Abstract
Context:
Skeletal muscle fatigue and exercise performance are novel areas of research and clinical application in the photobiomodulation field, and positive outcomes have been reported in several studies; however, the optimal measures have not been fully established.
Objective:
To assess the acute effect of photobiomodulation therapy (PBMT) combining superpulsed lasers (low-level laser therapy) and light-emitting diodes (LEDs) on muscle performance during a progressive cardiopulmonary treadmill exercise test.
Design:
Crossover study.
Setting:
Laboratory.
Patients or Other Participants:
Twenty untrained male volunteers (age = 26.0 ± 6.0 years, height = 175.0 ± 10.0 cm, mass = 74.8 ± 10.9 kg).
Intervention(s):
Participants received PBMT with either combined superpulsed lasers and LED (active PBMT) or placebo at session 1 and the other treatment at session 2. All participants completed a cardiopulmonary test on a treadmill after each treatment. For active PBMT, we performed the irradiation at 17 sites on each lower limb (9 on the quadriceps, 6 on the hamstrings, and 2 on the gastrocnemius muscles), using a cluster with 12 diodes (four 905-nm superpulsed laser diodes with an average power of 0.3125 mW, peak power of 12.5 W for each diode, and frequency of 250 Hz; four 875-nm infrared LED diodes with an average power of 17.5 mW; and four 640-nm red LED diodes with an average power of 15 mW) and delivering a dose of 30 J per site.
Main Outcome Measure(s):
Distance covered, time until exhaustion, pulmonary ventilation, and dyspnea score.
Results:
The distance covered (1.96 ± 0.30 versus 1.84 ± 0.40 km, t19 = 2.119, P < .001) and time until exhaustion on the cardiopulmonary test (780.2 ± 91.0 versus 742.1 ± 94.0 seconds, t19 = 3.028, P < .001) was greater after active PBMT than after placebo. Pulmonary ventilation was greater (76.4 ± 21.9 versus 74.3 ± 19.8 L/min, t19 = 0.180, P = .004) and the score for dyspnea was lower (3.0 [interquartile range = 0.5–9.0] versus 4.0 [0.0–9.0], U = 184.000, P < .001) after active PBMT than after placebo.
Conclusions:
The combination of lasers and LEDs increased the time, distance, and pulmonary ventilation and decreased the score of dyspnea during a cardiopulmonary test.
Key Words: low-level laser therapy, light-emitting diode therapy, fatigue, progressive-intensity exercise
Key Points
Photobiomodulation therapy combines the use of superpulsed lasers and light-emitting diodes.
When applied before a progressive cardiopulmonary exercise test on a treadmill, photobiomodulation therapy increased distance covered, time to exhaustion, and pulmonary ventilation and decreased dyspnea sensation in healthy volunteers.
Skeletal muscle fatigue is characterized by decreased muscle capacity to generate or maintain power production during muscle activity. In submaximal activities, the initial intensity cannot be maintained.1 The complex, multifaceted process of developing muscle fatigue involves various physiologic and biomechanical components,2 including the types of muscle fiber and the intensity and duration of the activity.3
An important factor contributing to muscle fatigue during exercise is the decrease in circulating oxygen available for muscle contraction because physical exercise increases the consumption of oxygen from the circulating blood. An increased arteriovenous oxygen difference results from a more efficient distribution of the cardiac output to the muscles as well as an increased capacity of the muscle cells to consume and use oxygen.4 To meet this high demand for oxygen from the muscles, the cardiovascular system increases the transport of blood through the tissues.
Given the muscles' need for increased oxygen and energy during exercise, cardiorespiratory changes occur. The main cardiorespiratory changes during exercise are increased pulmonary ventilation and increased supply of oxygen to the muscles. Therefore, muscle fatigue during strenuous exercise is often accompanied by shortness of breath.5 During mild to moderate exercise at a steady pace, pulmonary ventilation increases linearly with oxygen consumption (Vॱo2 uptake) and carbon dioxide production. An increase in tidal volume and respiratory rate causes pulmonary ventilation to increase under these conditions.5
Recently, photobiomodulation therapy (PBMT) using low-level lasers or light-emitting diodes (LEDs) has been described as an electrophysical intervention that provides beneficial effects on muscular activity.6–8 Researchers studying the application of PBMT before high-intensity exercises9 have shown an increase in blood-lactate removal and a reduction in muscle injuries resulting in faster muscle recovery,10,11 increased mitochondrial function through the modulation of cytochrome c oxidase,12 and changes in the ionic gradient.13
In many studies,14–16 PBMT has been used as a noninvasive therapeutic modality to optimize vasodilation, improve collateral circulation, increase tissue oxygen levels, and enhance mitochondrial adenosine triphosphate levels. According to investigators,14,17,18 it acts mainly by increasing the local blood flow and neuromuscular activity. In this way, PBMT can be used to prevent and delay muscle fatigue because muscle fatigue results from inadequate tissue perfusion.19,20 In the first clinical study of PBMT and skeletal muscle performance, Leal-Junior et al21 observed that PBMT can delay the onset of muscle fatigue, possibly through local mechanisms such as minimizing oxidative stress or decreasing the production of reactive oxygen species.
Recently, researchers22,23 have shown an increase in performance levels with the use of PBMT during progressive cardiopulmonary exercise tests. However, different PBMT devices result in different effects even with the use of similar exercise protocols to induce fatigue,22–25 which makes it difficult for clinicians to determine which device should be used. Therefore, the purpose of our study was to assess the acute effect of PBMT combining superpulsed lasers and LEDs on muscle performance during a progressive cardiopulmonary treadmill exercise test.
METHODS
Participants
We recruited 20 healthy male volunteers between 18 and 35 years of age with any skin color26 and without training or involvement in a regular exercise program (ie, exercise more than once per week). Volunteers were excluded if they had any skeletal muscle injury, used any nutritional supplement or pharmacologic agent, presented with signs or symptoms of any disease (ie, neurologic, inflammatory, pulmonary, metabolic, oncologic), or had a history of cardiac arrest that might limit performance of high-intensity exercises. All participants provided written informed consent, and the study was approved by the Research Ethics Committee of Nove de Julho University (process 882.738).
Randomization and Blinding Procedures
The order of treatments was randomized. We generated codes to ensure that 50% (n = 10) of our participants received the active treatment at session 1 and 50% (n = 10) received the active treatment at session 2 to counterbalance participants between treatments tested (active and placebo) at the 2 sessions.
Randomization was performed by drawing lots, which we used to determine whether the active combination of superpulsed lasers and LEDs comprising PBMT or the placebo would be given at the first session. At the second session, participants received the treatment that was not given at the first session. Randomization labels were created by using a randomization table at a central office where a series of sealed, opaque, and numbered envelopes ensured confidentiality. A participating researcher (E.C.P.L.-J.) who programmed the PBMT device (Multi Radiance Medical, Solon, OH) based on the randomization results performed the randomization. He was instructed not to inform the participants or other researchers of the PBMT dose (active or placebo). Thus, the researcher who performed the PBMT (A.A.V.) was blinded to the dose given to the volunteers. To further maintain blinding, participants used opaque goggles to prevent them from seeing whether a light was being irradiated.
Study Design and Protocol
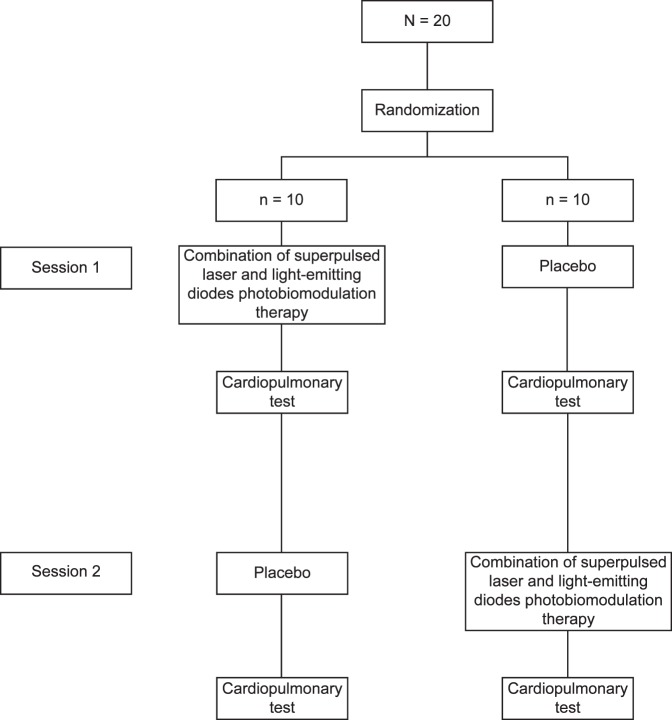
We carried out a crossover, double-blind, placebo-controlled randomized clinical trial. The study was conducted in the Laboratory of Phototherapy in Sports and Exercise at the Nove de Julho University, São Paulo, Brazil. Participants received either PBMT combining superpulsed lasers and LEDs or placebo on 2 visits 1 week apart. A progressive cardiopulmonary exercise test was performed on a motor-driven treadmill (Super ATL, Ibrasport, Porto Alegre, Brazil) 5 to 10 minutes after each therapeutic administration. A summary of the protocol is presented in Figure 1.
Figure 1. .
Protocol flow chart.
Procedures
Photobiomodulation Therapy
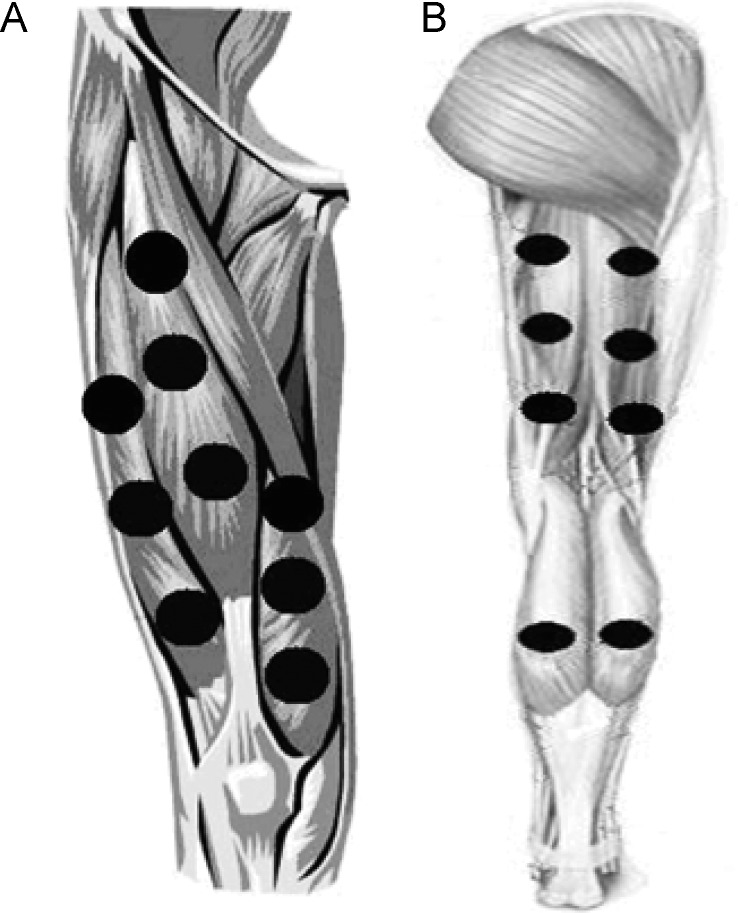
Active PBMT combining superpulsed lasers and LEDs or placebo was administered immediately before the progressive cardiopulmonary exercise test on a treadmill. We used a 12-diode cluster of superpulsed lasers and LEDs. Each cluster consisted of 4 diodes of 905-nm superpulsed laser with an average power of 0.3125 mW and a peak power of 12.5 W for each diode, 4 diodes of 875-nm infrared LEDs with an average power of 17.5 mW for each diode, and 4 diodes of 640-nm red LEDs with an average power of 15 mW for each diode. Given the large area of irradiation, the use of clusters was fundamental to application of the therapy. We applied the PBMT with the cluster in direct contact with the skin at 9 sites on the knee-extensor muscles (Figure 2A), 6 sites on the knee flexors, and 2 sites on the calf (Figure 2B).23 The same procedures were performed to administer the placebo but without effective irradiation.
Figure 2. .
A, Treatment points in knee extensor muscles. B, Treatment points in knee-flexor and ankle plantar-flexor muscles. Adapted from Lasers in Medical Science, Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress, volume 27, 2012, 231–236, Thiago De Marchi, Ernesto Cesar Pinto Leal Junior, Celiana Bortoli, Shaiane Silva Tomazoni, Rodrigo Álvaro Brandão Lopes-Martins, Marian Salvador, © Springer-Verlag London Ltd 2011, With permission of Springer.
We chose the PBMT measures based on previous studies24,27 that our research group has performed using the same device. A full description of the PBMT measures is provided in Table 1. The sounds emitted by the device were identical regardless of whether irradiation was present. A single researcher (A.A.V.) without knowledge of the randomization performed PBMT.
Table 1. .
Photobiomodulation Therapy Measures
| Measure |
Value |
| Superpulsed infrared lasers | |
| No. | 4 |
| Wavelength, nm ± SD | 905 ± 1 |
| Frequency, Hz | 250 |
| Peak power per diode, W | 12.5 |
| Average mean optical output per diode, mW | 0.3125 |
| Power density per diode, mW/cm2 | 0.71 |
| Dose per diode, J | 0.07125 |
| Spot size of laser per diode, cm2 | 0.44 |
| Red LEDs | |
| No. | 4 |
| Wavelength, nm ± SD | 640 ± 10 |
| Frequency, Hz | 2 |
| Average optical output per diode, mW | 15 |
| Power density per diode, mW/cm2 | 16.66 |
| Dose per diode, J | 3.42 |
| Spot size of LED per diode, cm2 | 0.9 |
| Infrared LEDs | |
| No. | 4 |
| Wavelength, nm ± SD | 875 ± 10 |
| Frequency, Hz | 16 |
| Average optical output per diode, mW | 17.5 |
| Power density per diode, mW/cm2 | 19.44 |
| Dose per diode, J | 3.99 |
| Spot size of LED per diode, cm2 | 0.9 |
| Magnetic field, mT | 35 |
| Irradiation time per site, s | 228 |
| Total dose per site, J | 30 |
| Total dose applied per lower limb, J | 510 |
| Aperture of device, cm2 | 20 |
Abbreviation: LED, light-emitting diode.
Progressive Cardiopulmonary Exercise Test on a Treadmill
Participants performed a standardized progressive cardiopulmonary exercise test on a treadmill with a fixed inclination of 1% until exhaustion. They began the test with a 3-minute warm-up at a velocity of 3 km/h. Next, at 1-minute intervals, we increased the velocity by 1 km/h until 16 km/h was reached. Participants were instructed to use hand signals to request termination of the test at any time. A 3-minute recovery phase at a velocity of 6 km/h followed the test.23 During testing, we monitored the rates of oxygen uptake (Vॱo2), carbon dioxide production measured with a VO 2000 gas analyzer (Inbrasport-Indústria Brasileira De Equipamentos Médico-Desportivos Ltd, Porto Alegre / Rio Grande do Sul, Brazil), total time until exhaustion, and heart rate measured with a digital electrocardiograph (Medical Graphs Ergomet, São Paulo, Brazil).
These data were used to evaluate the performance of participants during a progressive cardiopulmonary exercise test because they are currently the most widely used in the literature for this purpose.28 The entire test was monitored by electrocardiogram and blood pressure measurement. If any abnormal changes were observed in heart rate or blood pressure or if the test was terminated prematurely on request, the test was stopped, and the volunteer's data were deleted.
We used the modified Borg scale29 to assess the participants' perceived effort (dyspnea and fatigue in the lower limbs) before and after the progressive cardiopulmonary exercise test.
Statistical Analysis
The intention-to-treat analysis was performed. The Kolmogorov-Smirnov test was used to verify the normal distribution of data. Parametric data were expressed as means and standard deviations. Nonparametric data were expressed as medians and interquartile intervals. We used paired, 2-sided t tests to compare the variables of muscle function between the PBMT and placebo treatments. The Mann-Whitney test was used to compare the differences in the Borg scale. An additional responder analysis was performed for distance and time until exhaustion using the Fisher exact test. The α level was set at .05.
RESULTS
After data collection, we analyzed the results of 20 volunteers, and there were no dropouts. The characteristics of the volunteers are summarized in Table 2.
Table 2. .
Participant Characteristics
| Variable |
Mean ± SD |
| Age, y | 26.0 ± 6.0 |
| Height, cm | 175.0 ± 10.0 |
| Mass, kg | 74.8 ± 10.9 |
| Body mass indexa | 23.5 ± 3.0 |
| Heart rate, beats per minute | 95.0 ± 21.0 |
| Systolic blood pressure, mm Hg | 118.0 ± 8.0 |
| Diastolic blood pressure, mm Hg | 79.0 ± 4.0 |
Calculated as kg/m2.
The distance covered during the progressive cardiopulmonary exercise test on the treadmill was greater after the application of PBMT (1.96 ± 0.30 km) than after placebo (1.84 ± 0.40 km; t19 = 2.119, P < .001; Figure 3). Time until exhaustion was also longer after PBMT (780.2 ± 91.0 seconds) than after placebo (742.1 ± 94.0 seconds; t19 = 3.028, P < .001; Figure 4).
Figure 3. .
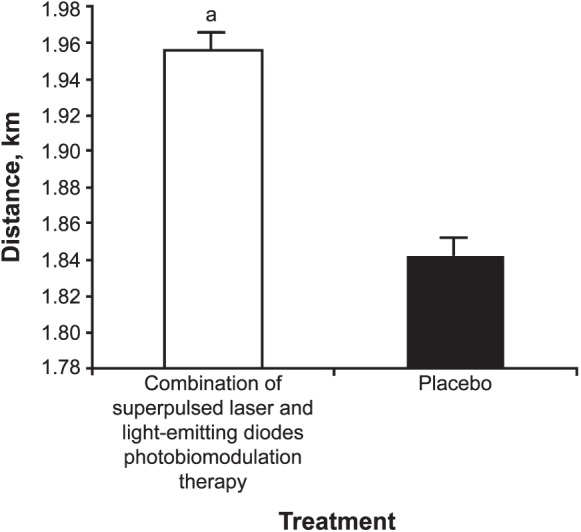
Distance covered during the progressive cardiopulmonary exercise test on a treadmill after photobiomodulation therapy or placebo. a Indicates difference (P < .001).
Figure 4. .
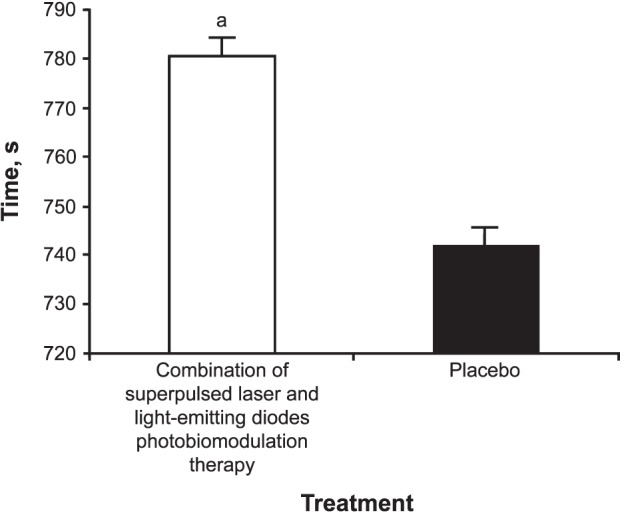
Total time of the progressive cardiopulmonary exercise test on a treadmill after photobiomodulation therapy or placebo. a Indicates difference (P < .001).
We observed that ventilation during the progressive cardiopulmonary exercise test on a treadmill was increased after PBMT (76.4 ± 21.9 L/min) compared with placebo (74.3 ± 19.8 L/min, t19 = 0.180, P = .004). In addition, the dyspnea score was lower after PBMT (3.0 [interquartile range = 0.5–9.0]) than after placebo (4.0 [0.0–9.0], U = 184.000, P < .001; Table 3).
Table 3. .
Data at the Peak of Exercise
| Variable |
Treatment |
|
| Placebo |
Combination of Superpulsed Laser and Light-Emitting Diodes Photobiomodulation Therapy |
|
| Metabolic rate, mL/kg/min (mean ± SD) | ||
| Oxygen uptake | 37.8 ± 6.9 | 38.0 ± 8.6 |
| Carbon dioxide production | 46.4 ± 8.9 | 48.3 ± 6.5 |
| Ventilatory rate, L/min (mean ± SD) | 74.3 ± 19.8 | 76.4 ± 21.9a |
| Borg29 scale score, median (interquartile range) | ||
| Dyspnea | 4.0 (0.0–9.0) | 3.0 (0.5–9.0)a |
| Lower limb fatigue | 3.5 (0.5–10.0) | 4.0 (0.5–9.0) |
Indicates difference (P < .05).
Finally, the responder analysis showed that 80% of volunteers (n = 16) increased the distance covered during the test with pre-exercise PBMT (P < .001), and 85% of volunteers (n = 17) increased their time until exhaustion with pre-exercise PBMT (P < .001; Tables 4 and 5).
Table 4. .
Responder Analysis for Distance Covered
| Group |
Treatment |
|
| Placebo |
Combination of Superpulsed Laser and Light-Emitting Diodes Photobiomodulation Therapy |
|
| Responders | 4 | 16a |
| Nonresponders | 16 | 4 |
Indicates difference (P < .001).
Table 5. .
Responder Analysis for Time
| Group |
Treatment |
|
| Placebo |
Combination of Superpulsed Laser and Light-Emitting Diodes Photobiomodulation Therapy |
|
| Responders | 3 | 17a |
| Nonresponders | 17 | 3 |
Indicates difference (P < .001).
DISCUSSION
To our knowledge, we are the first to analyze the acute effects of PBMT, using the combination of superpulsed lasers and LEDs, on muscle performance during a progressive cardiopulmonary exercise test on a treadmill. Briefly, we observed that the combination of superpulsed lasers and LEDs increased the distance covered, time to exhaustion, and ventilation and decreased the dyspnea score in sedentary volunteers during a progressive cardiopulmonary exercise test.
De Marchi et al23 were the first to investigate the effects of PBMT on muscle performance during a progressive cardiopulmonary exercise test on a treadmill. They reported increases in exercise time (14 seconds) and oxygen uptake (1.1 mL/kg/min) with PBMT compared with placebo. In our study, we noted a greater increase in exercise time (38 seconds, which was almost 3 times higher) after application of PBMT than after placebo. We believe this difference is due to the different light sources and measures used. Whereas De Marchi et al23 used 5 diodes of 810-nm infrared laser, we used different light sources simultaneously (4 × 905-nm superpulsed laser, 4 × 875-nm infrared LEDs, and 4 × 640-nm red LEDs) to irradiate our volunteers. Researchers have shown that a combination of different light sources enhances muscular performance,24,27 decreases pain,30 increases cytochrome c oxidase activity,12 decreases fatigue development, and protects muscles against gradually worsening damage.31 Whereas De Marchi et al23 reported a difference for oxygen uptake between groups, the clinical importance of these differences remains uncertain.32
Da Silva Alves et al22 assessed the acute effects of PBMT on physiologic responses and electromyography during cardiopulmonary exercise testing in healthy adults. The volunteers received PBMT or placebo to the quadriceps femoris and gastrocnemius muscles 10 minutes before a cycle-ergometer cardiopulmonary exercise test. Participants received PBMT using a multidiode cluster with a wavelength of 850 nm, average power of 100 mW per diode, and dose of 14 J per site for 20 seconds at each site. The PBMT (33 ± 10 mL/kg/min) increased oxygen uptake compared with the placebo (31 ± 9 mL/kg/min). However, they found no differences in the electromyography variables, test duration, loading, or Borg scale score. These data do not corroborate our findings, possibly due to differences in the cardiopulmonary exercise testing protocol (cycle ergometer versus treadmill) and the light sources and irradiation measures that were used. We believe our results are better and clinically sound because distance covered during the test, time until exhaustion, and ventilation were improved in our study, whereas da Silva Alves et al22 reported only a slight difference in oxygen consumption without changes in testing time and workload endpoints.
The difference observed between the outcomes of these 2 studies22,23 and our study can be attributed not only to different exercise protocols but also to the use of single wavelengths22,23 rather than the multiple wavelengths we used in our study.
Santos et al31 investigated the use of different wavelengths (660, 830, and 905 nm) immediately before tetanic contractions in the development of skeletal muscle fatigue and contraction-induced muscular damage in rats. They observed that PBMT could delay the development of muscle fatigue and protect muscle against damage. The optimal doses, in part, depended on specific wavelengths and, therefore, must be differentiated to obtain the optimal effects for preserving tissue and reducing muscular fatigue. However, to obtain both tissue preservation and performance enhancement, different wavelengths must be used simultaneously.
Albuquerque-Pontes et al12 irradiated the tibialis anterior muscle of rats using different wavelengths (660, 830, or 905 nm) and reported an increase in cytochrome c oxidase activity in different time windows (5 minutes to 24 hours after irradiation). This finding contributes to the understanding of mechanisms through which PBMT enhances muscular performance and protects muscles against fatigue and tissue damage.
Our responder analysis showed improvements after active PBMT in the distance covered during the test by 80% (n = 16) of volunteers and the time until exhaustion for 85% of volunteers (n = 17). This means that PBMT can enhance performance in 80% to 85% of athletes, physical practitioners, or sedentary people who use the combination of superpulsed lasers and LEDs for ergogenic effects. The beneficial effects of PBMT with the combination of lasers and LEDs have been described recently in the literature.33 The simultaneous use of different wavelengths may increase the effects of phototherapy on skeletal muscle performance and may represent a therapeutic advantage in the clinical situations observed in this study and previous studies.12,24,27,30,31 Finally, our results were due to desirable photobiomodulatory effects of light and not to thermal effects.26 They were also in line with current evidence that PBMT before exercise has ergogenic effects.9,33
We believe our outcomes are interesting because several training programs are used not only to improve athlete performance but also for recovery from different kinds of injuries. Our results showed that PBMT can acutely enhance treadmill exercise performance, which possibly can enhance long-term performance and consequently help athletes remain in games. However, further studies are needed to confirm this hypothesis and to establish optimal treatment protocols.
CONCLUSIONS
We demonstrated that PBMT with the combination of superpulsed lasers and LEDs applied before a progressive cardiopulmonary exercise test on a treadmill increased distance covered, time to exhaustion, and pulmonary ventilation and decreased dyspnea sensation in healthy volunteers.
ACKNOWLEDGMENTS
This study was supported by research grants 2010/52404-0 from the São Paulo Research Foundation–FAPESP (Professor Ernesto Cesar Pinto Leal-Junior), 472062/2013-1 and 307717/2014-3 from the Brazilian Council of Science and Technology Development–CNPq (Professor Ernesto Cesar Pinto Leal-Junior), and 2013/19355-3 from the São Paulo Research Foundation–FAPESP for PhD scholarship (Adriane Aver Vanin).
DISCLAIMER
Professor Ernesto Cesar Pinto Leal-Junior received research support from Multi Radiance Medical (Solon, OH), a laser device manufacturer.
REFERENCES
- 1. . Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008; 88 1: 287– 332. [DOI] [PubMed] [Google Scholar]
- 2. . Ivey FM, Roth SM, Ferrell RE, et al. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2000; 55 11: M641– M648. [DOI] [PubMed] [Google Scholar]
- 3. . Lamb GD, Stephenson DG. Point: counterpoint. Lactic acid accumulation is an advantage/disadvantage during muscle activity. J Appl Physiol (1985). 2006; 100 4: 1410– 1412. [DOI] [PubMed] [Google Scholar]
- 4. . Rowell L. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974; 54 1: 75– 159. [DOI] [PubMed] [Google Scholar]
- 5. . Bender PR, Martin BJ. Maximal ventilation after exhausting exercise. Med Sci Sports Exerc. 1985; 17 1: 164– 167. [PubMed] [Google Scholar]
- 6. . Smith KC. Laser (and LED) therapy is phototherapy. Photomed Laser Surg. 2005; 23 1: 78– 80. [DOI] [PubMed] [Google Scholar]
- 7. . Desmet KD, Paz DA, Corry JJ, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006; 24 2: 121– 128. [DOI] [PubMed] [Google Scholar]
- 8. . Takezaki S, Omi T, Sato S, Kawana S. Light-emitting diode phototherapy at 630 +/- 3 nm increases local levels of skin-homing T-cells in human subjects. J Nippon Med Sch. 2006; 73 2: 75– 81. [DOI] [PubMed] [Google Scholar]
- 9. . Leal-Junior EC, Vanin AA, Miranda EF. de Carvalho Pde T, Dal Corso S, Bjordal JM. Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci. 2015; 30 2: 925– 939. [DOI] [PubMed] [Google Scholar]
- 10. . Leal-Junior EC, Lopes-Martins RA, Vanin AA, et al. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci. 2009; 24 3: 425– 431. [DOI] [PubMed] [Google Scholar]
- 11. . Leal-Junior EC, Lopes-Martins RA, Baroni BM, et al. Effect of 830 nm low-level laser therapy applied before high-intensity exercises on skeletal muscle recovery in athletes. Lasers Med Sci. 2009; 24 6: 857– 863. [DOI] [PubMed] [Google Scholar]
- 12. . Albuquerque-Pontes GM, Vieira Rde P, Tomazoni SS, et al. Effect of pre-irradiation with different doses, wavelengths, and application intervals of low-level laser therapy on cytochrome c oxidase activity in intact skeletal muscle of rats. Lasers Med Sci. 2015; 30 1: 59– 66. [DOI] [PubMed] [Google Scholar]
- 13. . Karu TI, Afanasyeva NI, Kolyakov SF, Pyatibrat LV, Welser L. Changes in absorbance of monolayer of living cells induced by laser irradiation at 633, 670, and 820 nm. J Select Top Quantum Elect. 2001; 7 6: 982– 988. [Google Scholar]
- 14. . Ihsan FR. Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg. 2005; 23 3: 289– 294. [DOI] [PubMed] [Google Scholar]
- 15. . Passarella S. He-Ne laser irradiation of isolated mitochondria. J Photochem Photobiol B. 1989; 3 4: 642– 643. [DOI] [PubMed] [Google Scholar]
- 16. . Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg. 2007; 25 3: 180– 182. [DOI] [PubMed] [Google Scholar]
- 17. . Karu TI. Laser biostimulation: a photobiological phenomenon. J Photochem Photobiol B. 1989; 3 4: 638– 640. [DOI] [PubMed] [Google Scholar]
- 18. . Manteifel UM, Karu TI. Increase in the number of contacts of endoplasmic reticulum with mitochondria and plasma membrane in yeast cells stimulated to division with He-Ne laser light. Tsitologiia. 2004; 46 6: 498– 505. [PubMed] [Google Scholar]
- 19. . Behm DG, St-Pierre DM. The effects of strength training and disuse on the mechanisms of fatigue. Sports Med. 1998; 25 3: 173– 189. [DOI] [PubMed] [Google Scholar]
- 20. . Biasotto DC, Biasotto-Gonzales DA, Panhoca I. Correlation between the clinical phonoaudiological assessment and electromyographic activity of the masseter muscle. J Appl Oral Sci. 2005; 13 4: 424– 430. [DOI] [PubMed] [Google Scholar]
- 21. . Leal-Junior EC, Lopes-Martins RA, Dalan F, et al. Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg. 2008; 26 5: 419– 424. [DOI] [PubMed] [Google Scholar]
- 22. . da Silva Alves MA, Pinfildi CE, Neto LN, Lourenço RP, de Azevedo PH, Dourado VZ. Acute effects of low-level laser therapy on physiologic and electromyographic responses to the cardiopulmonary exercise testing in healthy untrained adults. Lasers Med Sci. 2014; 29 6: 1945– 1951. [DOI] [PubMed] [Google Scholar]
- 23. . De Marchi T, Leal Junior EC, Bortoli C, Tomazoni SS, Lopes-Martins RA, Salvador M. Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci. 2012; 27 1: 231– 236. [DOI] [PubMed] [Google Scholar]
- 24. . Antonialli FC, De Marchi T, Tomazoni SS, et al. Phototherapy in skeletal muscle performance and recovery after exercise: effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med Sci. 2014; 29 6: 1967– 1976. [DOI] [PubMed] [Google Scholar]
- 25. . Baroni BM, Leal Junior EC, De Marchi T, Lopes AL, Salvador M, Vaz MA. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol. 2010; 110 4: 789– 796. [DOI] [PubMed] [Google Scholar]
- 26. . Grandinétti Vdos S, EF, Mirand, Johnson DS, et al. The thermal impact of phototherapy with concurrent super-pulsed lasers and red and infrared LEDs on human skin. Lasers Med Sci. 2015; 30 5: 1575– 1581. [DOI] [PubMed] [Google Scholar]
- 27. . Miranda EF, de Oliveira LV, Antonialli FC, Vanin AA. de Carvalho Pde T, Leal-Junior EC. Phototherapy with combination of super-pulsed laser and light-emitting diodes is beneficial in improvement of muscular performance (strength and muscular endurance), dyspnea, and fatigue sensation in patients with chronic obstructive pulmonary disease. Lasers Med Sci. 2015; 30 1: 437– 443. [DOI] [PubMed] [Google Scholar]
- 28. . Powers SK, Howley ET. Exercise Physiology: Theory and Application to Fitness and Performance. 9th ed. New York, NY: McGraw-Hill; 2015. [Google Scholar]
- 29. . Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990; 16 suppl 1: 55– 58. [DOI] [PubMed] [Google Scholar]
- 30. . Leal-Junior EC, Johnson DS, Saltmarche A, Demchak T. Adjunctive use of combination of super-pulsed laser and light-emitting diodes phototherapy on nonspecific knee pain: double-blinded randomized placebo-controlled trial. Lasers Med Sci. 2014; 29 6: 1839– 1847. [DOI] [PubMed] [Google Scholar]
- 31. . Santos LA, Marcos RL, Tomazoni SS, et al. Effects of pre-irradiation of low-level laser therapy with different doses and wavelengths in skeletal muscle performance, fatigue, and skeletal muscle damage induced by tetanic contractions in rats. Lasers Med Sci. 2014; 29 5: 1617– 1626. [DOI] [PubMed] [Google Scholar]
- 32. . McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014; 312 13: 1342– 1343. [DOI] [PubMed] [Google Scholar]
- 33. . Leal-Junior EC. Photobiomodulation therapy in skeletal muscle: from exercise performance to muscular dystrophies. Photomed Laser Surg. 2015; 33 2: 53– 54. [DOI] [PMC free article] [PubMed] [Google Scholar]