Abstract
Context:
Some healthy athletes report high levels of baseline concussion symptoms, which may be attributable to several factors (eg, illness, personality, somaticizing). However, the role of baseline symptoms in outcomes after sport-related concussion (SRC) has not been empirically examined.
Objective:
To determine if athletes with high symptom scores at baseline performed worse than athletes without baseline symptoms on neurocognitive testing after SRC.
Design:
Cohort study.
Setting:
High school and collegiate athletic programs.
Patients or Other Participants:
A total of 670 high school and collegiate athletes participated in the study. Participants were divided into groups with either no baseline symptoms (Postconcussion Symptom Scale [PCSS] score = 0, n = 247) or a high level of baseline symptoms (PCSS score > 18 [top 10% of sample], n = 68).
Main Outcome Measure(s):
Participants were evaluated at baseline and 2 to 7 days after SRC with the Immediate Post-concussion Assessment and Cognitive Test and PCSS. Outcome measures were Immediate Post-concussion Assessment and Cognitive Test composite scores (verbal memory, visual memory, visual motor processing speed, and reaction time) and total symptom score on the PCSS. The groups were compared using repeated-measures analyses of variance with Bonferroni correction to assess interactions between group and time for symptoms and neurocognitive impairment.
Results:
The no-symptoms group represented 38% of the original sample, whereas the high-symptoms group represented 11% of the sample. The high-symptoms group experienced a larger decline from preinjury to postinjury than the no-symptoms group in verbal (P = .03) and visual memory (P = .05). However, total concussion-symptom scores increased from preinjury to postinjury for the no-symptoms group (P = .001) but remained stable for the high-symptoms group.
Conclusions:>
Reported baseline symptoms may help identify athletes at risk for worse outcomes after SRC. Clinicians should examine baseline symptom levels to better identify patients for earlier referral and treatment for their injury. Additional investigation of baseline symptoms is warranted to help delineate the type and severity of premorbid symptoms.
Key Words: traumatic brain injuries, baseline testing, neurocognitive impairment
Key Points
Athletes with a high level of symptoms at baseline reported the same level of symptoms after concussion.
Further baseline assessment of these athletes is important to identify any factors that may affect treatment and return to play after injury.
A high level of baseline concussion symptoms may aid clinicians in recognizing athletes at risk for cognitive impairment in the first week after a sport-related concussion.
As research expands our understanding of the premorbid risks and potential long-term effects of sport-related concussion (SRC), health care professionals are being challenged to accurately assess, diagnose, and treat this injury. A comprehensive, multifaceted evaluation approach has been established as the standard of care1 and includes the clinical interview, computerized neurocognitive testing,2 balance assessment,3 and self-report symptom checklists.4 Establishing baseline, or preinjury, levels of function and performance is especially important for athletes with identified premorbid risk factors for injury and prolonged recovery (eg, migraine, learning disability, attention-deficit hyperactivity disorder).5 Baseline neurocognitive tests typically consist of a computerized neurocognitive assessment and symptom report.
The importance of administering baseline symptom reports is supported by the notion that concussion symptoms are often present in the absence of injury and can be similar to symptoms of other conditions, including fatigue, physical illness, and orthopaedic injury.6 In fact, the occurrence of concussion symptoms among the nonconcussed population has been examined with several symptom scales and checklists.7–9 The most commonly reported concussion symptoms among this group include fatigue, irritability, headaches, nervousness, anxiety, poor concentration, depression, and sleeping problems.4 On baseline symptom reports, Covassin et al10 found that 68% of male and 76% of female collegiate athletes endorsed at least 1 symptom and 30% to 50% of participants endorsed mild degrees of fatigue, drowsiness, sleep disturbances, and concentration difficulties. Similarly, other researchers8 noted that 24% of healthy participants reported a symptom score greater than 6 on the Postconcussion Symptom Scale (PCSS). These findings suggest that baseline symptom reports may reflect variability similar to that in neurocognitive scores and having baseline symptom reports may inform management strategies of clinicians who provide clinical care postconcussion. For example, international return-to-play criteria state that an athlete must be asymptomatic at rest and after both cognitive and physical exertion before returning to play.1 However, defining asymptomatic status can be challenging, especially among athletes with premorbid somatic complaints and symptoms. Without a current operational definition of the term asymptomatic, symptom reports aim to establish baseline symptom levels for comparison with symptom levels after injury to provide a more accurate determination of asymptomatic status.11
Certain subpopulations of athletes, including those diagnosed with developmental conditions (eg, attention-deficit hyperactivity disorder or learning disabilities) or a history of headaches and previous concussions, report higher baseline symptom scores than those without these histories.12 Transient or situational factors including psychiatric distress, acute fatigue, physical illness, and orthopaedic injury also influence symptom reports.6,12,13 In other populations, symptom reporting has been established as a state-dependent phenomenon,14 with reporting subject to psychological and perceptual biases,15 medications,13 daily stress,16 and attentiveness to the body and measures of control.15 In addition, symptom reports may be influenced by personal factors including age, sex,10 education, personality,17 and general health status.18
The relationship between baseline symptoms and outcomes after SRC has only recently been examined by Merritt and Arnett.19 The results of this preliminary study suggested that baseline affective and physical symptom clusters on the PCSS and a baseline neurocognitive composite (derived from computerized and paper-and-pencil neuropsychological testing) predicted total PCSS symptom score within 1 week after SRC among collegiate athletes.19 It is interesting that physical symptoms at baseline were inversely related to total symptom score after injury. However, these authors did not examine postinjury neurocognitive performance. The frequency and magnitude of baseline symptom reports may have clinical value in postconcussion management and could be used to identify athletes who have undiagnosed conditions or are likely to exaggerate symptom reporting. Therefore, the purpose of our study was to examine differences in postconcussion symptom reports and neurocognitive test performance between athletes with no or a high level of baseline symptoms. We hypothesized that athletes with elevated baseline symptoms (ie, top 10% of the sample [PCSS > 18]) would report more symptoms on the PCSS and score lower on neurocognitive testing in the acute phase after SRC than those with no baseline symptoms.
METHODS
Participants and Recruitment
Between August 2009 and December 2011, we collected prospective, repeated-measures SRC data from 670 high school- and college-aged athletes who were part of a multisite study in California, Louisiana, Michigan, and Pennsylvania. All data were collected at baseline (ie, preinjury) and again within 2 to 7 days of injury. Study inclusion criteria were age 13 to 23 years, participation in a scholastic sport, and a valid baseline computerized neurocognitive and symptom assessment. Exclusion criteria, as documented by a brief medical history questionnaire, were a history of any of the following: moderate to severe traumatic brain injury, brain surgery, major psychiatric or neurologic disorder, substance abuse, attention-deficit hyperactivity disorder, learning disability, or 3 or more concussions. Athletes with a history of treatment for migraine were included, consistent with population prevalence estimates. We used baseline total symptom scores to categorize participants into no-symptoms and high-symptoms (top 10% of the sample) groups (see “Results” section). The study was approved by the university's institutional review board under an expedited protocol. After an informational meeting and before enrolling in the study, adult participants provided written informed consent; parents of minor participants provided consent, and minors provided assent.
Measurements
Definition of SRC
A concussion was defined as a “complex pathophysiological process affecting the brain, induced by biomechanical forces.”1 Athletes with suspected concussions were diagnosed by a licensed medical professional (eg, physician, neuropsychologist, certified athletic trainer) educated in the assessment and treatment of concussion using the following criteria: (1) clear mechanism of injury, (2) presence of signs at time of injury (eg, loss of consciousness, posttraumatic amnesia, disorientation, confusion), (3) current symptoms (eg, headache, dizziness, nausea), and (4) 1 or more areas of cognitive impairment (eg, memory, processing speed, reaction time). All concussions were the result of sport participation.
Demographic Data and Concussion Symptoms
We obtained self-reported demographic data from all participants, including age, sex, and number of previous concussions. The PCSS was used to assess SRC symptoms; it contains 22 self-reported SRC symptoms (eg, headache, fogginess, dizziness) rated on a 0 (none)- to 6 (severe)-point Likert scale. The maximum possible PCSS score is 132.
Neurocognitive Impairment
The Immediate Post-concussion Assessment and Cognitive Test (ImPACT; ImPACT Applications, Inc, Pittsburgh, PA) was used to assess cognitive impairment. This test consists of 6 subtests: verbal memory, design memory, X's and O's, symbol matching, color matching, and 3-letter memory. The 6 subtest results are collapsed into 4 composite scores: verbal memory (% correct), visual memory (% correct), visual motor processing speed (numeric value; a higher score is better), and reaction time (seconds).
Data Analysis
We calculated a series of χ2 independent-samples t tests and independent Mann-Whitney U tests to assess differences between the no-symptoms and high-symptoms groups for demographic variables and baseline symptoms. A series of repeated-measures (baseline and 2 to 7 days postinjury) analyses of variance with Bonferroni correction for multiple comparisons were conducted to compare groups for cognitive impairment and symptoms. All analyses were carried out using SPSS (version 22; IBM Corporation, Armonk, NY). We set statistical significance at P < .05 for all uncorrected analyses.
RESULTS
Demographic Data
Complete baseline and postinjury cognitive and symptom data were available for 656 of 670 athletes (98%) identified for inclusion in the study. A total of 315 of 656 participants (48%) were categorized into the no-symptoms (n = 247 [38%], total PCSS score = 0) or high-symptoms (n = 68 [10%], top 10% of total PCSS scores) baseline group. The remaining 341 of 656 (52%) eligible participants were excluded from the baseline comparisons. Key demographic data for the entire sample and both subgroups are provided in Table 1. The Mann-Whitney U test indicated that the no-symptoms group (17.9 ± 2.5 years) was older than the high-symptoms group (16.8 ± 2.3 years). As we expected, an independent-samples t test confirmed that the high-symptoms group (27.4 ± 11.25 symptoms) had more baseline symptoms than the no-symptoms group (0.0 ± 0.0 symptoms). No other differences between the groups were demonstrated.
Table 1. .
Demographic Data for the Entire Sample and the Baseline No-Symptoms and High-Symptoms Groups
| Variable |
Total Sample (N = 670) |
Group |
|
| No Symptoms (n = 247) |
High Symptoms (n = 68) |
||
| Sex, No. (%) | |||
| Males | 471 (70) | 175 (71) | 44 (65) |
| Females | 199 (30) | 72 (29) | 24 (35) |
| Mean ± SD |
|||
| Age, y | 17.4 ± 2.3 | 17.9 ± 2.5 | 16.8 ± 2.3 |
| Previous concussions, No. | 0.9 ± 1.2 | 0.8 ± 1.0 | 1.0 ± 1.2 |
| Days since injury | 2.9 ± 2.2 | 2.8 ± 2.4 | 3.3 ± 2.7 |
Comparison of Postinjury Neurocognitive Impairment and Symptoms
We found main effects for time for all 4 neurocognitive composite scores and total symptoms, as well as for interactions between group and time for neurocognitive impairment and total symptoms (PCSS score; Table 2). Specifically, all neurocognitive scores were worse (verbal memory: Wilks λ = 0.85, F1,305 = 52.48, P < .001, η2 = 0.15; visual memory: Wilks λ = 0.83, F1,305 = 61.18, P < .001, η2 = 0.17; visual motor processing speed: Wilks λ = 0.94, F1,305 = 20.36, P < .001, η2 = .06; reaction time: Wilks λ = 0.89, F1,305 = 39.65, P < .001, η2 = 0.12) and PCSS score was higher from baseline to 2 to 7 days postinjury (Wilks λ = 0.80, F1,305 = 75.41, P < .001, η2 = 0.20).
Table 2. .
Postinjury Cognitive Impairment and Symptoms in No-Symptoms and High-Symptoms Baseline Groups
| Variable |
Time (Mean ± SD) |
|||||
| Baseline |
2–7 Days Postinjury |
|||||
| No Symptoms (n = 247) |
High Symptoms (n = 68) |
Total (n = 315) |
No Symptoms (n = 247) |
High Symptoms (n = 68) |
Total (n = 315) |
|
| Verbal memory, % correct | 84.0 ± 10.8 | 85.5 ± 8.9 | 84.3 ± 10.5 | 78.7 ± 14.9 | 75.9 ± 14.2 | 78.1 ± 14.8a |
| Visual memory, % correct | 74.4 ± 12.8 | 73.5 ± 13.9 | 74.2 ± 13.0 | 67.8 ± 16.2 | 62.5 ± 16.0 | 66.7 ± 16.3a |
| Motor processing speed, numeric valueb | 38.8 ± 6.7 | 37.0 ± 7.3 | 38.4 ± 6.9 | 35.9 ± 0.2 | 34.7 ± 8.2 | 35.6 ± 9.0a |
| Reaction time, s | 0.57 ± 0.07 | 0.60 ± 0.07 | 0.58 ± 0.07 | 0.64 ± 0.16 | 0.67 ± 0.14 | 0.65 ± 0.16a |
| Total symptom score | 0 ± 0 | 26.7 ± 10.3 | 5.7 ± 12.0 | 19.6 ± 16.4 | 27.1 ± 19.6 | 21.2 ± 17.4 |
Decline from baseline, P < .001.
Higher score is better.
Significant interactions were present between time and group for verbal memory (Wilks λ = 0.99, F1,305 = 4.54, P = .03, η2 = 0.02), visual memory (Wilks λ = 0.99, F1,305 = 3.85, P = .05, η2 = 0.01), and PCSS score (Wilks λ = 0.82, F1,305 = 68.31, P < .001, η2 = 0.18; Figures 1 through 3). Specifically, participants in the high-symptoms group experienced a more significant decline in verbal and visual memory from baseline to 2 to 7 days postinjury compared with the no-symptoms group (Figures 2 and 3). However, although the PCSS score for the no-symptoms group increased from baseline to 2 to 7 days postinjury, it did not change for the high-symptoms group (Figure 3).
Figure 1. .
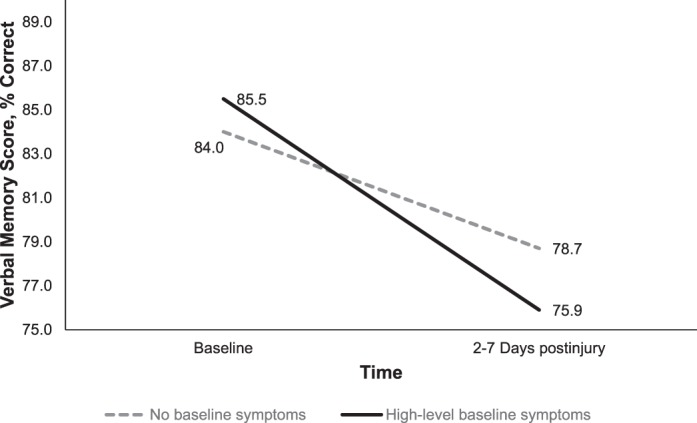
Group-by-time interaction for verbal memory from preinjury to postinjury for the baseline high-symptoms group (n = 68) and no-symptoms groups (n = 247, P = .03).
Figure 2. .
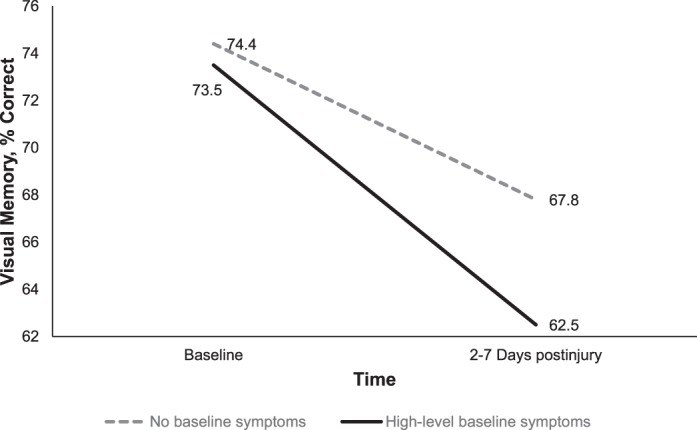
Group-by-time interaction for visual memory from preinjury to postinjury for the baseline high-symptoms (n = 68) and no-symptoms groups (n = 247, P = .05).
Figure 3. .
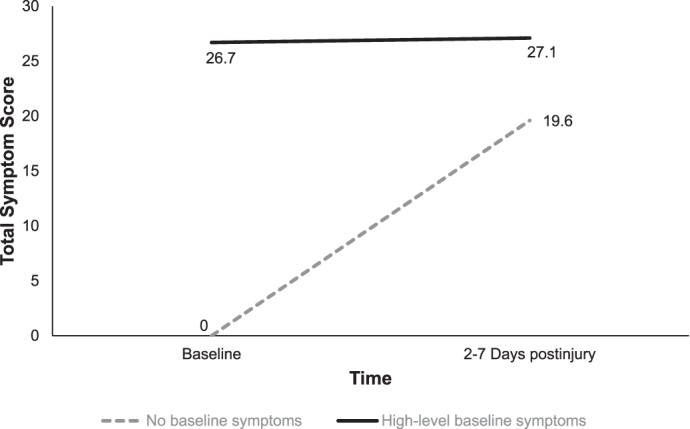
Group-by-time interaction for total symptom score from preinjury to postinjury for the baseline high-symptoms group (n = 68) and no-symptoms group (n = 247, P = .05).
DISCUSSION
To our knowledge, we are the first to compare postconcussion symptom reports and neurocognitive performance between athletes with no symptoms and those with a high level of symptoms at baseline. The primary finding was that the high-symptoms group performed worse than the no-symptoms group for verbal and visual memory during the acute phase (2–7 days) after SRC. It was surprising that athletes with a high level of baseline symptoms reported essentially the same scores during the acute period after SRC as they did at baseline. In contrast, those athletes with no baseline symptoms reported a substantial increase in symptoms during the acute period after SRC. Therefore, baseline symptom scores may be useful for clinicians in distinguishing athletes at risk for worse performance on neurocognitive testing in the acute phase postinjury. These results also highlight the limitations of relying on postconcussion symptom scores in the absence of baseline scores.
Subpopulations of athletes with preexisting conditions (eg, migraine, learning disability) reported more concussion-related symptoms in the absence of concussion12 and were at increased risk for worse impairments and outcomes after concussion.20,21 Our findings support these results. One explanation is that athletes in the high-symptoms group may have a subclinical constellation of symptoms related to other conditions (eg, cognitive complaints consistent with attention-deficit hyperactivity disorder but without meeting the full criteria) or may be in the prodromal phase of developing a condition (eg, migraine, clinical depression). In addition, concussion-symptom reports may capture a profile representative of other conditions that are not yet established as risk factors for poor outcomes (eg, somatization, sleep disorder, motion sensitivity, personality style). For example, researchers22 have proposed that type D personality, which is characterized by a negative affect and social inhibition, is linked to postconcussion syndrome. Individuals with these characteristics are somatically preoccupied and prone to negative health-related outcomes and behaviors, including help seeking, overuse of health care, and poor adjustment after psychosocial stressors.23 Our subpopulation of high-level symptom reporters may be vulnerable to distress or somatic preoccupation, leading to inadequate effort on testing as a result of anxiety24 secondary to stereotypes or an expectation of cognitive impairment25 or as a way of communicating or conveying the degree of impairment or the need for help to an examiner. Similarly, they may be hypervigilant or have enhanced sensory sensitivity to symptoms that contribute to cognitive difficulties. For example, anxiety symptoms have been linked to impaired cognitive functioning and selective memory encoding, especially when an individual perceives a situation as emotionally threatening.26,27 Sustaining a concussion, particularly with respect to return to play and time away from the team during recovery, can create emotionally threatening situations for athletes. In a related finding, the emotional-distress response may exacerbate the pathophysiologic nature of the injury through several posited mechanisms, including oxidative stress, chronic inflammation, and excitotoxicity.28 Alternatively, emotional stress may mediate other pathophysiologic outcomes, such as posttraumatic migraine, which is associated with worse performance on memory-composite aspects of neurocognitive testing.29
In contrast to neurocognitive test findings, high-level symptom reporters displayed no increase in total symptom scores after SRC. However, athletes who reported no baseline symptoms reported higher levels of symptoms after injury. Moreover, total symptom scores for the no-symptoms and high-symptoms groups were similar after SRC. The use of symptom reports in athletes after SRC is complicated by the potential minimization of symptoms related to a desire to return to play.30 Alternatively, this pattern of findings may represent the potential challenges of using symptom reports in both research and clinical settings. The PCSS lacks context for responses with regard to time period (eg, currently experiencing could be interpreted as the exact moment or past week), relative versus absolute ratings (eg, symptom intensity compared with the previous week or over the lifetime), and ceiling effects (eg, a baseline report of 6/6 symptom intensity cannot increase after injury). An unexpected decrease in physical symptoms after SRC among high-level symptom reporters was evident in another study and is difficult to explain.19 In short, transient and chronic influences on symptom reporting may be difficult to differentiate with current postconcussion measures or tools.
Limitations
The current study had several limitations. Because of the time lapse between baseline testing and injury, situational and transient stressors that influence symptom reporting could have changed across time (eg, divorce of parents, academic pressure). We did not investigate on-field injury-severity markers (eg, loss of consciousness, on-field dizziness). An overrepresentation of these markers in 1 group or the other is a threat to the internal validity of the study. Also, we neither assessed nor controlled for other factors that may explain symptoms such as somatization and chronic pain. As such, our findings may reflect group differences based on these characteristics. The findings reflect extreme subpopulations of athletes with no symptoms or the highest level of symptoms at baseline. Patients in the middle range who reported low-level symptoms were excluded from the current analyses. Finally, we did not gather information regarding medical attention or acute treatment for concussion. Some athletes may have received treatment for their concussion, which could have influenced postconcussion neurocognitive performance and self-reported symptoms. Finally, the no-symptoms group was slightly younger than the high-symptoms group. However, in contrast to some studies that suggested younger athletes performed worse on neurocognitive testing31 and that high school students were symptomatic for a longer time than collegiate athletes after injury,32 our findings did not support this pattern, which may indicate that baseline symptoms overshadow the contribution of age.
Future Research
Given that we are among the first to examine baseline symptoms with respect to acute outcomes after SRC, we believe there are several plausible next steps in this preliminary line of inquiry. Moving forward, researchers should examine symptom-report scales concurrently with other standardized self-reported measures (eg, mood, personality) and comprehensive medical histories to gain insight into the reasons for healthy athletes to report a high level of symptoms. Follow-up assessments through the acute phase to clinical recovery would help determine if this subpopulation is at increased risk for protracted recovery or the development of specific subgroups of symptoms. In addition, it is important to examine specific symptom clusters or factors beyond the first week postinjury.33 Further assessments after recovery might also provide insight into whether symptoms return to premorbid levels or if they demonstrate a noticeable and potentially clinically relevant elevation (ie, a new postinjury baseline) that persists after injury and recovery. Finally, an assessment of the role of factors such as age, sex, and concussion and migraine history on baseline symptoms and their relationship with postinjury symptoms and impairment is warranted.
CONCLUSIONS
A high level of baseline concussion symptoms may help to identify athletes at risk for cognitive impairment in the first week after SRC. Given that as a group, athletes with a high level of symptoms at baseline report the same level of symptoms postinjury, the use of cognitive testing and baseline assessments may help clinicians to recognize and track concussion in these patients when symptoms remain unchanged. Investigating an athlete's baseline symptoms is also important for making more informed return-to-play decisions to account for different levels of “asymptomatic” status, which may depend on premorbid history and day-to-day fluctuations in symptoms. In short, each patient has his or her own baseline level of symptoms that should be taken into consideration when interpreting postinjury symptoms. Further investigation of baseline symptoms, perhaps by administering additional questionnaires (eg, headache inventory, somatization and pain scale, clinical measures of anxiety and depression or personality) in athletes reporting a high level of baseline symptoms may help to delineate the nature of premorbid symptoms and guide more targeted approaches to postinjury treatment.
ACKNOWLEDGMENTS
This research was supported in part by grants to the University of Pittsburgh from the National Institute on Deafness and Other Communication Disorders (1K01DC012332-01A1) to Dr Kontos.
REFERENCES
- 1. . McCrory P, Meeuwisse W, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013; 47 5: 250– 258. [DOI] [PubMed] [Google Scholar]
- 2. . Johnson EW, Kegel NE, Collins MW. Neuropsychological assessment of sport-related concussion. Clin Sports Med. 2011; 30 1: 73– 88. [DOI] [PubMed] [Google Scholar]
- 3. . Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001; 11 3: 182– 189. [DOI] [PubMed] [Google Scholar]
- 4. . Alla S, Sullivan SJ, Hale L, McCrory P. Self-report scales/checklists for the measurement of concussion symptoms: a systematic review. Br J Sports Med. 2009; 43 suppl 1: i3– i12. [DOI] [PubMed] [Google Scholar]
- 5. . Collins MW, Kontos AP, Reynolds E, Murawski CD, Fu FH. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014; 22 2: 235– 246. [DOI] [PubMed] [Google Scholar]
- 6. . Piland SG, Ferrara MS, Macciocchi SN, Broglio SP, Gould TE. Investigation of baseline self-report concussion symptom scores. J Athl Train. 2010; 45 3: 273– 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Gouvier WD, Uddo-Crane M, Brown LM. Base rates of post-concussional symptoms. Arch Clin Neuropsychol. 1988; 3 3: 273– 278. [PubMed] [Google Scholar]
- 8. . Iverson GL, Lange RT. Examination of “postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. 2003; 10 3: 137– 144. [DOI] [PubMed] [Google Scholar]
- 9. . Wang Y, Chan RC, Deng Y. Examination of postconcussion-like symptoms in healthy university students: relationships to subjective and objective neuropsychological function performance. Arch Clin Neuropsychol. 2006; 21 4: 339– 347. [DOI] [PubMed] [Google Scholar]
- 10. . Covassin T, Swanik CB, Sachs M, et al. Sex differences in baseline neuropsychological function and concussion symptoms of collegiate athletes. Br J Sports Med. 2006; 40 11: 923– 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Alla S, Sullivan SJ, McCrory P. Defining asymptomatic status following sports concussion: fact or fallacy? Br J Sports Med. 2012; 46 8: 562– 569. [DOI] [PubMed] [Google Scholar]
- 12. . Iverson G, Atkins J, Zafonte R, Berkner P. Factors influencing post-concussion-like symptom reporting in adolescent athletes. Brain Inj. 2014; 28 5–6: 667– 668. [Google Scholar]
- 13. . Garden N, Sullivan KA. An examination of the base rates of post-concussion symptoms: the influence of demographics and depression. Appl Neuropsychol. 2010; 17 1: 1– 7. [DOI] [PubMed] [Google Scholar]
- 14. . Matthews G, Deary I, Whiteman M. Stable traits and transient states. : Personality Traits. 3rd ed. New York, NY: Cambridge University Press; 2009. [Google Scholar]
- 15. . Pennebaker JW. The Psychology of Physical Symptoms. New York, NY: Springer; 1982. [Google Scholar]
- 16. . Larsen RJ, Kasimatis M. Day-to-day physical symptoms: individual differences in the occurrence, duration, and emotional concomitants of minor daily illnesses. J Pers. 1991; 59 3: 387– 423. [DOI] [PubMed] [Google Scholar]
- 17. . Greiffenstein FM, Baker JW. Comparison of premorbid and postinjury MMPI-2 profiles in late postconcussion claimants. Clin Neuropsychol. 2001; 15 2: 162– 170. [DOI] [PubMed] [Google Scholar]
- 18. . McLean SA, Kirsch NL, Tan-Schriner CU, et al. Health status, not head injury, predicts concussion symptoms after minor injury. Am J Emerg Med. 2009; 27 2: 182– 190. [DOI] [PubMed] [Google Scholar]
- 19. . Merritt VC, Arnett PA. Premorbid predictors of postconcussion symptoms in collegiate athletes. J Clin Exp Neuropsychol. 2014; 36 10: 1098– 1111. [DOI] [PubMed] [Google Scholar]
- 20. . Belanger HG, Curtiss G, Demery JA, Lebowitz BK, Vanderploeg RD. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc. 2005; 11 3: 215– 227. [DOI] [PubMed] [Google Scholar]
- 21. . Collins MW, Grindel SH, Lovell MR, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999; 282 10: 964– 970. [DOI] [PubMed] [Google Scholar]
- 22. . Silverberg ND, Iverson GL. Etiology of the post-concussion syndrome: physiogenesis and psychogenesis revisited. NeuroRehabilitation. 2011; 29 4: 317– 329. [DOI] [PubMed] [Google Scholar]
- 23. . Michal M, Wiltink J, Grande G, Beutel ME, Brahler E. Type D personality is independently associated with major psychosocial stressors and increased health care utilization in the general population. J Affect Disord. 2011; 134 1–3: 396– 403. [DOI] [PubMed] [Google Scholar]
- 24. . Owens M, Stevenson J, Hadwin JA, Norgate R. When does anxiety help or hinder cognitive test performance? The role of working memory capacity. Br J Psychol. 2014; 105 1: 92– 101. [DOI] [PubMed] [Google Scholar]
- 25. . Kit KA, Mateer CA, Tuokko HA, Spencer-Rodgers J. Influence of negative stereotypes and beliefs on neuropsychological test performance in a traumatic brain injury population. J Int Neuropsychol Soc. 2014; 20 2: 157– 167. [DOI] [PubMed] [Google Scholar]
- 26. . Macleod C. Clinical anxiety and the selective encoding of threatening information. Int Rev Psychiatry. 1991; 3 2: 279– 292. [Google Scholar]
- 27. . Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct. 2007; 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. . Prasad KN, Bondy SC. Common biochemical defects linkage between post-traumatic stress disorders, mild traumatic brain injury (TBI) and penetrating TBI. Brain Res. 2015; 1599: 103– 114. [DOI] [PubMed] [Google Scholar]
- 29. . Kontos AP, Elbin RJ, Lau B, et al. Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. Am J Sports Med. 2013; 41 7: 1497– 1504. [DOI] [PubMed] [Google Scholar]
- 30. . Bailey CM, Echemendia RJ, Arnett PA. The impact of motivation on neuropsychological performance in sports-related mild traumatic brain injury. J Int Neuropsychol Soc. 2006; 12 4: 475– 484. [DOI] [PubMed] [Google Scholar]
- 31. . Covassin T, Elbin RJ, III, Larson E, Kontos AP. Sex and age differences in depression and baseline sport-related concussion neurocognitive performance and symptoms. Clin J Sport Med. 2012; 22 2: 98– 104. [DOI] [PubMed] [Google Scholar]
- 32. . Williams RM, Puetz TW, Giza CC, Broglio SP. Concussion recovery time among high school and collegiate athletes: a systematic review and meta-analysis. Sports Med. 2015; 45 6: 893– 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. . Kontos AP, Elbin R, Schatz P, et al. A revised factor structure for the post-concussion symptom scale baseline and post-concussion factors. Am J Sports Med. 2012; 40 10: 2375– 2384. [DOI] [PubMed] [Google Scholar]


