Abstract
Background
Aromatase inhibitors are sometimes used in the treatment of selected patients with uterine leiomyosarcoma (LMS), but there are few data assessing the efficacy of aromatase inhibitors in this setting.
Methods
We performed a retrospective electronic medical record review of patients with uterine LMS treated with an aromatase inhibitor at Memorial Sloan-Kettering Cancer Center between 1998–2008. We assessed progression-free survival (PFS) and objective response among patients with measurable disease and explored the correlation of hormone receptor status with outcome.
Results
Forty patients with advanced or recurrent uterine LMS were treated with aromatase inhibitors. Thirty-four had measurable uterine disease. Hormone receptor status for these patients was as follows: estrogen receptor (ER) positive-22, ER negative-9, ER unknown-3, progesterone receptor (PR) positive-10, PR negative-10, PR unknown-14. Aromatase inhibitors used were: letrozole (in 74% of patients), anastrozole (21%), and exemestane (6%_. Median PFS was 2.9 months (95% CI: 1.8–5.1). The 1-year PFS rate was 28% (95% CI: 11–48%) for ER and/or PR positive uterine LMS. Best objective response was partial response (PR) in 3/34 patients (9%) (all of whom were ER positive).
Conclusions
In this population of patients with mostly low-volume and ER positive uterine LMS, aromatase inhibitors achieve objective response in only 9%. Relatively prolonged PFS was observed among ER positive uterine LMS patients. In the absence of a no-treatment control group, the prolonged PFS cannot be attributed solely to the activity of the aromatase inhibitor treatment since it may reflect the underlying biology of low-volume, ER positive uterine LMS.
Precis
Our study is the largest case series evaluating the efficacy of aromatase inhibitors in patients with uterine LMS.
INTRODUCTION
Uterine leiomyosarcoma (LMS) accounts for 1.3% of all uterine malignancies, with an estimated annual incidence of 0.55 per 100,000 women [1]. These tumors follow an aggressive clinical course with overall 5-year survival less than 50% in stage I-II uterine LMS, and less than 15% in patients with advanced stage disease [2]. Primary management of localized uterine LMS consists of total abdominal hysterectomy, bilateral salpingo-oophorectomy and excision of all resectable tumor [3]. There is no established standard for adjuvant chemotherapy or radiotherapy after resection of disease [4–6]. Chemotherapy is the mainstay of treatment for advanced, unresectable uterine LMS. However, the median duration of response is less than 8 months [7–15]. Therapeutic options are limited for patients who progress following standard cytotoxic regimens and there is an urgent need to identify new active agents.
Similar to other gynecological malignancies, many of these tumors express hormone receptors, including ER (7–71%) and PR (17–60%) [16–20]. In post-menopausal or oophorectomized women, aromatase inhibitors, including anastrozole, letrozole and exemestane, can induce a profound reduction in circulating estrogen levels through inhibition of the enzyme aromatase. Aromatase inhibitors have an established role in the treatment of early-stage and advanced breast cancer. Several small studies have reported responses to aromatase inhibitors in hormone-sensitive endometrial cancer, endometrial stromal sarcoma, and ovarian cancer [21–25].
Anecdotal evidence in the literature suggests a role for aromatase inhibitors in the management of metastatic uterine LMS. There is a report of two patients with recurrent, hormone receptor positive uterine LMS, and a case report of a patient with ER positive uterine LMS metastatic to lung, who were successfully treated with letrozole or anastrozole [26, 27]. Aromatase inhibitors are generally well-tolerated oral agents which represent an attractive, targeted treatment for uterine LMS; however, there are no data assessing their efficacy. We sought to evaluate the efficacy of aromatase inhibitors in a cohort of patients with uterine LMS treated at our institution.
METHODS
We performed a retrospective study of patients with advanced or recurrent leiomyosarcoma treated with an aromatase inhibitor at Memorial Sloan-Kettering Cancer Center (MSKCC) from January 1998 to June 2008. The study was approved by the Institutional Review Board. Patients were identified using the institutional database and pharmacy records. Patients were excluded if they received the aromatase inhibitor as treatment for breast cancer or received concomitant chemotherapy. Patients’ electronic medical records were reviewed for age at diagnosis, stage, sites of metastases, volume of metastatic disease, tumor grade, hormone receptor status, performance status, prior treatments, type and dose of aromatase inhibitor used and toxicities. In addition, we recorded the presence or absence of the following comorbidities: hypertension, ischemic heart disease, hypercholesterolemia, asthma, chronic obstructive airway disease, thyroid disease, renal failure, cerebrovascular disease, thromboembolic disease and other malignancies. Only patients with measurable disease on radiographic imaging prior to aromatase inhibitor treatment were included in our analyses.
All patients had surgical biopsies reviewed by the MSKCC Department of Pathology, which confirmed the diagnosis of uterine LMS and tumor grade. Tumors were diagnosed as “high grade leiomyosarcoma” if they met criteria established by Bell et al. [28]. Tumors diagnosed as “low grade leiomyosarcoma” met criteria for “atypical leiomyoma with low recurrence rate,” or “smooth muscle tumor of low malignant potential with limited experience” if metastasis was present at diagnosis or the patient experienced recurrence that failed to meet high grade leiomyosarcoma criteria [28].
Immunohistochemistry for ER and PR was performed on formalin-fixed, paraffin embedded, representative, whole sections of tumor. Monoclonal antibody ER1D5 (Beckman Coulter; Miami, FL) was used for ER, diluted 1:100, after antigen retrieval with citrate, pH 6. Monoclonal antibody 10A9 (Beckman Coulter; Miami, FL) was used for PR, diluted 1:200. Staining was performed using standard methodology on an automated Ventana stainer (Ventana; Tucson, AZ). Sections demonstrating nuclear immunoreactivity in up to 10% of tumor cells were scored as negative and those with greater than 10% staining were scored as positive. In 3 patients who had not previously had their tumors tested for hormone receptors, we performed ER and PR immunohistochemical staining on archival tissue.
The primary end-point of the study was progression-free survival, defined as time from the start of aromatase inhibitor treatment until disease progression or death. Patients who had reached neither endpoint were censored at date of last follow-up. The Kaplan-Meier method was used to estimate PFS. Objective response was evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) criteria [29]. We explored the correlation of ER/PR status with objective response and PFS. Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 [30].
RESULTS
Patient and Tumor Characteristics
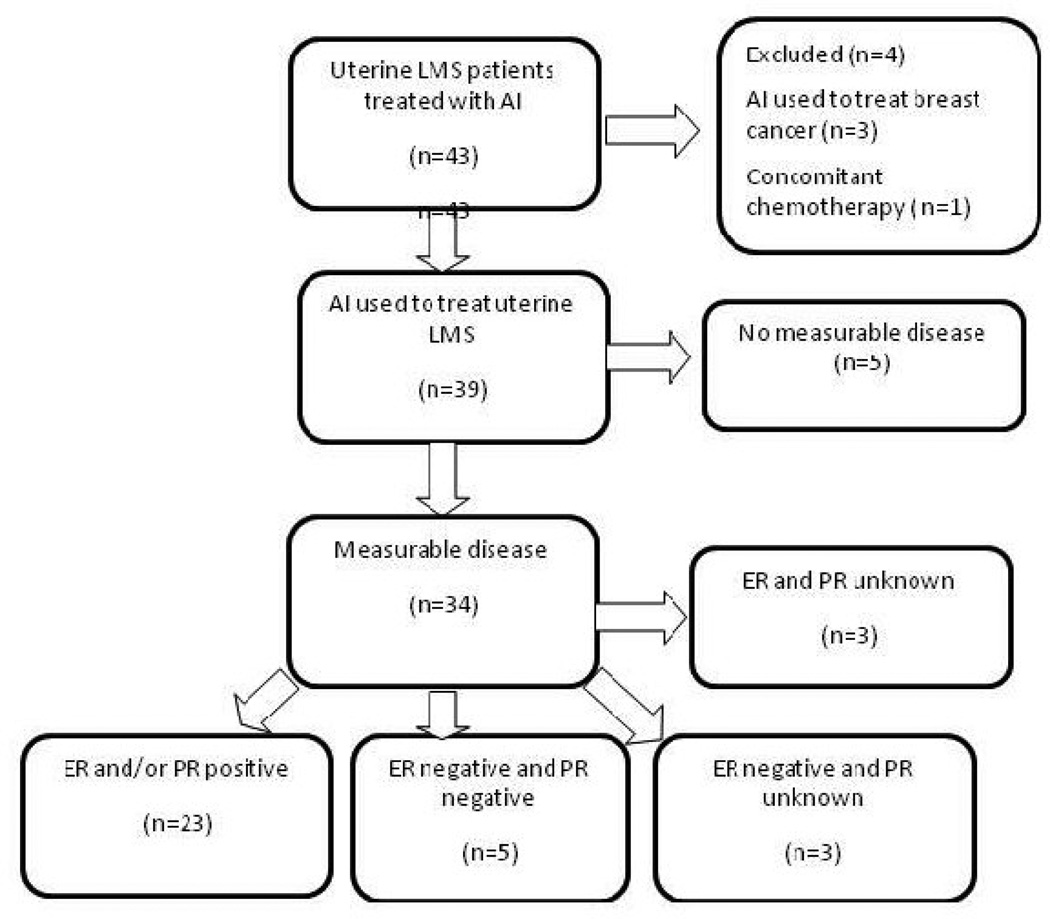
We identified 43 patients with uterine LMS treated with an aromatase inhibitor between January 1998 and June 2008 (Figure 1). Four of these patients were excluded: three with a history of uterine LMS who were prescribed an aromatase inhibitor for a subsequent diagnosis of breast cancer, and one with metastatic uterine LMS who received concomitant temozolamide with the aromatase inhibitor. Of the remaining 39 patients, 5 did not have evidence of measurable disease at the time of aromatase inhibitor initiation and were not included in the analyses. The demographics and tumor characteristics of the remaining 34 patients with measurable disease are listed in Table 1. Fifteen patients (44%) had uterine LMS confined to the uterus at initial diagnosis (FIGO stage I or II) and subsequently developed locally recurrent and/or metastatic disease. Nineteen patients (56%) had advanced disease at initial diagnosis. The median age at time of aromatase inhibitor initiation was 53 (range, 35 to 74). Ninety-one percent were postmenopausal at the time of treatment with the aromatase inhibitor. Tumors were classified as high grade LMS in twenty-nine patients (85%) and low-grade LMS in 15%.
Figure 1.
Flow Chart of uterine leiomyosarcoma Patients Treated with an Aromatase Inhibitor
Abbreviations: LMS, leiomyosarcoma; AI, aromatase inhibitor; ER, estrogen receptor; PR progesterone receptor.
Table 1.
Patient and Tumor Characteristics (n=34)
| Variable | n(%), Median(Range) |
|---|---|
| Median age at AI initiation | 53 years (35–74) |
| Performance status | |
| 0 | 2 (6%) |
| 1 | 30 (88%) |
| 2 | 1 (3%) |
| 3 | 1 (3%) |
| Ethnicity | |
| Caucasian | 27 (79%) |
| Hispanic | 4 (12%) |
| African American | 2 (6%) |
| Asian/Indian | 1 (3%) |
| Postmenopausal status | 31 (91%) |
| Median body mass index | 28 kg/m2 (16–52) |
| Number of comorbidities | |
| 0–1 | 14 (41%) |
| 2–3 | 18 (53%) |
| 4–5 | 2 (6%) |
| FIGO stage at diagnosis | |
| I | 11 (32%) |
| II | 4 (12%) |
| III | 3 (9%) |
| IV | 16 (47%) |
| Sites of metastases at time of AI initiation | |
| Lung | 30 (88%) |
| Lung as only site of metastases | 6 (18%) |
| Pelvis | 19 (56%) |
| Bone | 6 (18%) |
| Liver | 5 (15%) |
| Brain | 1 (3%) |
| Tumor volume at time of AI initiation | |
| Low | 19 (56%) |
| High | 15 (44%) |
| Histologic grade | |
| Low | 5 (15%) |
| High | 29 (85%) |
| Hormone receptor status | |
| ER | n=31 |
| Positive | 22 (71%) |
| Negative | 9 (29%) |
| PR | n=20 |
| Positive | 10 (50%) |
| Negative | 10 (50%) |
| ER and PR | n=20 |
| Positive | 9 (45%) |
| Negative | 5 (25%) |
Abbreviations: AI, Aromatase inhibitor; FIGO, International Federation of Gynecology and Obstetrics; ER, estrogen receptor; PR, progesterone receptor
ER status was determined in 31 of 34 patients. Twenty-two of these 31 patients (71%) had ER positive tumors. Progesterone receptor status was available for 20 patients: 10 were PR positive, and 10 were PR negative. Of the 10 patients with PR positive disease, all except one were also ER positive. Among the 20 patients whose biopsy specimen was stained for both ER and PR, 5 had tumors that were reported as negative for both hormone receptors.
Prior surgical and systemic treatment details are provided in Table 2. Thirty-one (91%) of the 34 patients had undergone hysterectomy; 27 (79%) had had bilateral salpingo-oophorectomy. Eighteen patients (53%) in the cohort had had further surgical debulking for recurrent disease immediately prior to starting the aromatase inhibitor. Despite such surgical debulking, all of these patients had measurable disease at time of aromatase inhibitor treatment. Eleven of these 18 patients had low volume, measurable disease (defined as the absence of any tumor deposit greater than 2 cm in longest diameter on radiographic imaging), and in another six patients the largest tumor deposit was less than 4 cm post-operatively.
Table 2.
Patient treatment details (n=34)
| Variable | n(%), Median(Range) |
|---|---|
| Initial management at diagnosis of uLMS | |
| Surgical resection alone | 22 (65%) |
| Surgical resection and chemotherapy | 11 (33%) |
| Chemotherapy alone | 1 (3%) |
| Number of prior chemotherapy regimens | |
| 0 | 11 (32%) |
| 1 | 10 (29%) |
| 2–3 | 11 (32%) |
| ≥ 4 | 2 (6%) |
| Prior hormonal treatment (medroxyprogesterone, tamoxifen) |
7 (31%) |
| Prior pelvic radiotherapy | 12 (35%) |
| Median interval between diagnosis and AI initiation |
1.2 years (0.02–22) |
| AI used | |
| Letrozole [with leuprolide] | 25 (74%) [3 (9%)] |
| Anastrozole | 7 (21%) |
| Exemestane | 2 (6%) |
Abbreviations: uLMS, uterine leiomyosarcoma; AI, aromatase inhibitor
Among all 34 patients with measurable recurrent or metastatic uterine LMS at the time of aromatase inhibitor commencement, 19 (56%) had only low volume disease. Sites of disease included lung in 30 patients (88%) and pelvis in 19 (56%). Other sites involved were liver, soft tissue, bone and brain. The aromatase inhibitor was prescribed as salvage therapy post disease progression in 20 patients (59%). In the remaining 14 women (41%), it was used as a form of consolidation therapy to treat small volume residual disease post chemotherapy or post surgical debulking
Sixty-eight percent of patients had been treated with at least one cytotoxic agent prior to aromatase inhibitor treatment. Sixteen patients (47%) had received gemcitabine plus docetaxel, 16 (47%) an anthracycline-containing regimen, and 10 (29%) both these regimens. Seven patients (21%) had received 3 or more chemotherapy regimens. Twelve patients (35%) had had pelvic radiotherapy prior to aromatase inhibitor, including two of the chemotherapy-naive patients.
Seven patients (21%) had previously received hormonal therapy; 4 of these had received megestrol acetate, 2 had received tamoxifen (1 as adjuvant treatment for breast cancer) and 1 had received both megestrol acetate and tamoxifen. The best response observed with these hormonal agents was disease stabilization in of the patients (3 with megestrol acetate and 1 with tamoxifen) and disease progression two others. None of these patients achieved an objective response to the subsequently prescribed aromatase inhibitor. Nevertheless, it is of interest to note that one patient who had early disease progression with megestrol acetate went on to derive a prolonged progression-free survival, lasting greater than 3 years, with letrozole.
Letrozole was prescribed for 25 of the 34 patients (74%). Ten of these 25 patients were subsequently prescribed anastrozole. Three patients were prescribed exemestane (2 as first-line and one as second-line aromatase inhibitor). Three patients (9%) were still premenopausal at the time of aromatase inhibitor treatment. Leuprolide, a gonadotropin-releasing hormone agonist, was administered to suppress ovarian function in these pre-menopausal women in order to permit treatment with an aromatase inhibitor.
Objective Responses to Aromatase Inhibitor Treatment
The duration of administered aromatase inhibitor therapy ranged from 1 to 84 months. No patient achieved a complete response. A partial response was observed in 3 patients (9%) (all three patients were ER positive; one was also PR positive and 2 were PR unknown). The durations of response in these 3 patients were 12.5, 9.5 and 5 months. Eleven patients (32%) had stable disease as best response on follow-up imaging. The duration of stable disease (median PFS) for these 11 patients was 13 months (95% CI: 3.1–40.3 months). Hormone receptor status for these 11 stable disease patients was: ER positive-7 patients; ER negative/PR positive-1 patient; ER negative/PR unknown-1 patient; ER negative/PR negative-1 patient; ER and PR both unknown-1 patient. Best response was progression of disease in 20 patients (59%).
Progression-free survival
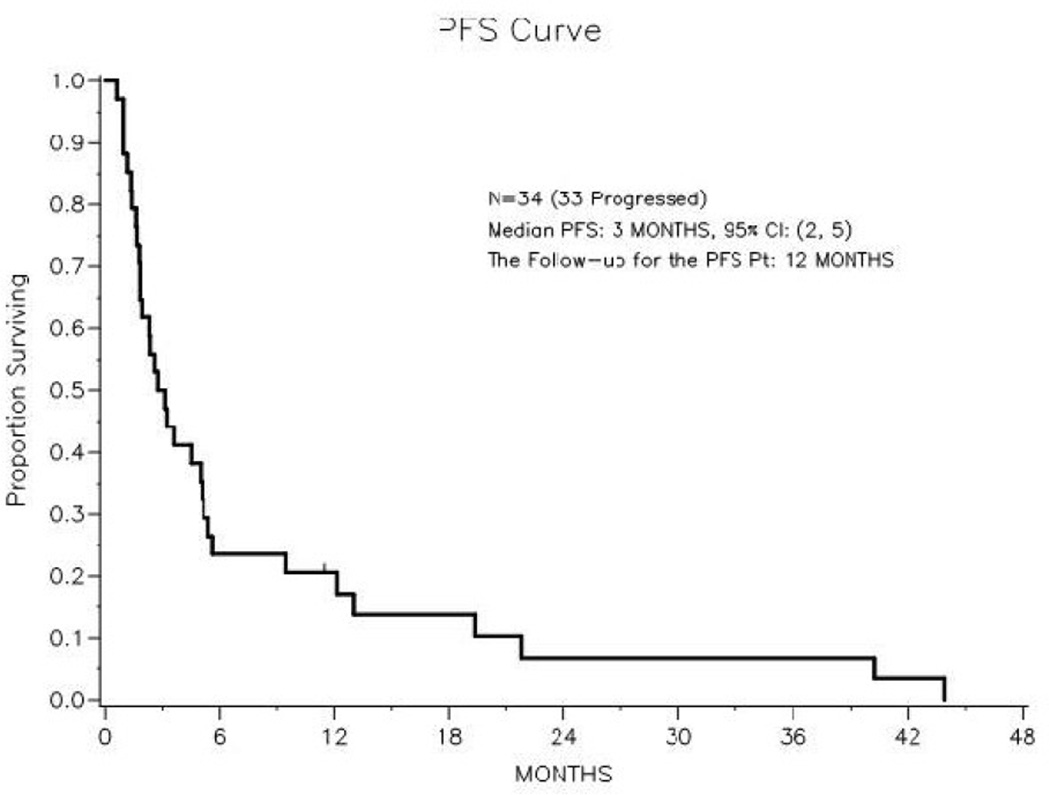
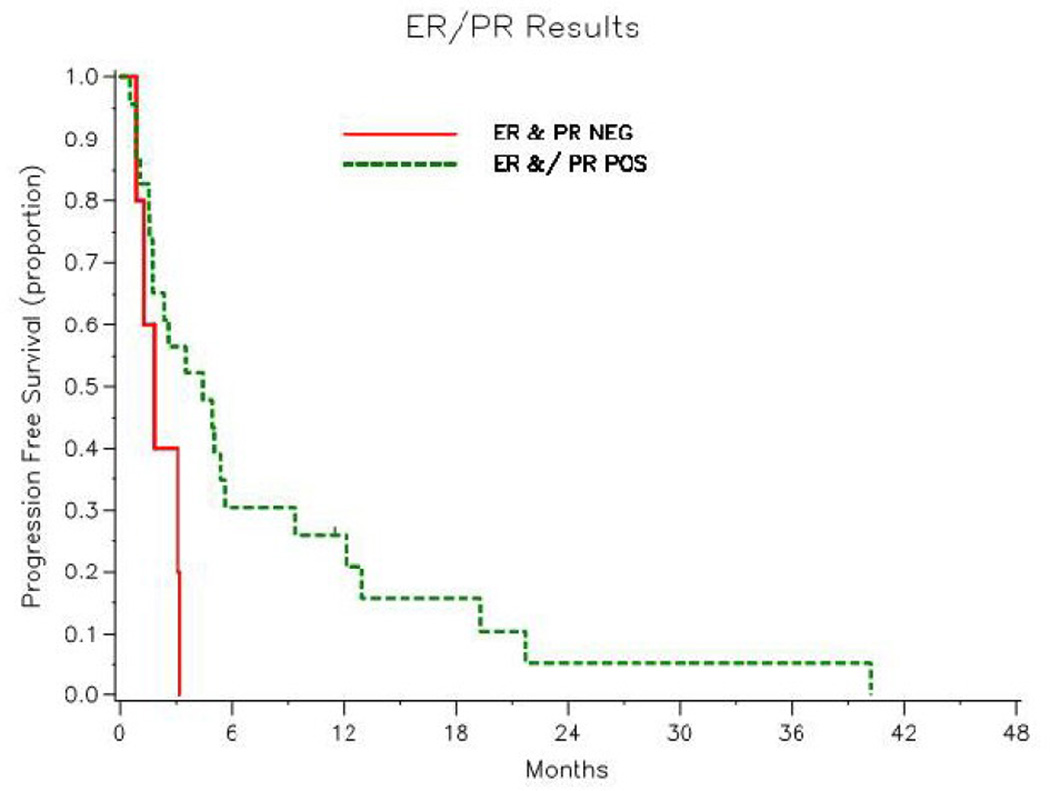
At last follow-up, 20 (59%) of the 34 patients had died with disease progression, 13 (38%) were alive with disease progression and 1 (3%) was alive without progression (Figure 2). Median PFS was 2.9 months (95% CI: 1.8–5.1 months). Figure 3 illustrates the difference in PFS between patients with ER and/or PR positive tumors and those with ER and PR negative tumors. The 1-year progression-free rate for the ER and/or PR tumor was 26% (95% CI: 11–45%). In the 5 patients with ER negative and PR negative tumors, the longest PFS recorded was 98 days.
Figure 2.
Figure 3.
Toxicities during aromatase inhibitor treatment
Toxicities observed during aromatase inhibitor treatment included grade 1–2 asthenia in 10 women (29%), hot flashes in 10 (29%) and arthralgias in 9 (26%). Six patients (18%) had significant weight gain (>5 kg) following aromatase inhibitor treatment. One patient (3%) was also observed to have new-onset peripheral edema. There were no reports of vaginal dryness, apart from 2 patients who had reported vaginal dryness prior to aromatase inhibitor treatment. Two patients (6%) with measurable disease developed significant joint pain following commencement of the aromatase inhibitor. The first patient who had stable disease at the time of anastrozole discontinuation was monitored expectantly off treatment. The second patient who had asymptomatic sub-centimeter progression while on the aromatase inhibitor was able to resume the same aromatase inhibitor, letrozole, after a brief interruption in therapy; she then went on to receive another 2 years of the drug before significant disease progression. Of the 8 patients (24%) who had pre-existing osteoarthritis, only three reported an increase in the severity of their musculoskeletal symptoms during aromatase inhibitor therapy. The rate of discontinuation of aromatase inhibitors secondary to toxicity among the 34 patients was 6%.
DISCUSSION
Our study represents the largest case series evaluating the efficacy of aromatase inhibitors in patients with uterine LMS. In a population of 34 patients with measurable, advanced uterine LMS, objective response was observed in only 9%. Median PFS was 2.9 months (95% CI: 1.8–5.1 months). Progestins and tamoxifen were among the first hormonal modulators to be explored in the management of metastatic uterine LMS. Medroxyprogesterone acetate, a synthetic derivative of progesterone, exerts an anti-estrogenic effect through binding to the PR. One case report depicted a protracted partial response to medroxyprogesterone (600 mg daily) in a patient with uterine LMS metastatic to lung [31]. Conversely, another series evaluating the efficacy of tamoxifen, medroxyprogesterone acetate or both in 28 patients with uterine LMS demonstrated only one objective responses [32]. In fact, tamoxifen has actually been linked to the subsequent development of uterine LMS in women prescribed the drug as adjuvant treatment for breast cancer [33–35]. This phenomenon is likely explained by tamoxifen’s estrogenic agonist activity in certain tissues of the female genital tract [36].
Mifepristone, a selective progesterone receptor modulator, has been shown to shrink benign uterine leiomyomata. In addition, it induced a prolonged response in a patient with disseminated osseous leiomyosarcoma [37]. This prompted the authors of one small case series to administer mifepristone at a dose of 50–200 mg daily to 3 patients with recurrent uterine LMS. One of these patients with a low-grade PR positive tumor obtained a durable objective response to the agent, exceeding 3 years. However, the other two patients, both with high-grade uterine LMS, had disease progression within 4 months of starting the drug [38, 39].
Aromatase inhibitor treatment had a favorable toxicity profile in this uterine LMS population. Joint-related toxicity was the reason for discontinuation of the drug in 2 (6%) of the 34 patients. While aromatase inhibitor treatment achieved a low objective response rate (9% partial response), 32% of patients had stable disease and thus may have had some clinical benefit. Objective response and prolonged PFS was more likely to be achieved among patients with ER and/or PR positive tumors. In other malignancies, it is widely accepted that patients are unlikely to derive benefit from anti-hormonal agents if their tumors do not express steroid receptors [40, 41].
Results of this study should be interpreted with several important caveats. The study is a retrospective cohort, and the patients who received aromatase inhibitor treatment represent a highly selected group of uterine LMS patients. Evidence of this patient selectivity is the high proportion of patients with low-volume disease, and with ER and/or PR positive disease. The expression of steroid receptors in uterine LMS has previously been associated with a better prognosis compared to those with hormone receptor negative tumors [17, 42]. It is possible that the more favorable outcome observed among our patients with ER and/or PR positive uterine LMS was merely due to tumor biology rather than a therapeutic effect of the aromatase inhibitor.
CONCLUSION
In summary, aromatase inhibitors achieved minimal rates of objective response in this highly selected population of women with advanced or recurrent uterine LMS. Objective responses were only observed among patients with ER positive tumors. Progression-free survival was longer among patients with ER and/or PR positive patients than among patients with ER and PR negative tumors. However, in the absence of a randomized, no-treatment, control group, this outcome cannot be attributed solely to the activity of the aromatase inhibitor treatment. Patients whose tumor did not express either ER or PR did not appear to derive benefit from aromatase inhibitor treatment.
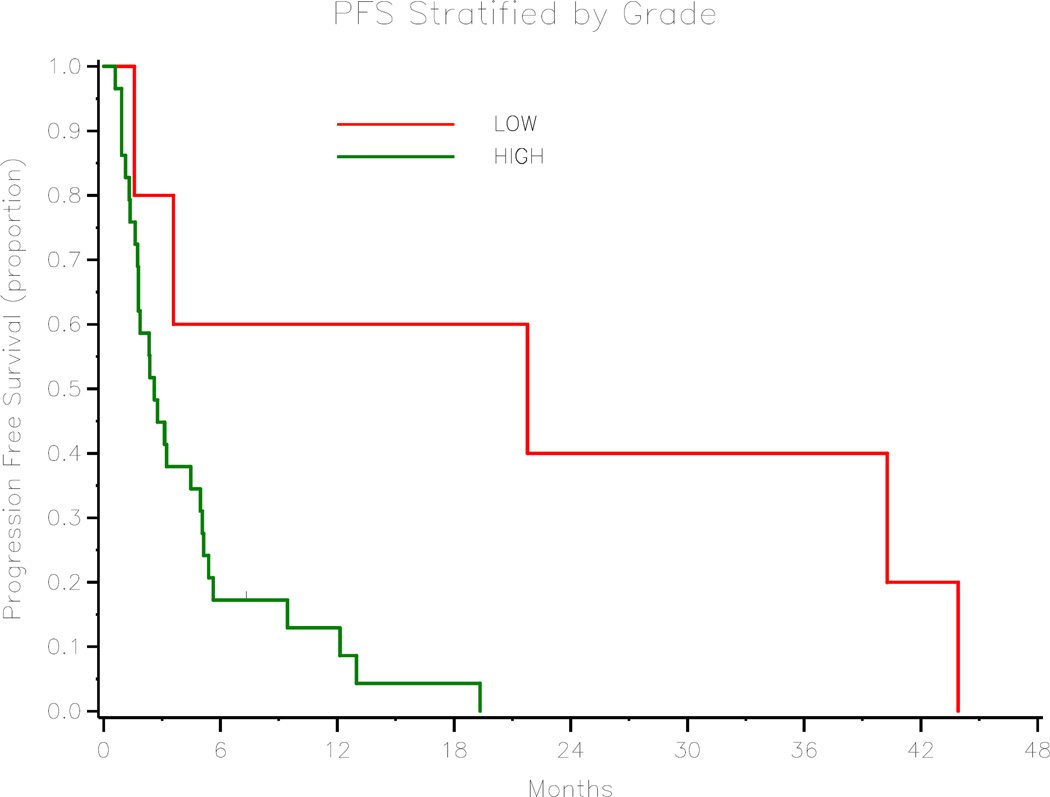
Figure 4.
Progression-free Survival Stratified by Tumor Grade
Table 3.
Best Objective Response to Aromatase Inhibitor treatment (n=34)
| Response | n (%) |
|---|---|
| Complete response | 0 (0%) |
| Partial response | 3 (9%) |
| Stable disease | 11 (32%) |
| Progressive disease | 20 (59%) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosure Statement The authors have no conflicts of interest to disclose.
References
- 1.Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 2.Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993;71(4 Suppl):1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 3.George M, Pejovic MH, Kramar A. Uterine sarcomas: prognostic factors and treatment modalities--study on 209 patients. Gynecol Oncol. 1986;24:58–67. doi: 10.1016/0090-8258(86)90008-9. [DOI] [PubMed] [Google Scholar]
- 4.Reed NS, Mangioni C, Malstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874) European Journal of Cancer. 2008;44:808–818. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Omura G, Blessing JA, Major F, et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. J Clin Oncol. 1985;3:1240–1245. doi: 10.1200/JCO.1985.3.9.1240. [DOI] [PubMed] [Google Scholar]
- 6.Hensley ML, Ishill N, Soslow R, et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I–IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecologic Oncology. 2009;112:563–567. doi: 10.1016/j.ygyno.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Hannigan EV, Freedman RS, Elder KW, Rutledge FM. Treatment of advanced uterine sarcoma with adriamycin. Gynecol Oncol. 1983;16:101–104. doi: 10.1016/0090-8258(83)90014-8. [DOI] [PubMed] [Google Scholar]
- 8.Omura GA, Major FG, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer. 1983;52:626–632. doi: 10.1002/1097-0142(19830815)52:4<626::aid-cncr2820520409>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Muss HB, Bundy B, DiSaia PJ, et al. Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group) Cancer. 1985;55:1648–1653. doi: 10.1002/1097-0142(19850415)55:8<1648::aid-cncr2820550806>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Sutton GP, Blessing JA, Barrett RJ, McGehee R. Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol. 1992;166:556–559. doi: 10.1016/0002-9378(92)91671-v. [DOI] [PubMed] [Google Scholar]
- 11.Anderson S, Aghajanian C. Temozolomide in uterine leiomyosarcomas. Gynecol Oncol. 2005;98:99–103. doi: 10.1016/j.ygyno.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Kanjeekal S, Chambers A, Fung MF, Verma S. Systemic therapy for advanced uterine sarcoma: a systematic review of the literature. Gynecol Oncol. 2005;97:624–637. doi: 10.1016/j.ygyno.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Sutton G, Blessing J, Hanjani P, Kramer P Gynecologic Oncology Group. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;96:749–752. doi: 10.1016/j.ygyno.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Tewari D, Saffari B, Cowan C, Wallick AC, Koontz MZ, Monk BJ. Activity of trabectedin (ET-743, Yondelis) in metastatic uterine leiomyosarcoma. Gynecol Oncol. 2006;102:421–424. doi: 10.1016/j.ygyno.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leitao MM, Soslow RA, Nonaka D, et al. Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer. 2004;101:1455–1462. doi: 10.1002/cncr.20521. [DOI] [PubMed] [Google Scholar]
- 17.Akhan SE, Yavuz E, Tecer A, et al. The expression of Ki-67, p53, estrogen and progesterone receptors affecting survival in uterine leiomyosarcomas. A clinicopathologic study. Gynecol Oncol. 2005;99:36–42. doi: 10.1016/j.ygyno.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Rao UN, Finkelstein SD, Jones SW. Comparative immunohistochemical and molecular analysis of uterine and extrauterine leiomyosarcomas. Mod Pathol. 1999;12:1001–1009. [PubMed] [Google Scholar]
- 19.Soper JT, McCarty KS, Jr, Hinshaw W, Creasman WT, McCarty KS, Sr, Clarke-Pearson DL. Cytoplasmic estrogen and progesterone receptor content of uterine sarcomas. Am J Obstet Gynecol. 1984;150:342–348. doi: 10.1016/s0002-9378(84)80135-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhai YL, Kobayashi Y, Mori A, et al. Expression of steroid receptors, Ki-67, and p53 in uterine leiomyosarcomas. Int J Gynecol Pathol. 1999;18:20–28. doi: 10.1097/00004347-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Spano JP, Soria JC, Kambouchner M, et al. Long-term survival of patients given hormonal therapy for metastatic endometrial stromal sarcoma. Med Oncol. 2003;20:87–93. doi: 10.1385/MO:20:1:87. [DOI] [PubMed] [Google Scholar]
- 22.Reich O, Regauer S. Hormonal therapy of endometrial stromal sarcoma. Curr Opin Oncol. 2007;19:347–352. doi: 10.1097/CCO.0b013e3281a7ef3a. [DOI] [PubMed] [Google Scholar]
- 23.Bowman A, Gabra H, Langdon SP, et al. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002;8:2233–2239. [PubMed] [Google Scholar]
- 24.Berstein L, Maximov S, Gershfeld E, et al. Neoadjuvant therapy of endometrial cancer with the aromatase inhibitor letrozole: endocrine and clinical effects. Eur J Obstet Gynecol Reprod Biol. 2002;105:161–165. doi: 10.1016/s0301-2115(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 25.Li YF, Hu W, Fu SQ, Li JD, Liu JH, Kavanagh JJ. Aromatase inhibitors in ovarian cancer: is there a role? International Journal of Gynecological Cancer. 2008;18:600–614. doi: 10.1111/j.1525-1438.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- 26.Fayette J, Ray-Coquard I, Bompas E, et al. Aromatase inhibitors (AI) are highly effective in uterine sarcomas (US) expressing estrogen receptors. J Clin Oncol (Meeting Abstracts) 2006;24:9576. [Google Scholar]
- 27.Hardman MP, Roman JJ, Burnett AF, Santin AD. Metastatic uterine leiomyosarcoma regression using an aromatase inhibitor. Obstet Gynecol. 2007;110:518–520. doi: 10.1097/01.AOG.0000267533.56546.c2. [DOI] [PubMed] [Google Scholar]
- 28.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- 29.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 30.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiation Oncology. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 31.Uchida T, Nakakawaji K, Sakamoto J, et al. The effectiveness of medroxyprogesterone in the treatment of multiple metastasizing leiomyosarcomas: report of a case. Surg Today. 1996;26:138–141. doi: 10.1007/BF00311780. [DOI] [PubMed] [Google Scholar]
- 32.Wade K, Quinn MA, Hammond I, Williams K, Cauchi M. Uterine sarcoma: steroid receptors and response to hormonal therapy. Gynecol Oncol. 1990;39:364–367. doi: 10.1016/0090-8258(90)90267-o. [DOI] [PubMed] [Google Scholar]
- 33.Silva EG, Tornos CS, Follen-Mitchell M. Malignant neoplasms of the uterine corpus in patients treated for breast carcinoma: the effects of tamoxifen. Int J Gynecol Pathol. 1994;13:248–258. doi: 10.1097/00004347-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Wickerham DL, Fisher B, Wolmark N, et al. Association of tamoxifen and uterine sarcoma. J Clin Oncol. 2002;20:2758–2760. doi: 10.1200/JCO.2002.20.11.2758. [DOI] [PubMed] [Google Scholar]
- 35.Lavie O, Barnett-Griness O, Narod SA, Rennert G. The risk of developing uterine sarcoma after tamoxifen use. Int J Gynecol Cancer. 2008;18:352–356. doi: 10.1111/j.1525-1438.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 36.Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet. 2000;356(9233):881–887. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- 37.Liote F, Lestang P, Pélissier-Langbort C, et al. Disseminated leiomyosarcoma of the bone with progesterone receptors. Rev Rhum Engl Ed. 1995;62:57–58. [PubMed] [Google Scholar]
- 38.Koivisto-Korander R, Leminen A, Heikinheimo O. Mifepristone as treatment of recurrent progesterone receptor-positive uterine leiomyosarcoma. Obstet Gynecol. 2007;109:512–514. doi: 10.1097/01.AOG.0000223228.23289.0f. [DOI] [PubMed] [Google Scholar]
- 39.Benagiano G, Bastianelli C, Farris M. Selective progesterone receptor modulators 3: use in oncology, endocrinology and psychiatry. Expert Opinion on Pharmacotherapy. 2008;9:2487–2496. doi: 10.1517/14656566.9.14.2487. [DOI] [PubMed] [Google Scholar]
- 40.Podczaski E, Mortel R. Hormonal treatment of endometrial cancer: past, present and future. Best Pract Res Clin Obstet Gynaecol. 2001;15:469–489. doi: 10.1053/beog.2000.0189. [DOI] [PubMed] [Google Scholar]
- 41.Gadducci A, Cosio S, Genazzani AR. Use of estrogen antagonists and aromatase inhibitors in breast cancer and hormonally sensitive tumors of the uterine body. Curr Opin Investig Drugs. 2004;5:1031–1044. [PubMed] [Google Scholar]
- 42.Sutton GP, Stehman FB, Michael H, Young PC, Ehrlich CE. Estrogen and progesterone receptors in uterine sarcomas. Obstet Gynecol. 1986;68:709–714. [PubMed] [Google Scholar]