was normalized to 10% of his or her height, lowered himself or herself until the contralateral heel touched a force plate, and then returned to the starting position. A 4-second time period was also used for this task.
Patterned electrical neuromuscular stimulation was administered using the Omnistim FX2 Pro Sport Electrotherapy system (Accelerated Care Plus Corporation) in a biphasic asymmetric square-wave pattern of 50-Hz frequency, 70-microsecond phase duration, and 200-millisecond stimulus train. Two channels were used to deliver alternating patterns, mimicking the agonist-antagonist-agonist pattern that is seen in healthy people during functional tasks.28,29 Channel 1 consisted of two 4- × 6-in (10.16 × 15.24-cm) electrodes positioned over the VMO and GM, whereas channel 2 consisted of two 4- × 6-in electrodes positioned over the middle of the adductor muscle group and the middle of the hamstrings muscle group (Figure 1). The patterned stimulus was a 200-millisecond contraction to channel 1, a 200-millisecond contraction to channel 2, and finally a 120-millisecond contraction to channel 1. Participants were positioned on a treatment table with the hip and knee flexed to approximately 90° for the 15-minute treatment.
Figure 1. .
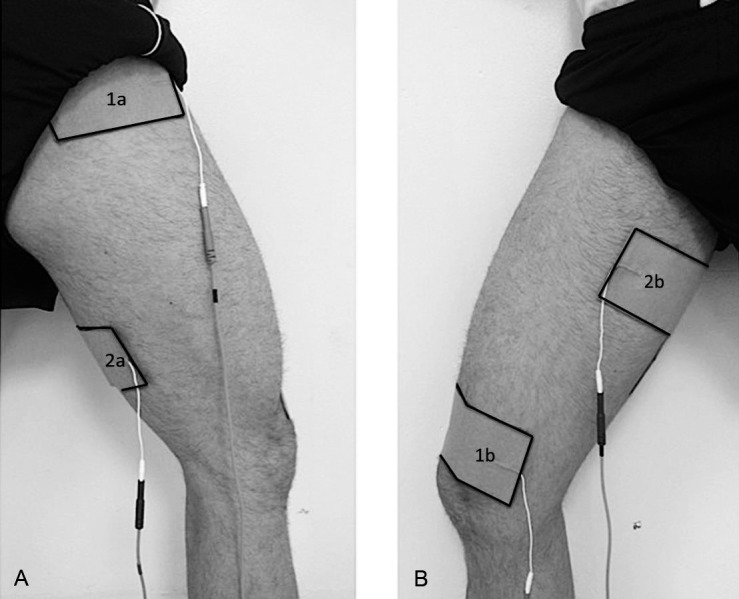
Electrode placement for patterned electrical neuromuscular stimulation. Channel 1: 1a, gluteus medius; 1b, vastus medialis oblique. Channel 2: 2a, hamstrings muscle group; 2b, adductor muscle group. A, Posterolateral view. B, Anteromedial view.
After each task, the participant described his or her pain levels (1) while completing the task and (2) immediately after the task using a visual analog scale (VAS). Once all preintervention data were collected, the primary researcher placed the PENS electrodes on the participant's limb and left the laboratory. Another researcher entered the laboratory and delivered either a PENS or a sham treatment. A third party concealed treatment interventions in envelopes, which were randomly allocated to participants before enrollment. Those assigned to the PENS group received a 15-minute treatment that resulted in a strong motor contraction visible to the researcher. For the sham treatment, the Omnistim FX2 Pro Sport Electrotherapy system with the identical settings was applied; however, the group received a 1-mA amplitude treatment, which is the minimal stimulus available with the unit. We selected this amplitude to allow the unit display, visible lights, and timer to replicate a true treatment, even though no participant could perceive stimulation. The sham treatment also lasted for 15 minutes, and participants were instructed that they were receiving a “subsensory” treatment. At the end of the intervention, the primary researcher reentered the laboratory and removed the PENS electrodes. Five additional single-legged squats and lateral step-down tasks were performed, as previously described, to collect EMG data and VAS scores for both tasks.
Data Processing
Before processing, we reduced the data for both the single-legged squat and lateral step down to 100 data points, which were normalized to the percentage of each task.37 For every trial, virtual event markers were manually inserted using the MotionMonitor software for both tasks when participants initiated knee flexion and ended with terminal extension. The data between those time points for each individual trial were exported, and the middle 3 trials were used for data processing.38 All data were collected and filtered using the MotionMonitor software, and 2 files containing all collected data were exported. To maintain the accuracy of the data, the files underwent processing by both EMGworks acquisition (version 4.1.1; Delsys, Inc) and AcqKnowledge (version 4; Biopac Systems, Inc, Goleta, CA) software.
Amplitude
Data from the middle 3 trials were averaged for both tasks. We calculated the root mean square using a 10-sample moving average and the area under the curve.39 The amplitude of both tasks was normalized to a 4-second MVIC assessment for each individual muscle because the 2 tasks were standardized to 4 seconds each. The EMGworks acquisition software was used to process the amplitude data.
Percentage of Activation
For percentage of activation, the mean and standard deviation for each individual muscle were identified during a 50-millisecond epoch of quiet standing. During a functional task, a muscle was determined to be “on” when the signal exceeded 5 standard deviations above the quiet-standing mean for at least 0.25 milliseconds.40 The average amount of time was calculated to obtain the percentage of the task for which the muscle exceeded the activation threshold. This value was averaged for the middle 3 trials of both tasks. We used AcqKnowledge software to process the data.
Onset of Activation
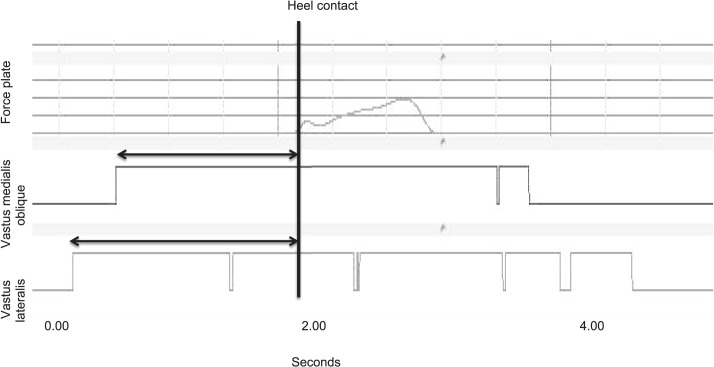
The VL and VMO onsets of activation were calculated for the lateral step-down task.39 The epoch for both the VMO and VL in the test limb was identified when the signal exceeded the activation threshold (5 standard deviations above the quiet-standing mean) before heel contact of the contralateral limb with the force plate39,40 (Figure 2). The duration of time between heel contact and the onset of activation was recorded as a positive value.18 The difference between the VMO and VL was used to identify timing differences between the muscles, with a negative value indicating a delay in the VMO onset of activation and a positive value indicating preactivation of the VMO.18 AcqKnowledge software was used for data processing.
Figure 2. .
Calculation of onset of muscle activation.
Pain
Participants placed a vertical mark on a 10-cm VAS line for the pain they experienced during both the preintervention and postintervention tasks. We measured the distance between the participant's mark and the start of the scale to obtain the pain level.
Statistical Analysis
The independent variable was treatment (PENS or sham). Outcome measures were EMG amplitude, EMG percentage of activation for the 6 muscles, onset of activation for the VMO and VL, and VAS scores during the 2 functional tasks. Independent repeated-measures analyses of variance for EMG measures and VAS scores between treatment groups were performed between the time points for the single-legged squat and lateral step-down task. We set the significance level a priori at P < .05. Post hoc analysis with the Tukey honestly significant difference test was performed on statistically significant findings. Correlations between the 6 muscle activations of interest and pain levels were also conducted for both functional tasks.
We calculated Cohen d effect sizes with 95% confidence intervals comparing preintervention and postintervention means with a pooled standard deviation. Effect sizes were interpreted as <0.20, trivial; 0.49 to 0.20, small; 0.79 to 0.50, moderate; and ≥0.80, large.41 Effect sizes were calculated for root mean square amplitude, percentage of activation, onset and duration of activation, and VAS pain scores. The signs of VAS pain scores were modified so that a positive effect size was in favor of the PENS group.
RESULTS
The preintervention (baseline) data are presented in Table 1. No differences were identified in baseline scores between the groups for amplitude, duration of activation, onset of activation, or VAS scores.
Amplitude
A time main effect in the sham group was found for the BF (P = .03; Table 2). No other statistical differences were noted for the single-legged squat or lateral step down (Table 3) between groups after the intervention.
Table 2. .
Single-Legged–Squat Root Mean Square Amplitude
| Muscle |
Group (Mean ± SD) |
P Value |
Effect Size (95% Confidence Interval) |
|||||
| Patterned Electrical Neuromuscular Stimulation |
Sham |
|||||||
| Preintervention |
Postintervention |
Preintervention |
Postintervention |
Time Main Effect |
Group Main Effect |
Group × Time Interaction |
||
| Vastus medialis oblique | 0.63 ± 0.27 | 0.63 ± 0.30 | 0.69 ± 0.56 | 0.65 ± 0.43 | .53 | .81 | .58 | 0.09 (−0.75, 0.93) |
| Vastus lateralis | 0.64 ± 0.37 | 0.69 ± 0.42 | 0.54 ± 0.29 | 0.53 ± 0.32 | .75 | .36 | .59 | 0.18 (−0.66, 1.02) |
| Gluteus medius | 0.23 ± 0.18 | 0.20 ± 0.14 | 0.27 ± 0.17 | 0.23 ± 0.15 | .17 | .60 | .89 | 0.06 (−0.78, 0.89) |
| Adductor longus | 0.35 ± 0.36 | 0.33 ± 0.27 | 0.33 ± 0.24 | 0.28 ± 0.21 | .31 | .80 | .65 | 0.10 (−0.74, 0.93) |
| Biceps femoris | 0.13 ± 0.09 | 0.09 ± 0.08 | 0.23 ± 0.24 | 0.10 ± 0.06 | .03a | .29 | .20 | 0.50 (−0.35, 1.35) |
| Medial gastrocnemius | 0.50 ± 0.50 | 0.41 ± 0.34 | 0.25 ± 0.13 | 0.26 ± 0.16 | .34 | .13 | .17 | −0.27 (−1.11, 0.57) |
Significant effect.
Table 3. .
Lateral Step-Down Root Mean Square Amplitude
| Muscle |
Group (Mean ± SD) |
P Value |
Effect Size (95% Confidence Interval) |
|||||
| Patterned Electrical Neuromuscular Stimulation |
Sham |
|||||||
| Preintervention |
Postintervention |
Preintervention |
Postintervention |
Time Main Effect |
Group Main Effect |
Group × Time Interaction |
||
| Vastus medialis oblique | 0.19 ± 0.14 | 0.17 ± 0.11 | 0.14 ± 0.14 | 0.13 ± 0.12 | .54 | .43 | .74 | −0.07 (−0.91, 0.76) |
| Vastus lateralis | 0.35 ± 0.26 | 0.38 ± 0.29 | 0.24 ± 0.19 | 0.21 ± 0.22 | .97 | .18 | .34 | 0.26 (−0.58, 1.10) |
| Gluteus medius | 0.58 ± 0.51 | 0.55 ± 0.46 | 0.57 ± 0.39 | 0.51 ± 0.50 | .30 | .89 | .71 | 0.06 (−0.77, 0.90) |
| Adductor longus | 0.94 ± 1.16 | 0.88 ± 0.89 | 0.63 ± 0.50 | 0.53 ± 0.44 | .47 | .32 | .83 | 0.04 (−0.67, 0.88) |
| Biceps femoris | 0.24 ± 0.24 | 0.25 ± 0.27 | 0.18 ± 0.08 | 0.16 ± 0.09 | .93 | .38 | .36 | 0.17 (−0.67, 1.00) |
| Medial gastrocnemius | 1.43 ± 1.57 | 1.15 ± 1.10 | 0.54 ± 0.43 | 0.49 ± 0.40 | .17 | .07 | .31 | −0.20 (−1.04, 0.64) |
Percentage of Activation
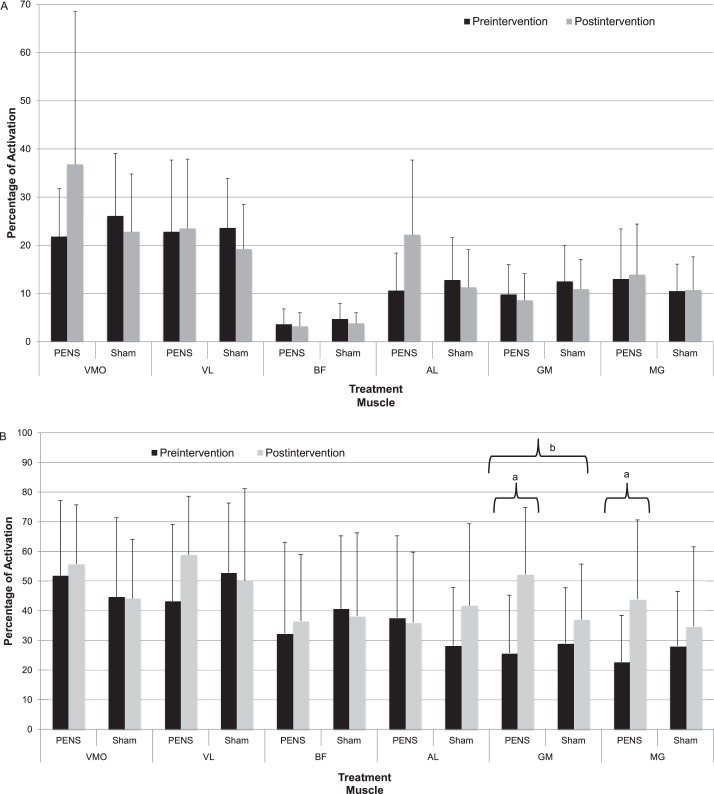
We observed no differences for percentage of activation during the single-legged squat (Figure 3A). The GM percentage of activation increased during the lateral step down in the PENS group (Figure 3B). A group × time interaction occurred for the GM (PENS: preintervention = 25.7% ± 19.5%, postintervention = 52.2% ± 22.6%; sham: preintervention = 29.0% ± 18.7%, postintervention = 37.0% ± 18.7%; P = .024), with the PENS group demonstrating a 102% increase in activation. Time main effects occurred for the GM (preintervention = 22.7 ± 15.7, postintervention = 52.2 ± 26.8; P = .01) and the MG (preintervention = 22.7 ± 15.7, postintervention = 43.8 ± 26.8; P < .001) for the PENS group. During the single-legged squat, we found large effect sizes in the VMO (1.58; 95% confidence interval [CI] = 0.62, 2.53), GM (0.96; 95% CI = 0.09, 1.85), and AL (1.45; 95% CI = 0.52, 2.40), which did not cross zero, in favor of the PENS treatment (Table 4).
Figure 3. .
Percentage of activation during functional tasks. A, Single-legged squat. B, Lateral step down. a Time main effect (P < .05). b Group × time interaction (P < .05). Abbreviations: AL, adductor longus; BF, biceps femoris; GM, gluteus medius; MG, medial gastrocnemius; PENS, patterned electrical neuromuscular stimulation; VL, vastus lateralis; VMO, vastus medialis oblique.
Table 4. .
Percentage of Electromyographic Activation During the Single-Legged Squat and Lateral Step-Down Tasks
| Muscle |
Task, Effect Size (95% Confidence Interval) |
|
| Single-Legged Squat |
Lateral Step Down |
|
| Vastus medialis oblique | 1.58 (0.62, 2.53) | 0.17 (−0.67, 1.01) |
| Vastus lateralis | 0.30 (−0.54, 1.15) | 0.74 (−0.12, 1.61) |
| Gluteus medius | 0.12 (−0.72, 0.95) | 0.97 (0.09, 1.85) |
| Adductor longus | 1.45 (0.52, 2.40) | −0.64 (−1.49, 0.22) |
| Biceps femoris | 0.15 (−0.68, 0.99) | 0.24 (−0.60, 1.08) |
| Medial gastrocnemius | 0.08 (−0.75, 0.92) | 0.85 (−0.02, 1.73) |
Onset of Activation
The onset of activation of the quadriceps muscles and the VMO duration of activation did not differ after the treatment (Table 5). Timing of the VMO/VL delay also did not change. A group × time interaction was found for the VL duration of activation during the lateral step down (P = .04). A large effect size was seen for the VL duration of activation in favor of PENS (0.91; 95% CI = 0.03, 1.78).
Table 5. .
Quadriceps Muscles Onset and Duration of Activation (Milliseconds) During the Lateral Step Down Before and After Patterned Electrical Neuromuscular Stimulation
| Muscle |
Onset or Duration of Activation, ms |
Group (Mean ± SD) |
P Value |
Effect Size (95% Confidence Interval) |
|||||
| Patterned Electrical Neuromuscular Stimulation |
Sham |
||||||||
| Preintervention |
Postintervention |
Preintervention |
Postintervention |
Time Main Effect |
Group Main Effect |
Group × Time Interaction |
|||
| Vastus medialis oblique | Onset | 0.46 ± 0.19 | 0.52 ± 0.22 | 0.40 ± 0.12 | 0.45 ± 0.18 | .14 | .42 | .86 | 0.19 (−0.65, 1.03) |
| Duration | 0.92 ± 0.49 | 1.2 ± 0.48 | 0.86 ± 0.38 | 0.99 ± 0.48 | .07 | .41 | .48 | 0.34 (−0.50, 1.18) | |
| Vastus lateralis | Onset | 0.50 ± 0.15 | 0.55 ± 0.23 | 0.58 ± 0.32 | 0.47 ± 0.25 | .39 | .97 | .07 | 0.64 (−0.22, 1.50) |
| Duration | 0.95 ± 0.45 | 1.28 ± 0.47 | 1.17 ± 0.52 | 1.06 ± 0.61 | .29 | .98 | .04a | 0.91 (0.03, 1.78) | |
| Vastus medialis oblique/vastus lateralis delay | −0.04 ± 0.10 | −0.03 ± 0.08 | −0.17 ± 0.28 | −0.04 ± 0.09 | .14 | .17 | .25 | −0.57 (−1.42, 0.28) | |
Significant group × time interaction.
Pain
We saw no differences in baseline pain or preintervention pain for the tasks. A group × time interaction was noted in the VAS scores for both the single-legged squat (P = .041) and lateral step down (P = .023; Figure 4). Those individuals who received the PENS treatment had a 65.3% reduction in pain (preintervention = 2.7 ± 1.9, postintervention = 0.9 ± 0.7) during the single-legged squat compared with the sham group's 13.6% reduction (preintervention = 3.2 ± 1.6, postintervention = 2.8 ± 1.9; Figure 4A). The PENS group also had a 67.4% reduction in pain during the lateral step down (preintervention = 3.4 ± 2.4, postintervention = 1.1 ± 0.8), compared with a 16.6% reduction in the sham group (preintervention = 3.9 ± 1.7, postintervention = 3.3 ± 2.0; Figure 4B). Time main effects for pain scores occurred for both the single-legged squat and lateral step down (P = .001 and P < .001, respectively). We also found large effect sizes for VAS pain scores in favor of the PENS treatment during both the single-legged squat (0.90; 95% CI = 0.03, 1.79) and lateral step down (0.98; 95% CI = 0.10, 1.87).
Figure 4. .
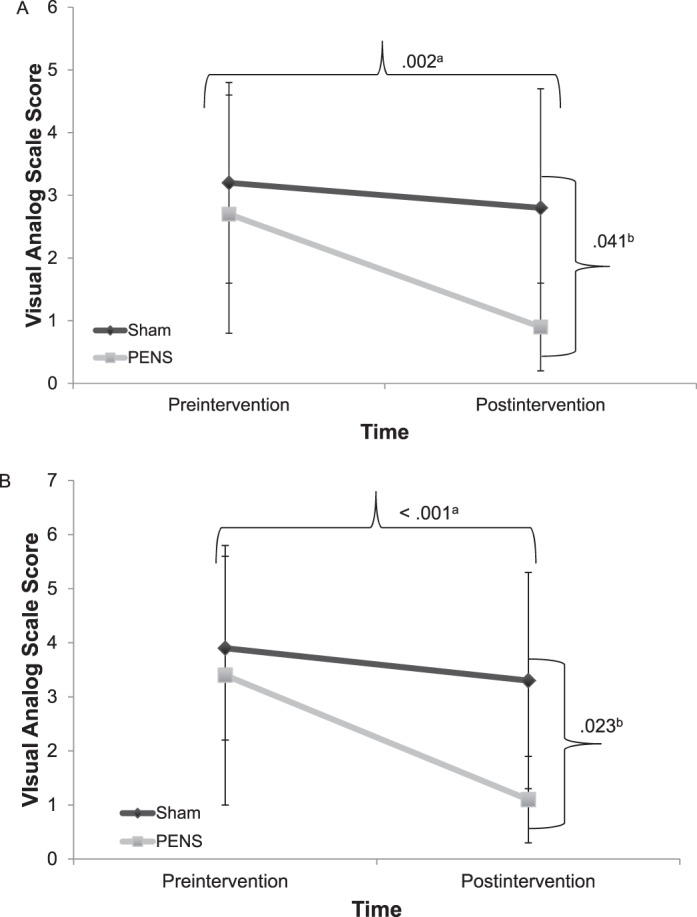
Preintervention and postintervention visual analog scores during functional tasks. A, Single-legged squat. B, Lateral step down. a Time main effect (P < .05). b Group × time interaction (P < .05). Abbreviation: PENS, patterned electrical neuromuscular stimulation.
Correlations between the activation change scores for the individual muscles and the change scores for pain during both activities were examined. Improved activation of the VMO produced a nearly significant moderately negative correlation with pain during the lateral step down (r = −0.419, P = .05). A moderate negative correlation was also seen with the VL activation and pain scores during the single-legged squat (r = −0.403, P = .06).
DISCUSSION
The purpose of our study was to assess the immediate effect of PENS on lower extremity muscle activation and pain during functional activities in individuals with PFP. We hypothesized that those in the PENS group would display increased activation of the VMO, VL, and GM muscle groups and decreased pain during both functional tasks. Our results confirmed that a single PENS treatment did improve the GM percentage of activation and the VL duration of activation during the lateral step-down task and resulted in decreased pain during both activities.
Although smaller studies have shown conflicting results regarding altered lower extremity EMG patterns in patients with PFP during functional tasks, systematic reviews14,16 support the existence of altered firing patterns. This discrepancy may be due to the participants enrolled within the studies, given that those with PFP were heterogeneous in their symptom presentation and functional limitations. Our participants demonstrated a delay in the VMO during the lateral step down, which may suggest the importance of both subjective and objective screening criteria. Boling et al17 observed that those with PFP activated the VMO 36 milliseconds after the VL, compared with the healthy group, which preactivated the VMO by 59 milliseconds. They also found that a 6-week rehabilitation program improved VMO preactivation by 39 milliseconds in the pathologic group and reduced their pain levels by the fourth week.17 Even though we did not show a statistical improvement in VMO preactivation with a single treatment session, we did note a reduction in pain levels. The weight-bearing demands between a lateral step-down task and true stair ambulation (Boling et al17) may reflect a task-dependent difference.17 Familiarity with the tasks may also influence the strategies participants use to complete them; stair ambulation is a fairly common daily activity, whereas the lateral step-down task is not. In addition, a moderate negative correlation was present between the change in VMO and VL activity and pain reduction during the lateral step down.42 These correlations may indicate the value of improving muscle activity in those with PFP to help decrease pain during functional activities. However, quadriceps strengthening should not be the only focus during clinical intervention.
The AL and MG have received attention for their potentially negative roles in individuals with PFP.15,43 Soft tissue restrictions in both muscles have been theorized to incidentally increase hip adduction, which can increase patellofemoral joint force.43 Although we found no group × time interaction for either muscle, we demonstrated a time main effect for MG percentage of activation during the lateral step down after PENS. These results are interesting because the MG did not receive any direct electrical stimulation, suggesting that changes in movement strategies may be due to improved proximal muscle activation. We did not calculate kinematic values, but increasing hip-abductor activation may help to maintain a more optimal hip-abducted position during the task and thereby influence distal muscle activation. Aminaka et al15 observed earlier hip-adductor activation during ascending stair ambulation in the asymptomatic limb than in the symptomatic limb. Although they did not find differences during stair descent, they identified earlier activation than in healthy individuals and theorized that the muscle acts as a pulley and alters the VMO's mechanical efficiency.15 Additional research is needed on the role of these muscles during other functional tasks that are often problematic for individuals suffering from PFP.
To our knowledge, no authors have examined functional EMG activity after neuromuscular electrical stimulation (NMES) in individuals with PFP. Studies of NMES and EMG activity in other conditions have produced conflicting results.44,45 Arya et al44 assessed surface EMG during gait in individuals with cerebral palsy and found no changes in the EMG activity of the quadriceps femoris or tibialis anterior. However, Chisari et al45 identified an abnormal EMG pattern in individuals with myotonic dystrophy and noted an improved firing pattern during functional tasks after electrical stimulation. Although PFP and myotonic dystrophy have different symptoms and disease progressions, they may both present with abnormal muscle-firing patterns. Using electrical stimulation to improve motor impairments is a possible step for clinical intervention, and researchers should examine its role in improving muscle function. Altered neuromuscular control may be a reason why strength training alone has not produced consistent kinematic results during functional activities in those with PFP.27
The use of NMES in individuals with PFP is limited to the quadriceps muscles, primarily the VMO. Of the 3 groups46–48 that have used NMES, 2 demonstrated less pain reduction than we did and 1 saw a greater pain reduction. Callaghan et al47 conducted 2 studies to evaluate 2 forms of electrical stimulation to the quadriceps in individuals with PFP. The first47 looked at daily treatments of 2 pulse durations (20 and 250–350 microseconds) over 6 weeks and found decreases of 1.2 and 1.5, respectively, in VAS scores. In the second study,46 Callaghan and Oldham administered 60-minute daily stimulus treatments over 6 weeks and observed a 33% reduction in pain in those individuals with PFP; however, the VAS change scores accounted for only 1 unit. Bily et al48 were among the investigators who noted greater pain relief; however, they examined 1 group that received standard physical therapy (reduction of 2.8 units) and 1 group that received physical therapy and electrical stimulation (reduction of 3.4 units). Yet it is difficult to ascertain whether the pain reduction was from the electrical stimulation alone or the therapeutic exercise that participants also performed during the 12-week protocol.48
Despite the moderate evidence of NMES' effectiveness in improving strength in patients with PFP, some authors31,49 have suggested that this modality has limitations such as fatigue and decreased functional application. Fatigue is proposed to occur due to the spatially recruited α motor neurons during repetitive electrically stimulated muscular contractions.49 When comparing fatigue with both electrical stimulation units, previous authors have shown a decrease in torque production as high as 20% after NMES49 but no such fatigue after PENS.33 Also, NMES often uses a 1:5 duty cycle of tetanic maximum contraction to rest, which is not functionally applicable. This protocol differs from that of PENS, which can be used during functional tasks, such as a vertical jump, to provide improved motor-neuron recruitment and increase jump height by almost 10%.31
We found a reduction in pain during both the single-legged squat (1.8 cm) and lateral step down (2.3 cm) in the PENS group. These values are greater than the VAS minimal clinically important difference, which is the smallest change that is a meaningful clinical improvement.50 The minimal clinically important difference for VAS scores during activity for individuals with PFP has been previously established as 1.3 cm, which is less than we demonstrated with both functional tasks.51 The difference in the VAS change score between the single-legged squat and lateral step down may reflect the level of difficulty of each task. The immediate decrease in pain from PENS during functional tasks offers an intervention for clinicians who are treating patients with this difficult condition. By decreasing pain, clinicians can progress functional training or increase sets and repetitions during the rehabilitation process. It may also help decrease fear avoidance during activities that are often painful for those with PFP. Fear avoidance has been identified as the strongest predictor of effective rehabilitation outcomes for individuals with PFP.43
Two theories can explain the immediate reduction in pain during tasks that are often difficult for people with PFP. The first is the rhythmic contraction of the electrical stimulation the PENS group received. Rhythmic motor contractions have been identified in motor pain modulation, when A-δ fibers are stimulated and release β lipotropin from the pituitary gland; this breaks down into β endorphins, resulting in pain reduction.52,53 The pain modulation is often seen in motor transcutanueous electrical nerve stimulation (TENS), when a strong motor response is generated.52 Although the settings used in motor TENS (pulse frequency = 2–4 Hz and phase duration = 200–300 microseconds) differ from the PENS settings (pulse frequency = 50 Hz and phase duration = 70 microseconds), the PENS triphasic pattern is a continuous stimulation that results in a strong, rhythmic motor contraction that mimics motor TENS and may be responsible for the immediate decrease in pain. The other possible explanation for the pain reduction is improved muscle activation during the functional tasks. During the single-legged squat, we did observe significant correlations between improved knee-extensor muscle activation and pain reduction and a trend toward significance for muscle activation of the GM and BF. Our findings are similar to those of Lack et al,19 who found a relationship between improved GM muscle activation and self-reported pain during stair ambulation.
Although our results were favorable, we only examined the influence of a single PENS treatment on pain and muscle activity. Therefore, this study is not without limitations. First, our sample size was 22 participants, which may explain why we had large effect sizes in the single-legged squat without any significant P values. Second, there was no difference in mass between the groups, but the mass of the sham group did appear to be greater than that of the PENS group. A difference in mass can influence EMG activity, which may be a potential confounder of our results. Third, the intervention involved a single treatment; long-term experience with this novel form of electrical stimulation is limited and should be addressed. It would be interesting to examine the effects of PENS in conjunction with a rehabilitation program to determine whether greater EMG changes are possible. Pain during functional activities was on the lower end of the VAS scale. It is unclear whether those with more severe pain would have the same beneficial results.
CONCLUSIONS
A single PENS treatment produced an immediate decrease in pain and increase in the GM activation percentage during functional tasks in individuals with PFP. Future researchers should examine the cumulative effect of this modality on pain, muscle activation, and kinematics during functional tasks, which are often problematic for those with PFP.
ACKNOWLEDGMENTS
We thank Luke Donovan, PhD, ATC; Mark Feger, PhD, ATC; and Grant Norte, MEd, ATC, CSCS, OTC, for administering the PENS treatments in the study.
REFERENCES
- 1. . Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011; 6 2: 112– 125. [PMC free article] [PubMed] [Google Scholar]
- 2. . Devereaux MD, Lachmann SM. Patello-femoral arthralgia in athletes attending a sports injury clinic. Br J Sports Med. 1984; 18 1: 18– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002; 36 2: 95– 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. . Witvrouw E, Callaghan MJ, Stefanik JJ, et al. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. Br J Sports Med. 2014; 48 6: 411– 414. [DOI] [PubMed] [Google Scholar]
- 5. . Glaviano NR, Kew M, Hart JM, Saliba S. Demographic and epidemiological trends in patellofemoral pain. Int J Sports Phys Ther. 2015; 10 3: 281– 290. [PMC free article] [PubMed] [Google Scholar]
- 6. . Loudon JK, Wiesner D, Goist-Foley HL, Asjes C, Loudon KL. Intrarater reliability of functional performance tests for subjects with patellofemoral pain syndrome. J Athl Train. 2002; 37 3: 256– 261. [PMC free article] [PubMed] [Google Scholar]
- 7. . Thomee R, Renstrom P, Karlsson J, Grimby G. Patellofemoral pain syndrome in young women, II: muscle function in patients and healthy controls. Scand J Med Sci Sports. 1995; 5 4: 245– 251. [PubMed] [Google Scholar]
- 8. . Heintjes E, Berger M, Bierma-Zeinstra SM, Bernsen RM, Verhaar JA, Koes BW. Exercise therapy for patellofemoral pain syndrome. Cochrane Database Syst Rev. 2003;(4):CD003472. [DOI] [PubMed] [Google Scholar]
- 9. . Rowland B, Brantingham J. The efficacy of patella mobilization in patients suffering from patellofemoral pain syndrome. J Neuromusculoskelet Syst. 1999; 7 pt 4: 142– 149. [Google Scholar]
- 10. . Eng JJ, Pierrynowski MR. Evaluation of soft foot orthotics in the treatment of patellofemoral pain syndrome. Phys Ther. 1993; 73 2: 62– 68. [DOI] [PubMed] [Google Scholar]
- 11. . Bolgla LA, Malone TR, Umberger BR, Uhl TL. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008; 38 1: 12– 18. [DOI] [PubMed] [Google Scholar]
- 12. . Ireland ML, Willson JD, Ballantyne BT, Davis IM. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003; 33 11: 671– 676. [DOI] [PubMed] [Google Scholar]
- 13. . Nakagawa TH, Moriya ET, Maciel CD, Serrão FV. Trunk, pelvis, hip, and knee kinematics, hip strength, and gluteal muscle activation during a single-leg squat in males and females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2012; 42 6: 491– 501. [DOI] [PubMed] [Google Scholar]
- 14. . Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013; 47 4: 207– 214. [DOI] [PubMed] [Google Scholar]
- 15. . Aminaka N, Pietrosimone BG, Armstrong CW, Meszaros A, Gribble PA. Patellofemoral pain syndrome alters neuromuscular control and kinetics during stair ambulation. J Electromyogr Kinesiol. 2011; 21 4: 645– 651. [DOI] [PubMed] [Google Scholar]
- 16. . Chester R, Smith TO, Sweeting D, Dixon J, Wood S, Song F. The relative timing of VMO and VL in the aetiology of anterior knee pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2008; 9: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. . Boling MC, Bolgla LA, Mattacola CG, Uhl TL, Hosey RG. Outcomes of a weight-bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2006; 87 11: 1428– 1435. [DOI] [PubMed] [Google Scholar]
- 18. . Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011; 6 4: 285– 296. [PMC free article] [PubMed] [Google Scholar]
- 19. . Lack S, Barton C, Woledge R, Laupheimer M, Morrissey D. The immediate effects of foot orthoses on hip and knee kinematics and muscle activity during a functional step-up task in individuals with patellofemoral pain. Clin Biomech (Bristol, Avon). 2014; 29 9: 1056– 1062. [DOI] [PubMed] [Google Scholar]
- 20. . Earl JE, Hoch AZ. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011; 39 1: 154– 163. [DOI] [PubMed] [Google Scholar]
- 21. . Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and hip strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012; 42 1: 22– 29. [DOI] [PubMed] [Google Scholar]
- 22. . Aminaka N, Gribble PA. Patellar taping, patellofemoral pain syndrome, lower extremity kinematics, and dynamic postural control. J Athl Train. 2008; 43 1: 21– 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. . Stathopulu E, Baildam E. Anterior knee pain: a long-term follow-up. Rheumatology (Oxford). 2003; 42 2: 380– 382. [DOI] [PubMed] [Google Scholar]
- 24. . Willson JD, Davis IS. Lower extremity strength and mechanics during jumping in women with patellofemoral pain. J Sport Rehabil. 2009; 18 1: 76– 90. [DOI] [PubMed] [Google Scholar]
- 25. . Willson JD, Davis IS. Lower extremity mechanics of females with and without patellofemoral pain across activities with progressively greater task demands. Clin Biomech (Bristol, Avon). 2008; 23 2: 203– 211. [DOI] [PubMed] [Google Scholar]
- 26. . Baldon Rde M, Serrão FV, Scattone Silva R, Piva SR. Effects of functional stabilization training on pain, function, and lower extremity biomechanics in females with patellofemoral pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2014; 44 4: 240– A8. [DOI] [PubMed] [Google Scholar]
- 27. . Willy RW, Davis IS. The effect of a hip-strengthening program on mechanics during running and during a single-leg squat. J Orthop Sports Phys Ther. 2011; 41 9: 625– 632. [DOI] [PubMed] [Google Scholar]
- 28. . Cooke JD, Brown SH. Movement-related phasic muscle activation. II. Generation and functional role of the triphasic pattern. J Neurophysiol. 1990; 63 3: 465– 472. [DOI] [PubMed] [Google Scholar]
- 29. . Hallett M, Shahani BT, Young RR. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975; 38 12: 1154– 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. . McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008; 57 1: 134– 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. . Gulick DT, Castel JC, Palermo FX, Draper DO. Effect of patterned electrical neuromuscular stimulation on vertical jump in collegiate athletes. Sports Health. 2011; 3 2: 152– 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. . Nakagawa TH, Serrão FV, Maciel CD, Powers CM. Hip and knee kinematics are associated with pain and self-reported functional status in males and females with patellofemoral pain. Int J Sports Med. 2013; 34 11: 997– 1002. [DOI] [PubMed] [Google Scholar]
- 33. . Glaviano NR, Langston WT, Hart JM, Saliba S. Influence of patterned electrical neuromuscular stimulation on quadriceps activation in individuals with knee joint injury. Int J Sports Phys Ther. 2014; 9 7: 915– 923. [PMC free article] [PubMed] [Google Scholar]
- 34. . Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000; 10 5: 361– 374. [DOI] [PubMed] [Google Scholar]
- 35. . Kelln BM, McKeon PO, Gontkof LM, Hertel J. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil. 2008; 17 2: 160– 170. [DOI] [PubMed] [Google Scholar]
- 36. . Zeller BL, McCrory JL, Kibler WB, Uhl TL. Differences in kinematics and electromyographic activity between men and women during the single-legged squat. Am J Sports Med. 2003; 31 3: 449– 456. [DOI] [PubMed] [Google Scholar]
- 37. . Nakagawa TH, Moriya ET, Maciel CD, Serrão AF. Frontal plane biomechanics in males and females with and without patellofemoral pain. Med Sci Sports Exerc. 2012; 44 9: 1747– 1755. [DOI] [PubMed] [Google Scholar]
- 38. . Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000; 80 3: 276– 291. [PubMed] [Google Scholar]
- 39. . Earl JE, Hertel J, Denegar CR. Patterns of dynamic malalignment, muscle activation, joint motion, and patellofemoral-pain syndrome. J Sport Rehabil. 2005; 14 3: 216– 234. [Google Scholar]
- 40. . Brindle TJ, Mattacola C, McCrory J. Electromyographic changes in the gluteus medius during stair ascent and descent in subjects with anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 2003; 11 4: 244– 251. [DOI] [PubMed] [Google Scholar]
- 41. . McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond P values. Psychiatry (Edgmont). 2009; 6 10: 21– 29. [PMC free article] [PubMed] [Google Scholar]
- 42. . Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009; 41 1: 3– 13. [DOI] [PubMed] [Google Scholar]
- 43. . Piva SR, Fitzgerald GK, Irrgang JJ, et al. Associates of physical function and pain in patients with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2009; 90 2: 285– 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. . Arya BK, Mohapatra J, Subramanya K, Prasad H, Kumar R, Mahadevappa M. Surface EMG analysis and changes in gait following electrical stimulation of quadriceps femoris and tibialis anterior in children with spastic cerebral palsy. Conf Proc IEEE Eng Med Biol Soc. 2012; 2012: 5726– 5729. [DOI] [PubMed] [Google Scholar]
- 45. . Chisari C, Bertolucci F, Dalise S, Rossi B. Chronic muscle stimulation improves muscle function and reverts the abnormal surface EMG pattern in myotonic dystrophy: a pilot study. J Neuroeng Rehabil. 2013; 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. . Callaghan MJ, Oldham JA. Electric muscle stimulation of the quadriceps in the treatment of patellofemoral pain. Arch Phys Med Rehabil. 2004; 85 6: 956– 962. [DOI] [PubMed] [Google Scholar]
- 47. . Callaghan MJ, Oldham JA, Winstanley J. A comparison of two types of electrical stimulation of the quadriceps in the treatment of patellofemoral pain syndrome: a pilot study. Clin Rehabil. 2001; 15 6: 637– 646. [DOI] [PubMed] [Google Scholar]
- 48. . Bily W, Trimmel L, Modlin M, Kaider A, Kern H. Training program and additional electric muscle stimulation for patellofemoral pain syndrome: a pilot study. Arch Phys Med Rehabil. 2008; 89 7: 1230– 1236. [DOI] [PubMed] [Google Scholar]
- 49. . Zory R, Boerio D, Jubeau M, Maffiuletti NA. Central and peripheral fatigue of the knee extensor muscles induced by electromyostimulation. Int J Sports Med. 2005; 26 10: 847– 853. [DOI] [PubMed] [Google Scholar]
- 50. . Jaeschke R, Singer J, Guyatt GH. Measurement of health status. ascertaining the minimal clinically important difference. Control Clin Trials. 1989; 10 4: 407– 415. [DOI] [PubMed] [Google Scholar]
- 51. . Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Arch Phys Med Rehabil. 2004; 85 5: 815– 822. [DOI] [PubMed] [Google Scholar]
- 52. Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. Pain. 2007; 130 1–2: 157– 165. [DOI] [PubMed] [Google Scholar]
- 53. . Sjolund B, Eriksson M. Electro-acupunture and endogenous morphines. Lancet. 1976; 2 7994: 1085. [DOI] [PubMed] [Google Scholar]