Abstract
Background
Individuals with posttraumatic stress disorder (PTSD) are more likely to smoke and more likely to relapse following a quit attempt than individuals without PTSD. Thus, there is a significant need to study promising interventions that might improve quit rates for smokers with PTSD. One such intervention, supplemental nicotine patch-preloading, entails the use of nicotine replacement therapy prior to quitting.
Objective
The objective of this study was to conduct a randomized controlled trial of the efficacy of supplemental nicotine patch-preloading among smokers with PTSD. We hypothesized that, relative to participants in the placebo condition, participants in the nicotine patch-preloading condition would: (1) smoke less and experience reduced craving for cigarettes during the nicotine patch-preloading phase; (2) experience less smoking-associated relief from PTSD symptoms and negative affect during the preloading phase; and (3) exhibit greater latency to lapse, and higher short- and long-term abstinence rates.
Methods
Sixty-three smokers with PTSD were randomized to either nicotine or placebo patch for three weeks prior to their quit date. Ecological momentary assessment was used to assess craving, smoking, PTSD symptoms, and negative affect during the preloading period.
Results
Nicotine patch-preloading failed to reduce smoking or craving during the preloading phase, nor was it associated with less smoking-associated relief from PTSD symptoms and negative affect. Moreover, no differences were observed between the treatment conditions for time to lapse, 6-week abstinence, or 6-month abstinence.
Conclusions
The findings from the present research suggest that supplemental nicotine patch-preloading is unlikely to substantially enhance quit rates among smokers with PTSD.
Keywords: Smoking, Nicotine, Posttraumatic stress disorder, Preloading, Cessation, Treatment
1. Introduction
Posttraumatic stress disorder (PTSD) is a prevalent mental illness that is associated with a high rate of smoking (Beckham et al., 1997; Breslau, Davis, & Schultz, 2003; Cook, McFall, Calhoun, & Beckham, 2007; Feldner, Babson, & Zvolensky, 2007; Koenen et al., 2005; Lasser et al., 2000; Morissette, Tull, Gulliver, Kamholz, & Zimering, 2007; Rasmusson, Picciotto, & Krishnan-Sarin, 2006). For example, Breslau et al. (2003) found that the odds of smoking among persons with PTSD were approximately 4 times higher than persons without PTSD. Evidence from laboratory-based and ambulatory monitoring indicates that, among patients with PTSD, trauma-related stimuli, negative affect, and PTSD symptoms are associated with urges to smoke and are significant antecedents of smoking (Beckham et al., 2005; Beckham et al., 2007). Moreover, emotional reactivity to trauma-related stimuli is associated with early relapse in smokers with PTSD (Calhoun, Dennis, & Beckham, 2007). Thus, some have suggested that smoking serves as a means for managing PTSD symptoms.
Nicotine-replacement therapy (NRT) has long been used to relieve cravings during quit attempts (Fiore et al., 2008). Some have reasoned that initiating NRT prior to the quit date (i.e., supplemental nicotine patch-preloading) may increase the efficacy of NRT by diminishing the reinforcing effects of inhaled nicotine, thereby making it easier to quit smoking. Rose, Behm, Westman, and Kukovich (2006) found that prior to a quit attempt smokers rated cigarettes as less rewarding when smoking while wearing nicotine patches, and that they were twice as likely to demonstrate continuous abstinence at 4 weeks post-quit than smokers who were administered a placebo patch. Other trials testing this strategy have yielded somewhat mixed results, with some (Rose, Herskovic, Behm, & Westman, 2009; Schuurmans, Diacon, van Biljon, & Bolliger, 2004), but not all (Bullen et al., 2010), reporting significantly improved long-term (i.e., 6 months or more) smoking abstinence. More recently, Stead et al. (2012) conducted a review of this literature and concluded that supplemental nicotine patch-preloading resulted in a moderate increase in abstinence rates. Previous studies have excluded smokers with psychiatric conditions. To date, no study has examined the effects of supplemental nicotine patch-preloading on smoking abstinence among smokers with PTSD.
1.1. Objective and hypotheses
The objective of the present study was to conduct the first randomized controlled trial of the efficacy of supplemental nicotine patch-preloading among smokers with PTSD. Based on prior research in this area, we hypothesized that smokers with PTSD who were assigned to the nicotine patch condition would experience reduced craving for cigarettes and decreased smoking during the supplemental nicotine patch-preloading phase of the study relative to smokers who were given a placebo patch during the preloading phase of the study. We further hypothesized that smokers with PTSD assigned to the nicotine patch condition would experience significantly less smoking-associated relief from PTSD symptoms and negative affect during the patch-preloading phase of the study due to their increased levels of baseline nicotine. Finally, we hypothesized that smokers assigned to the nicotine patch-preloading condition would exhibit greater latency to lapse as well as higher six-week and six-month abstinence rates during the post-quit phase of the study relative to participants in the placebo patch condition.
2. Method
2.1. Participants
Participants were 63 individuals with PTSD. Eligibility criteria included smoking at least 10 cigarettes daily for the past year, willingness to make an attempt to quit smoking within the next 30 days, age 18–70 years, and fluency in English. Potential participants were excluded if they used non-cigarette forms of nicotine, were pregnant, had major unstable medical problems or unstable medication regimens, major respiratory disorders, used bupropion or benzodiazepines, or met criteria for current manic syndrome, current psychotic disorder, or current substance abuse/dependence including substance use in the three months immediately preceding screening.
2.2. Procedures
Participants were recruited from outpatient clinic referrals and by Institutional Review Board-approved flyers and letters advertising a study on PTSD and smoking cessation posted in local hospitals. Participants were compensated up to $650 for complete participation, including incentive payments for adhering to the ecological momentary assessment (EMA) protocol. Participants completed a screening session and one week of baseline EMA monitoring to measure ad lib smoking behavior. After the “ad lib smoking period”, all participants completed a two-week “pre-quit period” during which they received either active nicotine patches or placebo patches and brief cognitive-behavioral therapy (CBT). A two-week pre-loading period is typical for prior patch pre-loading trials (Bullen et al., 2010; Rose et al., 2006; Rose et al., 1994; Rose et al., 2009; Schuurmans et al., 2004). EMA monitoring continued to track symptoms and smoking behavior for six weeks after each participant’s quit date or until the participant relapsed to smoking (i.e., “post-quit period”). Lapse dates and times were recorded via EMA and at weekly study visits. Study visits were scheduled at weeks 1–6 post-quit, during which 7-day abstinence was self-reported and verified by exhaled carbon-monoxide (CO). Participants whose CO levels exceeded 10 ppm were considered non-abstinent (Croghan, 2011). Thirty-day smoking abstinence was assessed by self-report at the six-month follow-up assessment, with positive reports of abstinence to be confirmed by saliva cotinine levels ≤10 ng/ml, as suggested by Benowitz (1983).
2.3. PTSD assessment
At the screening session, each participant provided sociodemographic information, smoking history, and completed the Commitment to Quitting Smoking Scale (Kahler et al., 2007), Relapse Situation Efficacy Questionnaire (Gwaltney, 2001), Fagerström Test of Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). PTSD was assessed with the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995), using established guidelines (Weathers & Keane, 1999). The presence of current major depressive disorder (MDD) was assessed with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (First, Spitzer, Gibbon, & Williams, 1996). Study interviewers completed an extensive training program, demonstrating strong inter-rater reliability at its completion on seven video-recorded interviews (Fleiss’ kappa = 0.96).
2.4. Randomization to nicotine preloading treatment groups
Randomization to active nicotine patch or placebo patch was stratified by gender and presence of current MDD. Participants were randomized to receive either 21 mg/24 h patch or placebo for 2 weeks prior to a target quit date in a double blind fashion. Patch allocation was concealed by maintaining a list through the pharmacy that was unavailable to study investigators and coordinators.
2.5. Behavioral counseling and post-quit NRT
During the pre-quit period, all participants received two individual sessions of cognitive-behavioral smoking-cessation counseling. CBT counseling sessions lasted 50 min each and included psychoeducation about the physiological effects of smoking, behavioral strategies for coping with withdrawal symptoms, relaxation training, identification of social support, plans for reinforcing abstinence and relapse prevention. Beginning at the quit date, all participants received six weeks of active nicotine replacement therapy (starting with 21 mg/24 h nicotine patches) and one form of rescue nicotine replacement (e.g., gum, lozenge).
2.6. Ecological momentary assessments
EMA data were collected on a PalmOne Treo 755p handheld computer (PalmOne, Inc.). EMA data collection procedures were designed to evaluate the influence of patch-preloading on 1) smoking frequency and craving throughout the ad lib, patch-preloading, and post-quit periods of a quit attempt, and 2) smoking-related changes in craving and PTSD symptoms during the patch-preloading phase. Diary entries were time-stamped to ensure temporal accuracy and to promote protocol adherence.
Participants responded to random alarms throughout the day and initiated their own assessments before and after smoking. Random readings assessed time of most recent cigarette and current craving via a single item from MNWS capturing “desire of craving to smoke” on a range from 1 (“not at all”) to 5 (“extremely”). Self-initiated readings included smoking craving, mood, setting, activity, and PTSD symptoms. PTSD symptoms were assessed with four questions corresponding to King, Leskin, King, and Weathers’s (1998) PTSD factors: “Right now, how much are you bothered by…” “disturbing thoughts, images, or feelings related to your traumatic event” (PTSD cluster B); “avoiding thoughts, activities, or feelings related to your traumatic event” (PTSD cluster C1); “feeling distant or cut off from other people and/or feeling emotionally numb” (PTSD cluster C2); “difficulty concentrating, feeling jumpy or easily startled, feeling overtly alert, or feeling irritably or angry?” (PTSD cluster D). Responses to each question ranged from 1 (“not at all”) to 5 (“extremely”). Negative affect was assessed by four items from the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986). Specifically, participants indicated the extent to which they felt a) angry, irritable, and frustrated, b) anxious and nervous, c) depressed mood and sad, and d) difficulty concentrating on a scale of 0 (“none”) to 4 (“severe”) (Cappelleri et al., 2005).
Smoking frequency was assessed using the self-initiated smoking entries in addition to nightly entries in which participants recorded the total number of cigarettes smoked that day. Both sets of estimates were summed over the course of each pre-quit phase (ad lib and patch). The larger estimate of the two was retained and divided by time elapsed to generate an estimate of the number of cigarettes smoked per day. These exact EMA procedures were used in prior research examining the association between smoking and PTSD symptomatology (Dedert, Dennis, Calhoun, Dennis, & Beckham, 2014a; Dedert et al., 2014b; Volz et al., 2014).
2.7. Analysis plan
Multilevel modeling (MLM) (Snijders & Bosker, 1999) was used to analyze the data, which included multiple EMA readings nested within individual participants. Because effects are estimated at the lowest level of the analysis (i.e., diary entry) while accounting for clustering at higher levels (i.e., individual participant), MLM is uniquely suited for unbalanced data (i.e., data missing at random and with differing numbers of cases per individual). MLM can also be used to model various distributions, including normal, dichotomous, and count distributions.
To model change in symptoms from pre- to post-cigarette, change scores were tabulated by subtracting pre-cigarette symptoms from post-cigarette symptoms. As such, a negative score would represent greater symptom reduction and relief following smoking. Because change scores are often related to initial levels (Francis, Fletcher, Stuebing, Davidson, & Thompson, 1991), change scores were modeled controlling for pre-cigarette symptoms.
MLM was conducted using PROC MIXED for linear models and PROC GLIMMIX for non-linear models, both available via SAS 9.2. To test potential treatment effects on time to lapse, survival analysis was conducted using PROC PHREG. Patch pre-treatment effects on odds of 6-week and 6-month abstinence were tested using PROC LOGISTIC.
3. Results
3.1. Participant characteristics
Participant characteristics are displayed in Table 1. No group differences were observed with regard to age, sex, minority status, PTSD symptoms severity, MDD status, or nicotine dependence.
Table 1.
Participant characteristics by patch condition.
| Placebo patch (n = 31) | Active patch (n = 32) | Difference test | |
|---|---|---|---|
| Age (years) | 42.8 (7.9) | 42.3 (10.7) | t(58) = 0.19, p = 0.85 |
| Sex (n, % female) | 16 (53%) | 18 (55%) | X2(1) = 0.01, p = 0.92 |
| Minorities | 23 (79%) | 20 (63%) | X2(1) = 2.07, p = 0.15 |
| Veterans | 4 (13%) | 6 (18%) | X2(1) = 0.34, p = 0.56 |
| PTSD Severity (CAPS) | 68.0 (20.2) | 66.2 (21.8) | t(61) = 0.34, p = 0.73 |
| MDD | 14 (47%) | 9 (27%) | X2(1) = 2.55, p = 0.11 |
| Commitment to quit | 30.3 (6.0) | 31.9 (5.5) | t(57) = 1.09, p = 0.28 |
| Relapse situation efficacy | 143.6 (43.9) | 158.9 (49.4) | t(56) = 1.25, p = 0.22 |
| Nicotine dependence (FTND) | 4.1 (1.8) | 4.0 (3.4) | t(39) = 0.21, p = 0.83 |
Note. Means/frequencies and standard deviations/percentages (in parentheses). CAPS = Clinician Administered PTSD Scale; MDD = major depressive disorder; FTND = Fagerström Test for Nicotine Dependence
3.2. Electronic diary entries
Participants completed a total of 5126 random-alarm and self-initiated smoking entries during the ad lib pre-quit period, which spanned a mean of 10.31 days (SD = 9.48), 5736 entries during the patch pre-quit period (M = 16.04 days, SD = 5.12), and 4832 entries during the post-quit period (M = 33.72 days, SD = 14.88) (see Table 2 for a breakdown of total entries and entries per day by treatment condition). The response rate to random alarms was high across all three periods (73%). Mean total entries per days did not vary by treatment condition during any of the three study phases, all ps > 0.76.
Table 2.
Electronic diary entries by patch condition.
| Placebo patch n/n per day | Active patch n/n per day | |
|---|---|---|
| Pre-quit (ad lib) | ||
| Self-initiated (smoking) | 1649/6.30 | 1684/7.02 |
| Random alarm (smoking) | 169/0.67 | 144/0.62 |
| Random alarm (non-smoking) | 675/2.83 | 805/3.17 |
| Missed alarms | 311/1.11 | 398/1.28 |
| Pre-quit (patch pre-treatment) | ||
| Self-initiated (smoking) | 1530/3.33 | 1520/4.29 |
| Random alarm (smoking) | 171/0.40 | 213/0.60 |
| Random alarm (non-smoking) | 1,111/2.62 | 1,191/3.17 |
| Missed alarms | 466/1.07 | 471/1.17 |
| Post-quit | ||
| Self-initiated (smoking) | 182/0.29 | 266/0.38 |
| Random alarm (smoking) | 47/0.17 | 105/0.19 |
| Random alarm (non-smoking) | 1649/2.22 | 2663/3.05 |
| Missed alarms | 692/0.93 | 992/1.20 |
3.3. Pre-quit nicotine patch effects
3.3.1. Nicotine patch effects on nicotine craving
To determine whether active nicotine versus placebo patch condition influenced smoking craving in the pre-quit phase, momentary craving ratings recorded during random alarm and self-initiated entries were modeled via linear MLM as a function of patch condition, pre-quit phase (ad lib vs. patch pre-treatment), and the interaction between the two. The main effect of the active patch was not significant, t (59) = −1.17, p = 0.25. However, the effect of pre-quit phase was, t (52) = −7.87, p < 0.001, indicating that nicotine craving dropped by 0.26 points once participants started patch pre-treatment. The interaction between patch condition and phase was not significant, t (52) = −1.03, p = 0.31, indicating that the decrease in craving from the ad lib to the patch phase did not vary by patch condition.
3.3.2. Patch effects on smoking
To determine whether patch condition influenced smoking in the pre-quit phase, daily cigarette counts reported by each participant during a nightly EMA reading were submitted to a negative-binomial MLM as a function of patch condition, pre-quit phase, and the interaction between the two. The main effect of the active patch was not significant, t (1340) = −0.74, p = 0.46. However, the effect of phase was, t (1340) = −9.64, p < 0.001, indicating that the modeled mean cigarettes per day reported in the ad lib phase, 17.67, was significantly higher than that reported in the patch pre-treatment phase, 10.63. This effect was not moderated by treatment group, t(1340) = 0.75, p = 0.45.
3.3.3. Patch effects on smoking-related symptom relief
Across the ad lib phase, participants in both treatment groups recorded small yet significant decreases in re-experiencing (M = −0.07, p < 0.001), avoidance (M = −0.08, p < 0.001), numbing (M = −0.06, p < 0.001), and hyperarousal symptoms (M = −0.07, p < 0.001), as well as negative affect (M = −0.33, p < 0.001) directly following smoking. To determine how nicotine replacement may have influenced smoking-related symptom relief, different scores were tabulated from each of the four PTSD symptoms clusters and negative affect collected immediately prior to and following each cigarette recording during self-initiated smoking entries. These were modeled via multivariate MLM as a function of patch condition, phase, and the interaction of the two. Grand-mean standardized (i.e., z-scored) pre-cigarette symptom/affect scores were controlled as were individual-mean standardized scores to account for individual differences in overall symptom level as well as intra-individual variability. According to the model, the multivariate effect of the individual-mean standardized scores was significant, F(5,16,161) = 852.77, p < 0.001, indicating that the greatest relief in PTSD symptoms/negative affect was observed when individuals were experiencing relatively high levels of symptoms and negative affect. The multivariate effect of grand-mean standardized pre-cigarette symptoms/affect was also significant, F(5,230) = 6.66, p < 0.001, indicating that the individuals with the highest overall levels of PTSD symptoms and negative affect demonstrated the greatest relief from smoking. Neither the main effect of patch condition, F(5,230) = 0.47, p = 0.80, nor pre-quit phase, F(5,255) = 0.77, p = 0.58, were significant. The interaction between patch condition and phase was also not significant, F(5,255) = 0.40, p = 0.85, indicating that smoking-related symptom relief did not vary by patch condition. The univariate effects are depicted in Fig. 1. According to those, only one pretreatment effect was observed: Participants in the placebo patch condition reported greater relief of avoidance symptoms during patch-pretreatment. Compared to the placebo patch, the nicotine patch was not associated with decreased relief of PTSD symptoms or negative affect from the ad lib to the patch phase (p > 0.07).
Fig. 1.
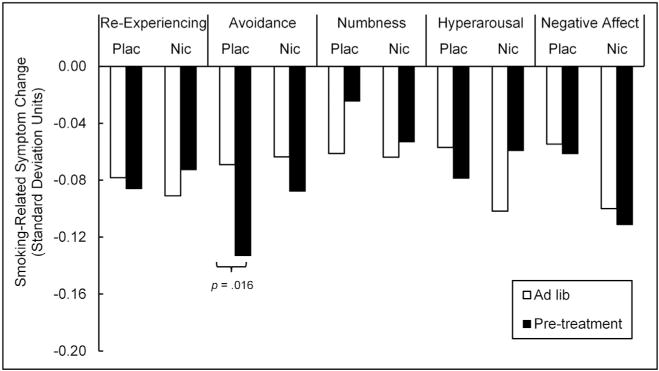
Patch effects on smoking-related symptom change. Negative change reflects decrease in symptoms after smoking. For ease of interpretation, change scores were linearly transformed by dividing them by grand (i.e., sample-wide) standard deviations in pre-smoking symptom scores recorded during the ad lib phase (re-experiencing SD = 1.00, avoidance SD = 1.09, numbness SD = 1.22, hyperarousal SD = 1.09, negative affect SD = 4.15). Plac = placebo patch; Nic = nicotine patch.
3.4. Post-quit patch effects
Nine participants in the placebo-patch condition (29%) and 7 in the active-patch condition (22%) dropped out of the study prior to the quit date. No differences in age, t(55) = 0.67, p = 0.51, sex, X2(1, n = 59) = 1.51, p = 0.22, minority status, X2(1, n = 60) = 2.60, p = 0.11, veteran status, X2(1, n = 60) = 2.93, p = 0.09, total CAPS scores, t(57) = 0.54, p = 0.59, MDD status, X2(1, n = 59) = 0.12, p = 0.73, or nicotine dependence, t(57) = 0.34, p = 0.73, were observed between the 16 participants who dropped out and the 47 who remained in the study post-quit. Of the 47 participants who underwent post-quit monitoring, 42 (21 in each arm) yielded reliable lapse data.
3.4.1. Time to first lapse
During the post-quit period, all 21 of the participants on the placebo patch and all but 2 of the 21 participants on the active patch recorded a lapse, Fisher’s exact test p = 0.49. Mean time to lapse for the 40 participants who lapsed was 4.33 days (SD = 6.43). To determine whether the active patch forestalled smoking in the post-quit period, survival analysis was conducted on time to lapse as a function of patch condition, with baseline nicotine dependence controlled. According to the survival analysis, neither nicotine dependence (HR = 1.09, p = 0.51) nor active-patch pre-treatment (HR = 0.97, p = 0.91) was associated with time to lapse.
3.4.2. Abstinence at 6 weeks and 6 months
Analysis of abstinence rates were based on intent-to-treat principles, with missing data being treated as smoking. At 6 weeks post-quit, 29 participants (12 in the placebo-patch condition and 17 in the active-patch condition) provided bio-verified 7-day abstinence data. Two participants (17%) in the placebo-patch condition and 4 (24%) in the active-patch condition were abstinent. Odds of 6-week abstinence were not significantly greater for the active-patch condition in comparison to the placebo-patch condition, OR = 1.54, 95% CI: 0.23–10.15. Twenty-six participants (12 in the placebo-patch condition and 14 in the active-patch condition) were reached for the 6-month follow-up. All 26 reported smoking in the prior 30 days.
4. Discussion
In contrast with our hypotheses, as well as several previous studies that have demonstrated positive effects for supplemental nicotine patch-preloading in other groups of smokers (e.g. Rose et al., 2006; Rose et al., 2009; Schuurmans et al., 2004), the present research found that supplemental nicotine patch-preloading did not improve time to lapse, 6-week abstinence rates, or 6-month abstinence rates among smokers with PTSD. Supplemental nicotine patch-preloading also failed to reduce smoking-associated relief from PTSD symptoms and negative affect in smokers with PTSD. Taken together, these findings suggest that supplemental nicotine patch-preloading is unlikely to substantially enhance quit rates for smokers with PTSD.
One possible explanation for the present findings is that smokers with PTSD may be less likely to benefit from standard smoking-cessation techniques that are effective in the general population due to their high levels of PTSD symptoms and negative affect. Prior studies of nicotine preloading have been conducted in samples with little to no psychopathology. Smokers with PTSD may require significantly more intensive treatment approaches that combine behavioral treatment such as contingency management (CM) with other evidenced-based treatment focused on relapse prevention such as CBT. Recently, Hertzberg et al. (2013) piloted an intervention that paired mobile CM with CBT and smoking-cessation aids (e.g., NRT, and bupropion). This high-intensity intervention was well-tolerated and led to high quit rates among smokers with PTSD at both 4-weeks (82%) and three-months (50%). Although the findings from Hertzberg et al. (2013) are based on a small sample and are in need of replication, they are consistent with the idea that many smokers with PTSD and other forms of serious mental illness may require substantially more intensive treatment approaches in order to successfully quit smoking.
4.1. Limitations
The present findings should be interpreted within the context of several limitations. First, this was a relatively small study. It is possible that a larger sample size with greater statistical power would have enabled us to identify statistically significant group differences; however, the use of MLM to analyze the pre-quit data maximized statistical power by taking into account repeated measurements. Moreover, given how similar the results were across the treatment arms, we believe that insufficient power is unlikely to blame for lack of treatment effects. Given the relatively poor outcomes, another possible limitation was low motivation to quit. However, only smokers willing to make a quit attempt within the next 30 days were enrolled in the study. Another factor potentially contributing to the minimal impact of the nicotine patch pre-treatment was the use of 21 mg/24 h patches, which may have been insufficient to assuage the craving of smokers with higher nicotine metabolism rates (Schnoll et al., 2009). This limitation was imposed by our IRB over safety concerns. That said, overall effects of higher dose patch pre-treatment are fairly minimal (Stead et al., 2012), and individual differences in nicotine metabolism were likely evenly distributed over the two treatment groups due to randomization, thus minimizing any effects on treatment outcomes. This study is also limited in that a single item was used to measure craving. Although the single item of craving used in this study has been successfully used previously to distinguish craving effects (Volz et al., 2014), the use of a single item could have limited variance compared to longer multiitem scales. Given evidence that integrated treatment of PTSD and smoking leads to improved smoking abstinence outcomes (e.g. Hertzberg, Moore, Feldman, & Beckham, 2001; McFall et al., 2005), future studies might consider studying supplemental nicotine patch-preloading within the context of an integrated treatment protocol.
4.2. Conclusion
We found that supplemental nicotine patch-preloading did not lead to reductions in craving and smoking during the preloading phase, nor was it associated with reductions in smoking-associated relief from PTSD symptoms and negative affect. Supplemental nicotine patchpreloading also failed to improve time to lapse, or abstinence rates among smokers with PTSD. Thus, our findings suggest that supplemental nicotine patch-preloading is unlikely to substantially improve quit rates for smokers with PTSD. It is likely that more intensive, multi-component treatment approaches will be necessary to improve smoking-cessation outcomes within this important subset of smokers.
HIGHLIGHTS.
Smokers with PTSD received an active or placebo patch prior to a quit attempt.
Active patch preloading was expected to diminish nicotine-based rewards of smoking.
Active patch was not associated with reduced craving or smoking prior to quit.
Active patch was not associated with reduced symptom relief from smoking pre-quit.
Active patch did not increase time to lapse or short- and long-term abstinence.
Acknowledgments
We would like to express our gratitude to the participants who volunteered to participate in this study.
Role of funding sources
This work was supported by the National Institutes of Health (R21CA128965; R01CA037220; R34DA038272), by the Department of Veterans Affairs (VA) Office of Research and Development (ORD) Health Services Research and Development Service (HSR&D; I01HX000132; I01HX001109), and by the VA Mid-Atlantic Mental Illness Research, Education, and Clinical Center. Drs. Kimbrel and Dedert were supported by Career Development Awards (IK2CX000525 and IK2CX000718) from the Clinical Science Research and Development Service (CSR&D) of the VA ORD. Dr. Beckham was supported by a VA Research Career Scientist Award (11S-RCS-009) from the CSR&D of the VA ORD. Funding sources had no role in the design, execution, analysis, interpretation of the data, or the decision to submit results for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the VA or US Government, or any of the institutions with which the authors are affiliated.
Abbreviations
- PTSD
posttraumatic stress disorder
- NRT
nicotine-replacement therapy
- EMA
ecological momentary assessment,
- CBT
cognitive-behavioral therapy
- CO
carbon monoxide
- CAPS
Clinician Administered PTSD Scale
- MDD
major depressive disorder
- MNWS
Minnesota Nicotine Withdrawal Scale
- MLM
multilevel modeling
- CM
contingency management
Footnotes
Contributors
Paul Dennis conducted the statistical analyses and wrote the initial draft of the manuscript. Nathan Kimbrel and Eric Dedert provided substantial edits and content to subsequent drafts. Jean Beckham was a co-investigator and provided input on study design and analysis. Patrick Calhoun, the primary investigator, designed and managed all aspects of the study including publication of results. Michelle Dennis oversaw data collection and management. All authors have approved the final manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, Fairbank JA. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addictive Behaviors. 1997;22:637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Feldman ME, Vrana SR, Mozley SL, Erkanli A, Clancy CP, Rose JE. Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: A preliminary study. Experimental and Clinical Psychopharmacology. 2005;13:218–228. doi: 10.1037/1064-1297.13.3.219. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, Vrana SR. The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addictive Behaviors. 2007;32(12):2900–2915. doi: 10.1016/j.addbeh.2007.04.026. http://dx.doi.org/10.1016/j.addbeh.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. The use of biologic fluid samples in assessing tobacco smoke consumption. In: Grabowski J, Bell CS, editors. NIDA Research Monograph. Vol. 48. Washington, D.C: Department of Health and Human Services; 1983. pp. 6–26. [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered posttraumatic stress disorder scale. Journal of Traumatic Stress. 1995;8:75–80. doi: 10.1007/BF02105408. doi: 0894-9867/95/0100-U075507,50/1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Bullen C, Howe C, Lin R, Grigg M, Laugesen M, McRobbie H, Whittaker R. Pre-cessation nicotine replacement therapy: Pragmatic randomized trial. Addiction. 2010;105(8):1474–1483. doi: 10.1111/j.1360-0443.2010.02989.x. [DOI] [PubMed] [Google Scholar]
- Calhoun PS, Dennis MF, Beckham JC. Emotional reactivity to trauma stimuli and duration of past smoking cessation attempts in smokers with posttraumatic stress disorder. Experimental and Clinical Psychopharmacology. 2007;15:256–263. doi: 10.1037/1064-1297.15.3.256. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade A, Gilbert DG. Revealing the multidimensional framework of the Minnesota nicotine withdrawal scale. Current Medical Research and Opinion. 2005;21(5):749–760. doi: 10.1185/030079905X43712. [DOI] [PubMed] [Google Scholar]
- Cook JW, McFall MM, Calhoun PS, Beckham JC. Posttraumatic stress disorder and smoking relapse: A theoretical model. Journal of Traumatic Stress. 2007;20(6):989–998. doi: 10.1002/jts.20275. [DOI] [PubMed] [Google Scholar]
- Croghan E. Local Stop Smoking Services: Service delivery and monitoring guidance 2011/2012. London, UK: Department of Health and Human Services; 2011. [Google Scholar]
- Dedert EA, Dennis PA, Calhoun PS, Dennis MF, Beckham JC. Nicotine preloading for smoking cessation in posttraumatic stress disorder. Manuscript under review. 2014a doi: 10.1016/j.addbeh.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedert EA, Dennis PA, Swinkels CM, Calhoun PS, Dennis MF, Beckham JC. Ecological momentary assessment of posttraumatic stress disorder symptoms during a smoking quit attempt. Nicotine & Tobacco Research. 2014b;16(4):430–436. doi: 10.1093/ntr/ntt167. http://dx.doi.org/10.1093/ntr/ntt167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and posttraumatic stress: A critical review of the empirical literature. Clinical Psychology Review. 2007;27:14–45. doi: 10.1016/j.cpr.2006.08.004. http://dx.doi.org/10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, Wewers ME. Treating tobacco use and dependence: 2008 update. United States Department of Health and Human Services; 2008. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV Axis I Disorders. New York, NY: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- Francis D, Fletcher J, Stuebing K, Davidson K, Thompson N. Analysis of change: Modeling individual growth. Journal of Consulting and Clinical Psychology. 1991;59(1):27–37. doi: 10.1037//0022-006x.59.1.27. [DOI] [PubMed] [Google Scholar]
- Gwaltney J. Relapse situation efficacy questionnaire (RSEQ) Journal of Consulting and Clinical Psychology. 2001;69:516–527. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hertzberg MA, Moore SD, Feldman ME, Beckham JC. A preliminary study of bupropion sustained-release for smoking cessation in patients with chronic post-traumatic stress disorder. Journal of Clinical Psychopharmacology. 2001;21:94–98. doi: 10.1097/00004714-200102000-00017. [DOI] [PubMed] [Google Scholar]
- Hertzberg JS, Carpenter VL, Kirby AC, Calhoun PS, Moore SD, Dennis MF, Beckham JC. Mobile contingency management as an adjunctive smoking cessation treatment for smokers with posttraumatic stress disorder. Nicotine & Tobacco Research. 2013;15(11):1934–1938. doi: 10.1093/ntr/ntt060. http://dx.doi.org/10.1093/ntr/ntt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kahler C, LaChance H, Strong D, Ramsey SE, Monti P, Brown R. The commitment to quitting smoking scale: Initial validation in a smoking cessation trial for heavy social drinkers. Addictive Behaviors. 2007;32(10):2420–2424. doi: 10.1016/j.addbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DW, Leskin GA, King LA, Weathers FW. Confirmatory factor analysis of the clinician-administered PTSD scale: Evidence for the dimensionality of posttraumatic stress disorder. Psychological Assessment. 1998;10(2):90–96. http://dx.doi.org/10.1037/1040-3590.10.2.90. [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Archives of General Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. http://dx.doi.org/10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Thompson CE, Yoshimoto D, Malte C, Straits-Troster K, Steele B. Improving the rates of quitting smoking for veterans with posttraumatic stress disorder. American Journal of Psychiatry. 2005;162:1311–1319. doi: 10.1176/appi.ajp.162.7.1311. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychological Bulletin. 2007;133(2):245–272. doi: 10.1037/0033-2909.133.2.245. http://dx.doi.org/10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Picciotto MR, Krishnan-Sarin S. Smoking as a complex but critical covariate in neurobiological studies of posttraumatic stress disorders: A review. Journal of Psychopharmacology. 2006;20:693–707. doi: 10.1177/0269881106060193. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clinical Pharmacology Therapy. 1994 doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm F, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine & Tobacco Research. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine & Tobacco Research. 2009;11(9):1067–1075. doi: 10.1093/ntr/ntp103. http://dx.doi.org/10.1093/ntr/ntp103. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto E, Tyndale R, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: A validation study. Pharmacology Biochemistry and Behavior. 2009;92(1):6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans MM, Diacon AH, van Biljon X, Bolliger CT. Effect of pretreatment with nicotine patch on withdrawal symptoms and abstinence rates in smokers subsequently quitting with the nicotine patch: A randomized controlled trial. Addiction. 2004;99:634–640. doi: 10.1111/j.1360-0443.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel analysis: An introduction to basic and advanced multilevel modeling. London: Sage Publications; 1999. [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2012;14(11):CD000146. doi: 10.1002/14651858.CD000146.pub4. http://dx.doi.org/10.1002/14651858.CD00146.pub4. [DOI] [PubMed] [Google Scholar]
- Volz AR, Dennis PA, Dennis MF, Calhoun PS, Wilson SM, Beckham JC. The role of daily hassles and distress tolerance in predicting cigarette craving during a quit attempt. Nicotine & Tobacco Research. 2014;16(6):872–875. doi: 10.1093/ntr/ntt286. http://dx.doi.org/10.1093/ntr/ntt286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM. Psychological assessment of traumatized adults. In: Saigh PA, Brenner JD, editors. Posttraumatic stress disorder: a comprehensive text. Needham Heights, MA: Allyn & Bacon; 1999. pp. 219–247. [Google Scholar]


