Abstract
Lipoprotein lipase (LPL) is a rate-limiting enzyme for hydrolysing circulating triglycerides (TG) into free fatty acids that are taken up by peripheral tissues. Postprandial LPL activity rises in white adipose tissue (WAT), but declines in the heart and skeletal muscle, thereby directing circulating TG to WAT for storage; the reverse is true during fasting. However, the mechanism for the tissue-specific regulation of LPL activity during the fed–fast cycle has been elusive. Recent identification of lipasin/angiopoietin-like 8 (Angptl8), a feeding-induced hepatokine, together with Angptl3 and Angptl4, provides intriguing, yet puzzling, insights, because all the three Angptl members are LPL inhibitors, and the deficiency (overexpression) of any one causes hypotriglyceridaemia (hypertriglyceridaemia). Then, why does nature need all of the three? Our recent data that Angptl8 negatively regulates LPL activity specifically in cardiac and skeletal muscles suggest an Angptl3-4-8 model: feeding induces Angptl8, activating the Angptl8–Angptl3 pathway, which inhibits LPL in cardiac and skeletal muscles, thereby making circulating TG available for uptake by WAT, in which LPL activity is elevated owing to diminished Angptl4; the reverse is true during fasting, which suppresses Angptl8 but induces Angptl4, thereby directing TG to muscles. The model suggests a general framework for how TG trafficking is regulated.
Keywords: Angptl3, Angptl4, Angptl8, lipasin, lipoprotein lipase, triglyceride
1. Lipoprotein lipase
Triglycerides (TG), the main form of lipids to store and provide energy to the body, are essential to human life. To allow TG to circulate in the blood system, lipids are emulsified by proteins, forming lipoproteins. Chylomicrons and very-low-density lipoprotein (VLDL) are the two major TG-rich lipoprotein classes. Following a meal, chylomicrons are formed from dietary TG in mucosal cells within the villi of the duodenum, and reach the bloodstream through the lymphatic system. During fasting, VLDL is produced in the liver by TG synthesis, and is secreted directly into the bloodstream. These TG-rich lipoproteins transport and distribute TG to various tissues for either storage or oxidation to generate energy. In capillaries of these tissues, TG hydrolysis and uptake of the resulting fatty acids are largely dependent on a single enzyme, lipoprotein lipase (LPL) [1–5].
The discovery of LPL stemmed from a serendipitous observation made by Hahn, more than seven decades ago, that heparinized plasma cleared diet-induced lipaemia in dogs, but heparin by itself did not have this effect, indicating that heparin injection released a factor that cleared fat in the plasma [6]. As Hahn [6] noted ‘This phenomenon was so striking, even in the instances where the degree of lipemia was such that the plasma was suggestive of light cream’. This heparin-released clearing factor was later identified as LPL [7], because its activation depends on apolipoprotein C2, a component of lipoproteins, including VLDL, high-density lipoproteins (HDL) and chylomicrons [1–5].
LPL is a rate-limiting enzyme for hydrolysing TG presenting in circulating lipoproteins, generating free fatty acids that are taken up by peripheral tissues [8], including the heart [9–11], muscle [12–14] and fat [4]. LPL is abundantly expressed in the heart and skeletal muscle, which mainly depend on fatty acid oxidation for energy production, and in white adipose tissue (WAT), which stores energy by re-synthesis of TG from absorbed fatty acids [15]. In both humans and mice, deficiency of LPL results in severe hypertriglyceridaemia [16–18]. Because of the critical role that LPL plays in lipoprotein metabolism and tissue-specific substrate delivery and utilization, LPL activity is carefully orchestrated in a tissue-specific manner to meet the energy demands of various tissues at different nutritional statuses. For instance, feeding upregulates LPL activity in WAT but downregulates its activity in the heart and skeletal muscle; the reverse is true during fasting [4]. It is generally accepted that most physiological variations in LPL activity, such as during the fed–fast cycle, are determined by post-translational mechanisms involving interacting proteins, including apolipoproteins and members of angiopoietin-like protein family (Angptl) [4].
2. GPIHBP1
LPL hydrolyses TG in TG-rich lipoproteins on the surface of capillaries of peripheral tissues, including the heart, skeletal muscle and WAT; however, LPL is not expressed by capillary endothelial cells, but is produced by the parenchymal cells, myocytes and adipocytes [3]. Therefore, LPL must be transported across endothelial cells to the luminal surface of capillaries. An important discovery regarding LPL biology is that an endothelial cell protein glycosylphosphatidylinositol-anchored high density lipoprotein binding protein 1 (GPIHBP1) transports LPL into capillaries, where LPL remains anchored to the capillary wall by GPIHBP1 [19–21]. In Gpihbp1 knockout (KO) mice, LPL is mislocalized to the interstitial spaces surrounding myocytes and adipocytes, and the KO mice exhibit severe hypertriglyceridaemia (chylomicronaemia) [20,21]. In humans, GPIHBP1 loss-of-function mutations result in familial chylomicronaemia [22–25]. Without GPIHBP1, LPL cannot reach the capillary lumen, and TG-rich lipoproteins do not bind to the lumen of capillaries [19]. GPIHBP1 is, therefore, required for LPL to function on the capillary surface and is a key platform for the lipolytic processing of TG-rich lipoproteins [26–28].
3. Angptl3 and Angptl4
Angptl3 and Angptl4 are well-established inhibitors of LPL [29]. The first hint for involvement of Angptl proteins in lipid metabolism was from the study of KK/San mice, which exhibit extremely low serum TG levels. By performing positional cloning, Koishi et al. [30] identified a loss-of-function mutation in Angptl3 in these mice, suggesting that the low TG level is due to Angptl3 deficiency. Angptl3 is a circulating factor secreted from the liver, where it is specifically expressed [30]. Furthermore, Angptl3 overexpression, either by adenovirus infection or by recombinant protein i.v. injection, rescues the low TG phenotypes of KK/San mice, and leads to hypertriglyceridaemia in wild-type mice [30]. Consistently, deletion of Angptl3 in mice lowers serum TG and cholesterol levels [29,31].
Mechanistically, Angptl3 increases circulating TG levels by inhibiting LPL activity. In mice lacking Angptl3, the clearance rate of VLDL-TG was increased, whereas VLDL-TG synthesis or secretion was not affected [32]. Angptl3 has two functional domains, an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain. Angptl3 is proteolytically cleaved by proprotein convertases via recognition at the position 221–224 to yield the N-terminal domain, which is sufficient and necessary for LPL inhibition [33,34]. An Angptl3 monoclonal antibody binding to the N-terminal domain, consistently, lowers serum TG levels in mice and monkeys [35,36]. In Angptl3 KO mice, LPL activity as well as VLDL-TG incorporation are increased in oxidative tissues, including heart, muscle and brown fat [37].
Angptl4 was identified as a novel Angptl family member induced by fasting via the peroxisome proliferator-activated receptor (PPAR) in adipocytes [38–40]. Angptl4 is a potent LPL inhibitor [29,41], and plays an important role in regulating LPL activity under conditions of fasting and exercise [42]. Similar to the domain structure of Angptl3, Angptl4 is cleaved at the conserved proprotein convertase recognition sequence at position 161–164, RRKP, to release the N-terminal coiled-coil domain, which potently inhibits LPL [43,44]. Different mechanisms by which Angptl4 inhibits LPL have been proposed [45–48]. The N-terminal domain of ANGPTL4 irreversibly inhibits LPL activity by disrupting its dimerization, converting the enzyme into inactive monomers [47,48]. Using a cell-culture system to examine LPL complexed to GPIHBP1 on the endothelial cell surface, Chi et al. [46] showed that Angptl4 can bind and inactivate LPL complexed to GPIHBP1 and that inactivation of LPL by Angptl4 greatly reduces the affinity of LPL for GPIHBP1.
Mice injected with a monoclonal antibody against the Angptl4 N-terminal domain exhibit phenotypes similar to those of Angptl4-null mice, such as low plasma TG levels [35,49]. Indeed, Angptl4-null mice exhibit lower plasma TG and increased post-heparin plasma LPL activity; conversely, injection of recombinant Angptl4 or its transgenic overexpression increases plasma TG [29,41]. Angptl4 appears to inhibit LPL in an adipose-specific manner [50,51]. For instance, by cold exposure, the amount of labelled TG incorporated into WAT and BAT was altered in Angptl4 KO mice, whereas TG incorporation into muscle was comparable between KO and wild-type mice [50].
Sequence variations of ANGPTL3 and ANGPTL4 are robustly linked to lipid profiles by genome-wide association studies (GWAS). In humans, homozygotes or compound heterozygotes for loss-of-function mutations of ANGPTL3 cause familial combined hypolipidaemia, characterized by a reduction of all lipoprotein classes, such as VLDL, LDL and HDL [52,53]. The E40K substitution in ANGPTL4 is associated with lower plasma TG and HDL-C concentrations [54,55]. Re-sequencing of protein-coding regions showed that 1% of the Dallas Heart Study (DHS) population and 4% of those participants with a plasma TG in the lowest quartile have loss-of-function mutations in ANGPTL3, ANGPTL4 or ANGPTL5 [56].
4. Lipasin/Angptl8
The functional roles in lipid metabolism of a previously uncharacterized gene, Gm6484, were discovered and reported by multiple groups in 2012, under various names, such as RIFL [57], lipasin [58], Angptl8 [59] and betatrophin [60]. In October 2015, the HUGO gene nomenclature committee [61] assigned the official name of this gene as ANGPTL8 (human) and Angptl8 (mouse), which are adopted in the current review. Active research on Angptl8 in the past years has provided critical information on its function, mechanism of action and therapeutic potential [62,63].
We overexpressed Angptl8 in the mouse liver using adenovirus through tail vein injection, and Angptl8 overexpression led to dramatically increased serum TG levels [58]. Quagliarini et al. [59] found that overexpressed Angptl8 increased serum TG levels in an Angptl3-dependent manner. Mice lacking Angptl8 consistently exhibit lower TG levels owing to enhanced plasma TG clearance by having increased post-heparin LPL activity [64,65]. We recently found that Angptl8 KO mice have higher LPL activity specifically in cardiac and skeletal muscles [66]. This result suggests that Angptl8 negatively regulates LPL activity in these two tissues. Furthermore, Angptl8 is a therapeutic target because its neutralization with a monoclonal antibody (epitope being E97IQVEE) lowers serum TG levels [66].
Angptl8 expression is highly enriched in the liver, WAT and BAT [57–59]. Furthermore, Angptl8 expression is reduced by fasting, and is highly induced by feeding in both liver and adipose tissues [57–59]. In brown fat, Angptl8 is upregulated by cold exposure [67]. Using mice lacking different isoforms of sterol regulatory element-binding protein (Srebp), Angptl8 was shown to be induced by feeding independent of Srebp [59]. AMP-activated protein kinase was shown to suppress LXR/SREBP-1 signalling-induced Angptl8 expression in HepG2 cells [68]. Angptl8 is highly regulated during adipogenesis, and its knockdown significantly suppresses adipocyte differentiation [57]. Additionally, Angptl8 is upregulated by thyroid hormone and modulates autophagy [69].
In humans, ANGPTL8 sequence variations have been demonstrated to be associated with lipid profiles by GWAS. Three ANGPTL8 SNPs are strongly associated with lipid profiles. The first SNP, rs2278426, represents a nucleotide transition (C versus T, from CGG to TGG) that results in a non-synonymous amino acid change, from arginine (R) to tryptophan (W) at residue 59. Quagliarini et al. [59] found that the 59W variant is associated with lower LDL-C and HDL-C levels in various ethnic groups. Consistently, in a study composed of 4361 Mexicans, Weissglas-Volkov et al. found that WW homozygotes had 14% lower HDL-C than RR homozygotes. African Americans in the DHS had 15% lower LDL-C in WW homozygotes than in RR homozygotes [70]. The second SNP, rs145464906, represents a nucleotide transition (C versus T, from CAG to TAG) that results in a premature stop codon at residue 121, and therefore a truncated ANGPTL8 is generated by this SNP. The carriers of this presumably partial loss-of-function mutation with European ancestry were 10 mg dl−1 higher in HDL-C and 15% lower in TG levels [71]. The third SNP, rs737337, has also been found to be associated with HDL-C levels [28], and this SNP is located in the upstream region of the ANGPTL8 transcription start site [72].
Circulating levels of ANGPTL8 in human physiology and pathology have been an area of active investigation. The circulating levels of ANGPTL8 in humans were found to be decreased by overnight fasting [59] and increased 2 h following a defined meal [73]. Circulating ANGPTL8 levels were found to increase in type 2 diabetes [73–80], gestational diabetes [81–83], obese children with insulin resistance [84] and type 1 diabetes [78,85]. Nevertheless, the relationship between circulating levels of ANGPTL8 and diabetes and obesity remains inconclusive [86,87]. ANGPTL8 levels were also found to be associated with other metabolic conditions [88–96].
Therefore, overwhelming evidence from both loss- and gain-of-function studies in mice as well as human GWAS has demonstrated that Angptl8 is a feeding-induced hepatokine that is a potent regulator of lipid metabolism.
5. The Angptl3-4-8 model
TG are directed to WAT for storage after feeding, and to the heart and skeletal muscle for oxidation to generate energy during fasting. It is now clear that the process of TG trafficking is critically determined by LPL. After feeding, LPL activity rises in WAT but declines in muscles; conversely, during fasting, LPL activity declines in WAT but rises in muscles. Nevertheless, the mechanism for regulating tissue-specific LPL activity during the fed–fast cycle remains largely unknown.
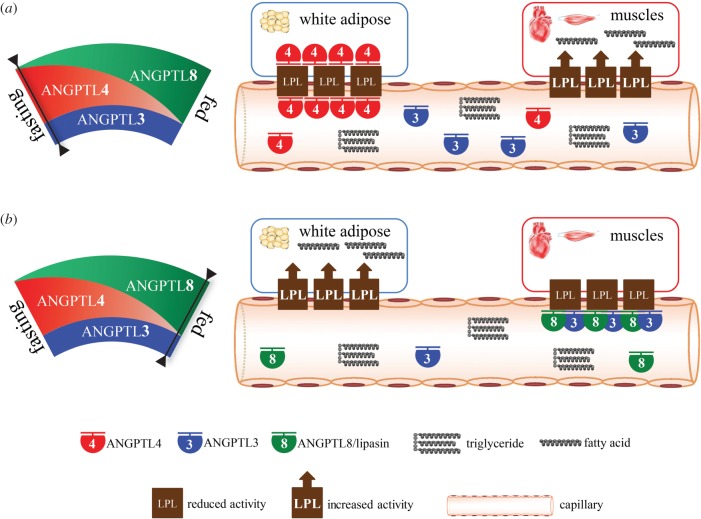
The discoveries of Angptl3 and Angptl4 have offered significant insights into this process, as both are potent LPL inhibitors. However, based on Angptl3 and Angptl4 only, the LPL regulation among WAT, the heart and skeletal muscle cannot be explained. The discovery of Angptl8 seems to complete the player set for LPL regulation, but it is puzzling that all the three Angptl members are LPL inhibitors, and that deficiency (overexpression) of any one of them results in hypotriglyceridaemia (hypertriglyceridaemia). Then why does nature need all of the three Angptl members for regulating LPL activity? Our finding that Angptl8 negatively regulates LPL activity specifically in the heart and skeletal muscle immediately suggested a model by which TG trafficking regulation is explained by Angptl3, Angptl4 and Angptl8 (Angptl3-4-8 model; figure 1) [66].
Figure 1.
The ANGPTL3-4-8 model. ANGPTL8, ANGPTL3 and ANGPTL4 regulate triglyceride (TG) trafficking by inhibiting lipoprotein lipase, in a tissue-specific manner, under different nutritional statuses. The level of ANGPTL3 is stable, regardless of nutritional status, but it requires activation by ANGPTL8. Fasting induces ANGPTL4, which inhibits LPL in WAT to direct circulating TG to cardiac and skeletal muscles for oxidation (a); conversely, feeding induces ANGPTL8, activating the ANGPTL8–ANGPTL3 pathway, which inhibits LPL in cardiac and skeletal muscles to direct circulating TG to WAT for storage (b).
According to this model, Angptl8 activates Angptl3, in an endocrine manner, to inhibit the activity of LPL in the heart and skeletal muscle, whereas Angptl4, involving intracellular and circulating species, inhibits LPL activity in WAT. Fasting upregulates Angptl4 but downregulates Angptl8, and consequently LPL activity in WAT is reduced but in muscles is increased, and therefore TG are directed to muscles for oxidation. Conversely, food intake downregulates Angptl4 but upregulates Angptl8, and consequently LPL activity in WAT is increased but in muscles is reduced, thereby directing circulating TG to WAT for storage (figure 1) [66].
Several provocative findings should be noted. In 1964, Eagle & Robinson [97] demonstrated that in WAT, blocking the transcription using actinomycin increases LPL activity during fasting. Consistently, Olivecrona and co-workers [98] showed that expression of a gene needs to turn on to downregulate adipose LPL activity. Now, it has become clear that this hypothesized fasting-induced protein that inhibits WAT LPL is Angptl4. By studying a wide array of mouse strains, Ben-Zeev et al. [99] suggested that separate genes regulate LPL activity in adipose tissue and in the heart. Importantly, Olivecrona and co-workers [100] found that, similar to adipose tissue, a transcription-dependent mechanism is involved in modulating heart LPL activity. Following actinomycin D injection, postprandial LPL activity in the heart was increased. Therefore, they proposed that feeding induced a protein that inhibits postprandial cardiac LPL activity [100]. It is likely that this hypothesized feeding-induced protein is Angptl8.
6. Angptl8 and Angptl3 function in the same pathway
Current evidence supports a notion that Angptl8 and Angptl3 function in the same pathway, that is, Angptl8 inhibits LPL, in an Angptl3-dependent manner, in cardiac and skeletal muscles, whereas Angptl3, although being abundant in the circulation regardless of nutritional status, needs to be activated by Angptl8, which is induced by feeding. By jointly considering the Angptl3-4-8 model and phenotypes of mice deficient in Angptl8 or Angptl3, we can obtain further insights into the relationship between the two Angptl members.
Angptl8 KO mice exhibited higher LPL activity in cardiac and skeletal muscles in the fed state, suggesting that Angptl8 is required for LPL inhibition in these tissues [66]. This result was obtained in mice with abundant Angptl3, suggesting that in the absence of Angptl8, Angptl3 does not effectively suppress LPL in these tissues. In other words, Angptl3 requires Angptl8 to be functionally active to inhibit LPL in cardiac and skeletal muscles.
Angptl3 KO mice exhibited higher LPL activity in cardiac and skeletal muscle in the fed state [37], suggesting that Angptl3 is required for LPL inhibition in these tissues. This result was obtained in the fed state, that is, Angptl8 was abundant, suggesting that Angptl8 required Angptl3 to inhibit muscle LPL. Consistently, hepatic Angptl8 overexpression in mice dramatically increased serum TG levels [58], but this increase was abolished in the Angptl3 KO mice [59].
It was shown that Angptl8 interacts with Angptl3, and enhances Angptl3 cleavage, releasing the N-terminal domain, which potently inhibits LPL [59]. This result leads to multiple possibilities for the mechanisms of how Angptl8 and Angptl3 function. One possibility is that Angptl8 enhances Angptl3 cleavage, releasing the N-terminal domain, which in turn targets muscle LPL, but Angptl8 itself remains in the circulation. Another possibility is that the two proteins form a complex that translocates to muscle capillaries to inhibit LPL. The latter seems more likely for the following reasons. Angptl8 KO mice did not exhibit reduced levels of the Angptl3 N-terminal domain [65], and thus Angptl8 is not required for Angptl3 cleavage. Furthermore, in mice with Angptl8 overexpression circulating Angptl3 levels were reduced [59], supporting the notion that exogenous Angptl8 formed complexes with Angptl3, which, in turn, translocated into the capillaries in the heart and skeletal muscle, resulting in lowered levels of circulating Angptl3.
Both Angptl8 and Angptl3 are secreted by the liver into the circulation, and are not expressed in the heart and skeletal muscle, and thus are likely to work in an endocrine manner. Taken together, these results strongly suggest that Angptl8, induced by feeding, binds and activates Angptl3 to inhibit LPL in cardiac and skeletal muscles, in an endocrine manner.
7. Explanation of triglyceride levels in mice with altered expression of Angptl8, Angptl3 or Angptl4 by the Angptl3-4-8 model
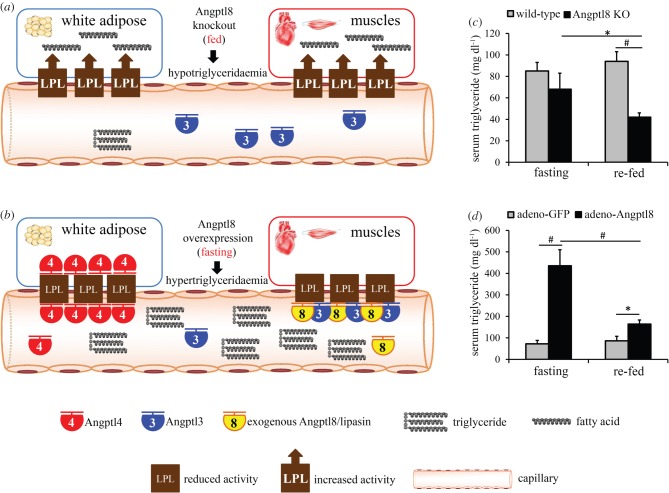
A striking phenotype in Angptl8 KO mice is that refeeding decreases serum TG levels [65,66] (figure 2c). This striking phenotype is nicely explained by the model. According to the Angptl3-4-8 model, in Angptl8 KO mice, LPL activity in the heart and skeletal muscle remains active in both fasting and fed states. However, in the fasting state abundant Angptl4 inhibits WAT LPL, while following refeeding Angptl4 is diminished, resulting in higher WAT LPL activity, enhanced WAT fatty acid uptake, enhanced circulating TG clearance and thus lower serum TG levels.
Figure 2.
Changes in triglyceride levels in Angptl8-deficient or overexpressing mice explained by the Angptl3-4-8 model. (a) In the fed state, Angptl8-null mice have high LPL activity in both WAT and muscles, resulting in lower circulating TG levels. (b) Conversely, in the fasting state, Angptl8-overexpressing mice have low LPL activity in both WAT and muscles, resulting in dramatically higher circulating TG levels. (c,d) TG levels in Angptl8 KO (c) and overexpressing (d) mice. Mice were fasted for 24 h or re-fed for 4 h following the fast. Panels (c,d) are reproduced from data in figure 3 of [66] with permission of Scientific Reports. Data are presented as mean ± s.e.m. N = 6–8 per group. KO, knockout; WAT, white adipose tissue. *p < 0.05; #p < 0.01.
In the fed state, Angptl8 KO mice also have low levels of Angptl4, resulting in higher activity of LPL in both WAT and muscles, and therefore circulating TG are effectively hydrolysed and taken up by both WAT and muscles, leading to hypotriglyceridaemia [65,66] (figure 2a,c). In the fasting state, because Angptl4 is induced, WAT LPL inhibition is retained, and therefore circulating TG levels showed no significant difference from those of wild-type mice (table 1 and figure 2c). In the case of Angptl8 overexpression, in the fed state, because LPL activity in WAT is still high in the absence of Angptl4, the elevation of circulating TG is modest. In the fasting state, however, LPL in both WAT and muscles is inhibited, resulting in striking elevation of circulating TG (figure 2b,d).
Table 1.
Serum triglyceride levels and LPL activity in mice with altered Angptl8 expression [66]. ↑, increased; ↓, decreased; −, unchanged; ^, slightly increased.
| serum triglycerides |
LPL activity in cardiac and skeletal muscles |
LPL activity in WAT |
||||
|---|---|---|---|---|---|---|
| Angptl8 levels | fed | fasting | fed | fasting | fed | fasting |
| deficiencya | ↓ | — | ↑ | — | — | — |
| overexpressionb | ^ | ↑ | — | ↓c | — | — |
aAngptl8 knockout.
bMice with adenovirus-Angptl8 injection.
cOnly in the heart.
In the Angptl3 KO mice, in the fed state, induced Angptl8 cannot inhibit muscle LPL because this inhibition is Angptl3-dependent, and therefore LPL activity is high in both WAT and muscles, resulting in hypotriglyceridaemia, whereas in the fasting state, inhibition of LPL by Angptl4 is retained, and therefore the hypotriglyceridaemia phenotype is relatively modest [37].
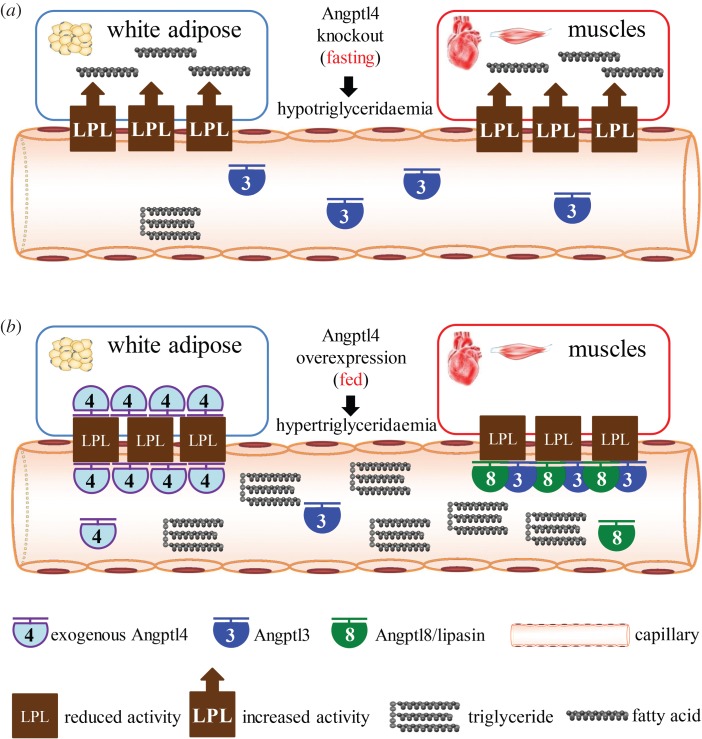
In Angptl4 KO mice, in the fasting state, LPL activity in both WAT and muscle is high, resulting in hypotriglyceridaemia. In the fed state, inhibition of LPL in muscles is retained, and therefore the low TG phenotype is relatively modest compared with that in the fasting state [29,51]. In the case of Angptl4 overexpression, in the fed state, LPL activity in both WAT and muscles is low, and therefore hypertriglyceridaemia results, whereas in the fasting state, we hypothesize that the hypertriglyceridaemia would be relatively modest because the low Angptl8 and associated high LPL activity in muscles would result in muscle uptake of TG-derived fatty acids (figure 3).
Figure 3.
Changes in triglyceride levels in Angptl4 deficient or overexpressing mice explained by the Angptl3-4-8 model. (a) In the fasting state, Angptl4-null mice have high LPL activity in both WAT and muscles, resulting in lower circulating TG levels. (b) Conversely, in the fed state, Angptl4 overexpressing mice have low LPL activity in both WAT and muscles, resulting in dramatically higher circulating TG levels.
8. Limitations of the Angptl3-4-8 model
The Angptl3-4-8 model, obviously, is not a perfect one. First, the model does not explain the functional role of Angptl8 in adipose tissues. In WAT, Angptl8 expression is strongly induced by feeding [57]. However, WAT LPL activity is upregulated by feeding as well [51]. In Angptl8 KO mice, we found that WAT LPL activity was not affected [66]. Furthermore, in Angptl8 KO mice, uptake of VLDL-TG by WAT was decreased [65]. These results are inconsistent with a role of Angptl8 in inhibiting WAT LPL. Therefore, Angptl8 in WAT may be involved in functions independent of LPL, such as adipogenesis [57]. Likewise, in BAT, LPL activity is upregulated by cold exposure, whereas Angptl8 is also upregulated [67]. Therefore, we hypothesize that Angptl8 may have LPL-independent functions in both WAT and BAT. Although it has been well established that Angptl8 is a circulating factor secreted from the liver, it should be noted that, thus far, there has been no evidence to suggest that Angptl8 is also secreted from adipose tissues. It is likely that Angptl8 in WAT and BAT functions in a non-endocrine manner.
Angptl3 KO mice exhibited elevated LPL activity in WAT, in addition to increased postprandial LPL activity in oxidative tissues [37]. This result suggests that Angptl3 may also play a role in inhibiting LPL in WAT. Because the Angptl3 level is not nutritionally regulated, it is possible that Angptl3 is a general LPL inhibitor needed by both Angptl4 and Angptl8. However, currently, there has been no evidence showing Angptl4 and Angptl3 can interact to regulate LPL.
Angptl4 has a relatively wide expression pattern, including expression in skeletal muscle and the heart [4]. In Angptl4 KO mice, however, in response to cold exposure, TG incorporation was altered specifically in adipose tissues, but not in muscles [50], consistently with the Angptl3-4-8 model. The contributions of intracellular versus circulating Angptl4, as well as Angptl4 expressed in non-adipose tissues, to LPL inhibition are unclear.
To fully prove the model, it is necessary to examine tissue-specific uptake of labelled TG in Angptl8 and Angptl4 KO mice during the fed–fast cycle. Future models will probably incorporate functional roles of Angptl8 and Angptl3 in WAT, Angptl4 in non-WAT tissues and other factors involved in LPL regulation, such as apoA-V and apoC-III [101].
9. Perspective
Since the discovery of Angptl8 in 2012 [57–59], significant progress has been made in elucidating the functional role, mechanism of action and therapeutic potential of this protein [62,63]. With the Angptl8 discovery, a model of how TG trafficking is coordinated at various nutritional states emerged [66]. Although the Angptl3-4-8 model still leaves much room to be improved, it provides a framework that links Angptl3, Angptl4, Angptl8 and LPL. Below are some outstanding questions to address.
9.1. Mechanism of action
The mechanism of LPL inhibition by Angptl8 remains obscure. One possible mechanism of action is that Angptl8 enhances cleavage of Angptl3, releasing the N-terminal domain, which, in turn, inhibits LPL. Another possibility is that Angptl8 binds to Angptl3, forming a complex that inhibits LPL in the heart and skeletal muscle. The two scenarios are distinct in that in the former Angptl8 does not translocate to the heart and skeletal muscle, but stays in the circulation only, whereas in the latter Angptl8 is physically located in these tissues and has interactions with Angptl3 and/or LPL. As discussed in §6, the second scenario seems more likely; however, direct experimental evidence, such as immunohistostaining showing the presence of Angptl8 and Angptl3 on the surface of capillaries in the heart and skeletal muscle, is lacking.
A related question is: what is the mechanism ensuring tissue specificity of LPL inhibition by the Angptl8–Angptl3 pathway in cardiac and skeletal muscles, and by Angptl4 in WAT? Because Angptl8 and Angptl3 are likely to function in an endocrine manner, it is almost certain that they encounter LPL in capillaries of peripheral tissues, in which LPL is anchored by GPIHBP1 to the capillary endothelial lumen. GPIHBP1 has been shown to play an important role in mediating inhibition of Angptl proteins on LPL [46,102]. However, the roles of GPIHBP1 on Angptl8- and Angptl3-mediated inhibition of LPL and on establishing their functional tissue specificity remain unknown.
9.2. Transcriptional regulation
Angptl8 is strongly induced by feeding and suppressed by fasting. As we pointed out, levels of Angptl8 and Angptl4 show opposite changes in response to various stimuli, such as fasting, feeding, insulin resistance and cold exposure [62,63,67]. Likewise, a recent report showed that glucagon receptor antagonists upregulate Angptl4, while downregulating Angptl8, and that the induction of adipose Angptl4 specifically promotes pancreatic α-cell proliferation [103]. This reciprocal regulation is critical to balance the abundance of Angptl8 versus Angptl4 to regulate cellular processes in different physiological and pathological settings. Because Angptl8 is expressed in liver and adipose tissues, it is likely that transcription factors mediating Angptl8 transcription are different in the two tissues. The carbohydrate-responsive element-binding protein (ChREBP), a glucose-responsive transcription factor [73], and PPARγ [57] were suggested to mediate Angptl8 transcription. However, the identity of transcription factors and their binding sites in the Angptl8 promoter region have not been clearly delineated. Another question is that Angptl8 protein during fasting must be degraded quickly, but the degradation pathway and its regulation remain elusive. Elucidation of the identity of transcription factors mediating Angptl8 transcription is critical in understanding the nutritional regulation of Angptl8, as well as the reciprocal regulation of Angptl8 versus Angptl4.
9.3. Functions in adipose tissues
Angptl8 is strongly upregulated in WAT following food intake [57,58]. Angptl8 is abundant in mouse BAT, and is highly upregulated by cold exposure [67]. In both cases, LPL activity is increased, and therefore it is likely that Angptl8 has LPL-independent functions in adipose tissues. Angptl8 is upregulated during adipocyte differentiation, and its knockdown impairs adipogenesis in 3T3-L1 cells [57]. However, the functional roles of Angptl8 in WAT and BAT in vivo remain unclear.
9.4. Human pathology
Accumulating evidence suggests that circulating ANGPTL8 is elevated in diabetes [73–85,104]. Diabetes is often associated with diabetic dyslipidaemia, characterized by hypertriglyceridaemia, lower HDL-C and postprandial lipaemia [105]. The direct implication of the ANGPTL3-4-8 model is that when ANGPTL8 is increased, LPL activity will be suppressed in the heart and skeletal muscle, resulting in accumulation of TG in the circulation (hypertriglyceridaemia). Then, is ANGPTL8 a contributing factor in causing diabetic dyslipidaemia? We have shown that an Angptl8 monoclonal antibody lowers serum TG in mice. Can ANGPTL8 inhibition be a therapeutic approach to treat diabetic dyslipidaemia?
Homozygous or compound heterozygous loss-of-function mutations in ANGPTL3 and ANGPTL4 have been identified in humans, providing valuable information on human pathology [56]. However, homozygous loss-of-function mutations have not been found in ANGPTL8. Searching the ExAC database [106], which is based on exome sequencing results of more than 60 000 human subjects, revealed no ANGPTL8 homozygous null mutations. According to phenotypes of Angptl8 KO mice, it is unlikely that null mutations are lethal. Therefore, with the expansion of exome databases, identification of homozygous null mutations of ANGPTL8 is possible, and will provide critical information on the pathophysiology of its functions in humans.
10. Concluding remarks
The partitioning of TG to specific tissues according to nutritional states is a fundamental biological process, and the elucidation of the molecular mechanism of TG trafficking will have profound implications for understanding metabolic disease. The ANGPTL3-4-8 model suggests that the three Angptl members are a key in balancing the partitioning of circulating TG between WAT and oxidative tissues. Breaking this balance may lead to obesity, lipotoxicity or hypertriglyceridaemia, representing excess TG in WAT, non-adipose tissues and plasma, respectively.
Inhibition of each of the three Angptl members, based on small molecular inhibitors or monoclonal antibodies, has tremendous therapeutic potential in treating dyslipidaemia. Of note, clinical trials evaluating an ANGPTL3 monoclonal antibody are ongoing (trial https://trialbulletin.com/lib/entry/ct-02265952; trial https://trialbulletin.com/lib/entry/ct-01749878; trial https://trialbulletin.com/lib/entry/ct-02107872). The ANGPTL3-4-8 model suggests that the key to the concept of LPL-based therapeutic strategy is the balance. The goal should not be to inhibit, e.g., ANGPTL3 to the maximum extent, which may lead to lipotoxicity, but to reduce its activity to a specific level, so that abnormal TG trafficking associated with pathological conditions is corrected.
Since the discovery of LPL seven decades ago, significant progress has been made in elucidating the functional role of LPL in regulating TG trafficking. Recent discovery of lipasin/ANGPTL8 results in an ANGPTL3-4-8 model, which provides a molecular mechanism by which tissue-specific LPL activity is regulated during the fed–fast cycle. Specifically, feeding induces ANGPTL8, activating the ANGPTL8–ANGPTL3 pathway, which inhibits LPL in cardiac and skeletal muscles, thereby making circulating TG available for uptake by WAT, in which LPL activity is elevated owing to diminished ANGPTL4; the reverse is true during fasting, which suppresses ANGPTL8 but induces ANGPTL4, thereby directing circulating TG to muscles. The model may provide significant insights into the understanding of TG metabolism and metabolic disease.
Acknowledgements
Stimulating discussions with Dr Brandon Davies are greatly appreciated.
Authors' contributions
R.Z. designed the study, interpreted data and wrote the manuscript.
Competing interests
The author has no competing interests.
Funding
This work was supported in part by a grant from Wayne State University to R.Z.
References
- 1.Eckel RH. 1989. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N. Engl. J. Med. 320, 1060–1068. (doi:10.1056/NEJM198904203201607) [DOI] [PubMed] [Google Scholar]
- 2.Goldberg IJ. 1996. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 37, 693–707. [PubMed] [Google Scholar]
- 3.Wang H, Eckel RH. 2009. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 297, E271–E288. (doi:10.1152/ajpendo.90920.2008) [DOI] [PubMed] [Google Scholar]
- 4.Kersten S. 2014. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta 1841, 919–933. (doi:10.1016/j.bbalip.2014.03.013) [DOI] [PubMed] [Google Scholar]
- 5.Merkel M, Eckel RH, Goldberg IJ. 2002. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 43, 1997–2006. (doi:10.1194/jlr.R200015-JLR200) [DOI] [PubMed] [Google Scholar]
- 6.Hahn PF. 1943. Abolishment of alimentary lipemia following injection of heparin. Science 98, 19–20. (doi:10.1126/science.98.2531.19) [DOI] [PubMed] [Google Scholar]
- 7.Korn ED. 1954. Properties of clearing factor obtained from rat heart acetone powder. Science 120, 399–400. (doi:10.1126/science.120.3114.399-a) [DOI] [PubMed] [Google Scholar]
- 8.Cryer A. 1981. Tissue lipoprotein lipase activity and its action in lipoprotein metabolism. Int. J. Biochem. 13, 525–541. (doi:10.1016/0020-711X(81)90177-4) [DOI] [PubMed] [Google Scholar]
- 9.Augustus A, Yagyu H, Haemmerle G, Bensadoun A, Vikramadithyan RK, Park SY, Kim JK, Zechner R, Goldberg IJ. 2004. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J. Biol. Chem. 279, 25 050–25 057. (doi:10.1074/jbc.M401028200) [DOI] [PubMed] [Google Scholar]
- 10.Khan RS, et al. 2013. Rescue of heart lipoprotein lipase-knockout mice confirms a role for triglyceride in optimal heart metabolism and function. Am. J. Physiol. Endocrinol. Metab. 305, E1339–E1347. (doi:10.1152/ajpendo.00349.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yagyu H, et al. 2003. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Invest. 111, 419–426. (doi:10.1172/JCI16751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, et al. 2009. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes 58, 116–124. (doi:10.2337/db07-1839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JK, et al. 2001. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl Acad. Sci. USA 98, 7522–7527. (doi:10.1073/pnas.121164498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira LD, Pulawa LK, Jensen DR, Eckel RH. 2001. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes 50, 1064–1068. (doi:10.2337/diabetes.50.5.1064) [DOI] [PubMed] [Google Scholar]
- 15.Goldberg IJ, Eckel RH, Abumrad NA. 2009. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 50 Suppl, S86–S90. (doi:10.1194/jlr.R800085-JLR200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman T, Seip RL, Gimble JM, Lee D, Maeda N, Semenkovich CF. 1995. COOH-terminal disruption of lipoprotein lipase in mice is lethal in homozygotes, but heterozygotes have elevated triglycerides and impaired enzyme activity. J. Biol. Chem. 270, 12 518–12 525. (doi:10.1074/jbc.270.21.12518) [DOI] [PubMed] [Google Scholar]
- 17.Santamarina-Fojo S, Brewer HB Jr. 1991. The familial hyperchylomicronemia syndrome. New insights into underlying genetic defects. JAMA 265, 904–908. (doi:10.1001/jama.1991.03460070086049) [PubMed] [Google Scholar]
- 18.Weinstock PH, Bisgaier CL, Aalto-Setala K, Radner H, Ramakrishnan R, Levak-Frank S, Essenburg AD, Zechner R, Breslow JL. 1995. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. Mild hypertriglyceridemia with impaired very low density lipoprotein clearance in heterozygotes. J. Clin. Invest. 96, 2555–2568. (doi:10.1172/JCI118319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulbourne CN, et al. 2014. The GPIHBP1–LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab. 19, 849–860. (doi:10.1016/j.cmet.2014.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beigneux AP, et al. 2007. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 5, 279–291. (doi:10.1016/j.cmet.2007.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies BS, et al. 2010. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12, 42–52. (doi:10.1016/j.cmet.2010.04.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigneux AP, et al. 2009. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler. Thromb. Vasc. Biol. 29, 956–962. (doi:10.1161/ATVBAHA.109.186577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buonuomo PS, Bartuli A, Rabacchi C, Bertolini S, Calandra S. 2015. A 3-day-old neonate with severe hypertriglyceridemia from novel mutations of the GPIHBP1 gene. J. Clin. Lipidol. 9, 265–270. (doi:10.1016/j.jacl.2014.10.003) [DOI] [PubMed] [Google Scholar]
- 24.Olivecrona G, et al. 2010. Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia. J. Lipid Res. 51, 1535–1545. (doi:10.1194/jlr.M002717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franssen R, et al. 2010. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ. Cardiovasc. Genet. 3, 169–178. (doi:10.1161/CIRCGENETICS.109.908905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young SG, Davies BS, Fong LG, Gin P, Weinstein MM, Bensadoun A, Beigneux AP. 2007. GPIHBP1: an endothelial cell molecule important for the lipolytic processing of chylomicrons. Curr. Opin. Lipidol. 18, 389–396. (doi:10.1097/MOL.0b013e3281527914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young SG, Zechner R. 2013. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 27, 459–484. (doi:10.1101/gad.209296.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies BS, Beigneux AP, Fong LG, Young SG. 2012. New wrinkles in lipoprotein lipase biology. Curr. Opin. Lipidol. 23, 35–42. (doi:10.1097/MOL.0b013e32834d0b33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koster A, et al. 2005. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146, 4943–4950. (doi:10.1210/en.2005-0476) [DOI] [PubMed] [Google Scholar]
- 30.Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, Horikoshi H, Furukawa H. 2002. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 30, 151–157. (doi:10.1038/ng814) [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto K, Koishi R, Shimizugawa T, Ando Y. 2006. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp. Anim. 55, 27–34. (doi:10.1538/expanim.55.27) [DOI] [PubMed] [Google Scholar]
- 32.Shimizugawa T, et al. 2002. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 277, 33 742–33 748. (doi:10.1074/jbc.M203215200) [DOI] [PubMed] [Google Scholar]
- 33.Ono M, Shimizugawa T, Shimamura M, Yoshida K, Noji-Sakikawa C, Ando Y, Koishi R, Furukawa H. 2003. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 278, 41 804–41 809. (doi:10.1074/jbc.M302861200) [DOI] [PubMed] [Google Scholar]
- 34.Jin W, Wang X, Millar JS, Quertermous T, Rothblat GH, Glick JM, Rader DJ. 2007. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 6, 129–136. (doi:10.1016/j.cmet.2007.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee EC, et al. 2009. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J. Biol. Chem. 284, 13 735–13 745. (doi:10.1074/jbc.M807899200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gusarova V, et al. 2015. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J. Lipid Res. 56, 1308–1317. (doi:10.1194/jlr.M054890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, McNutt MC, Banfi S, Levin MG, Holland WL, Gusarova V, Gromada J, Cohen JC, Hobbs HH. 2015. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc. Natl Acad. Sci. USA 112, 11 630–11 635. (doi:10.1073/pnas.1515374112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY. 2000. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem. J. 346, 603–610. (doi:10.1042/bj3460603) [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. 2000. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell Biol. 20, 5343–5349. (doi:10.1128/MCB.20.14.5343-5349.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. 2000. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275, 28 488–28 493. (doi:10.1074/jbc.M004029200) [DOI] [PubMed] [Google Scholar]
- 41.Yoshida K, Shimizugawa T, Ono M, Furukawa H. 2002. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 43, 1770–1772. (doi:10.1194/jlr.C200010-JLR200) [DOI] [PubMed] [Google Scholar]
- 42.Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HF, Hesselink MK, Schrauwen P, Muller M. 2009. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 29, 969–974. (doi:10.1161/ATVBAHA.108.182147) [DOI] [PubMed] [Google Scholar]
- 43.Mandard S, et al. 2004. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J. Biol. Chem. 279, 34 411–34 420. (doi:10.1074/jbc.M403058200) [DOI] [PubMed] [Google Scholar]
- 44.Ge H, Yang G, Huang L, Motola DL, Pourbahrami T, Li C. 2004. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J. Biol. Chem. 279, 2038–2045. (doi:10.1074/jbc.M307583200) [DOI] [PubMed] [Google Scholar]
- 45.Lafferty MJ, Bradford KC, Erie DA, Neher SB. 2013. Angiopoietin-like protein 4 inhibition of lipoprotein lipase: evidence for reversible complex formation. J. Biol. Chem. 288, 28 524–28 534. (doi:10.1074/jbc.M113.497602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chi X, Shetty SK, Shows HW, Hjelmaas AJ, Malcolm EK, Davies BS. 2015. Angiopoietin-like 4 modifies the interactions between lipoprotein lipase and its endothelial cell transporter GPIHBP1. J. Biol. Chem. 290, 11 865–11 877. (doi:10.1074/jbc.M114.623769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. 2006. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl Acad. Sci. USA 103, 17 450–17 455. (doi:10.1073/pnas.0604026103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shan L, Yu XC, Liu Z, Hu Y, Sturgis LT, Miranda ML, Liu Q. 2009. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J. Biol. Chem. 284, 1419–1424. (doi:10.1074/jbc.M808477200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desai U, et al. 2007. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc. Natl Acad. Sci. USA 104, 11 766–11 771. (doi:10.1073/pnas.0705041104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dijk W, et al. 2015. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. Elife 4, e8428 (doi:10.7554/eLife.08428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kroupa O, Vorrsjo E, Stienstra R, Mattijssen F, Nilsson SK, Sukonina V, Kersten S, Olivecrona G, Olivecrona T. 2012. Linking nutritional regulation of Angptl4, Gpihbp1, and Lmf1 to lipoprotein lipase activity in rodent adipose tissue. BMC Physiol. 12, 13 (doi:10.1186/1472-6793-12-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musunuru K, et al. 2010. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 363, 2220–2227. (doi:10.1056/NEJMoa1002926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pisciotta L, et al. 2012. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ. Cardiovasc. Genet. 5, 42–50. (doi:10.1161/CIRCGENETICS.111.960674) [DOI] [PubMed] [Google Scholar]
- 54.Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. 2007. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 39, 513–516. (doi:10.1038/ng1984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talmud PJ, et al. 2008. ANGPTL4 E40K and T266M: effects on plasma triglyceride and HDL levels, postprandial responses, and CHD risk. Arterioscler. Thromb. Vasc. Biol. 28, 2319–2325. (doi:10.1161/ATVBAHA.108.176917) [DOI] [PubMed] [Google Scholar]
- 56.Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, Cohen JC. 2009. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119, 70–79. (doi:10.1172/JCI37118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren G, Kim JY, Smas CM. 2012. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 303, E334–E351. (doi:10.1152/ajpendo.00084.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R. 2012. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 424, 786–792. (doi:10.1016/j.bbrc.2012.07.038) [DOI] [PubMed] [Google Scholar]
- 59.Quagliarini F, et al. 2012. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl Acad. Sci. USA 109, 19 751–19 756. (doi:10.1073/pnas.1217552109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi P, Park JS, Melton DA. 2013. Betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell 153, 747–758. (doi:10.1016/j.cell.2013.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Gray KA, Yates B, Seal RL, Wright MW, Bruford EA. 2015. Genenames.org: the HGNC resources in 2015. Nucleic Acids Res. 43, D1079–D1085. (doi:10.1093/nar/gku1071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang R, Abou-Samra AB. 2014. A dual role of lipasin (betatrophin) in lipid metabolism and glucose homeostasis: consensus and controversy. Cardiovasc. Diabetol. 13, 133 (doi:10.1186/s12933-014-0133-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang R, Abou-Samra AB. 2013. Emerging roles of lipasin as a critical lipid regulator. Biochem. Biophys. Res. Commun. 432, 401–405. (doi:10.1016/j.bbrc.2013.01.129) [DOI] [PubMed] [Google Scholar]
- 64.Tang T, et al. 2010. A mouse knockout library for secreted and transmembrane proteins. Nat. Biotechnol. 28, 749–755. (doi:10.1038/nbt.1644) [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Quagliarini F, Gusarova V, Gromada J, Valenzuela DM, Cohen JC, Hobbs HH. 2013. Mice lacking ANGPTL8 (betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl Acad. Sci. USA 110, 16 109–16 114. (doi:10.1073/pnas.1315292110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu Z, Abou-Samra AB, Zhang R. 2015. A lipasin/Angptl8 monoclonal antibody lowers mouse serum triglycerides involving increased postprandial activity of the cardiac lipoprotein lipase. Sci. Rep. 5, 18502 (doi:10.1038/srep18502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu Z, Yao F, Abou-Samra AB, Zhang R. 2013. Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem. Biophys. Res. Commun. 430, 1126–1131. (doi:10.1016/j.bbrc.2012.12.025) [DOI] [PubMed] [Google Scholar]
- 68.Lee J, Hong SW, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. 2015. AMP-activated protein kinase suppresses the expression of LXR/SREBP-1 signaling-induced ANGPTL8 in HepG2 cells. Mol. Cell Endocrinol. 414, 148–155. (doi:10.1016/j.mce.2015.07.031) [DOI] [PubMed] [Google Scholar]
- 69.Tseng YH, Ke PY, Liao CJ, Wu SM, Chi HC, Tsai CY, Chen CY, Lin YH, Lin KH. 2014. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy 10, 20–31. (doi:10.4161/auto.26126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weissglas-Volkov D, et al. 2013. Genomic study in Mexicans identifies a new locus for triglycerides and refines European lipid loci. J. Med. Genet. 50, 298–308. (doi:10.1136/jmedgenet-2012-101461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peloso GM, et al. 2014. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56 000 whites and blacks. Am. J. Hum. Genet. 94, 223–232. (doi:10.1016/j.ajhg.2014.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teslovich TM, et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713. (doi:10.1038/nature09270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu Z, Berhane F, Fite A, Seyoum B, Abou-Samra AB, Zhang R. 2014. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci. Rep. 4, 5013 (doi:10.1038/srep05013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Espes D, Martinell M, Carlsson PO. 2014. Increased circulating betatrophin concentrations in patients with type 2 diabetes. Int. J. Endocrinol. 2014, 323407 (doi:10.1155/2014/323407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu H, et al. 2014. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care 37, 2718–2722. (doi:10.2337/dc14-0602) [DOI] [PubMed] [Google Scholar]
- 76.Chen X, et al. 2015. Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J. Clin. Endocrinol. Metab. 100, E96–E100. (doi:10.1210/jc.2014-2300) [DOI] [PubMed] [Google Scholar]
- 77.Tokumoto S, et al. 2015. Correlation of circulating betatrophin concentrations with insulin secretion capacity, evaluated by glucagon stimulation tests. Diabetes Med. 32, 653–656. (doi:10.1111/dme.12696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamada H, et al. 2015. Circulating betatrophin is elevated in patients with type 1 and type 2 diabetes. Endocr. J. 62, 417–421. (doi:10.1507/endocrj.EJ14-0525) [DOI] [PubMed] [Google Scholar]
- 79.Gao T, Jin K, Chen P, Jin H, Yang L, Xie X, Yang M, Hu C, Yu X. 2015. Circulating betatrophin correlates with triglycerides and postprandial glucose among different glucose tolerance statuses: a case–control study. PLoS ONE 10, e0133640 (doi:10.1371/journal.pone.0133640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ebert T, et al. 2014. Circulating angiopoietin-like protein 8 is independently associated with fasting plasma glucose and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 99, E2510–E2517. (doi:10.1210/jc.2013-4349) [DOI] [PubMed] [Google Scholar]
- 81.Ebert T, Kralisch S, Wurst U, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Tonjes A, Fasshauer M. 2015. Betatrophin levels are increased in women with gestational diabetes mellitus compared to healthy pregnant controls. Eur. J. Endocrinol. 173, 1–7. (doi:10.1530/EJE-14-0815) [DOI] [PubMed] [Google Scholar]
- 82.Wawrusiewicz-Kurylonek N, et al. 2015. Increased maternal and cord blood betatrophin in gestational diabetes. PLoS ONE 10, e0131171 (doi:10.1371/journal.pone.0131171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trebotic LK, Klimek P, Thomas A, Fenzl A, Leitner K, Springer S, Kiefer FW, Kautzky-Willer A. 2015. Circulating betatrophin is strongly increased in pregnancy and gestational diabetes mellitus. PLoS ONE 10, e0136701 (doi:10.1371/journal.pone.0136701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu S, Gao H, Ma Y, Fu L, Zhang C, Luo X. 2014. Characterisation of betatrophin concentrations in childhood and adolescent obesity and insulin resistance. Pediatr. Diabetes. 17, 53–60. (doi:10.1111/pedi.12233) [DOI] [PubMed] [Google Scholar]
- 85.Espes D, Lau J, Carlsson PO. 2014. Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia 57, 50–53. (doi:10.1007/s00125-013-3071-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu Z, Abou-Samra AB, Zhang R. 2014. An explanation for recent discrepancies in levels of human circulating betatrophin. Diabetologia 57, 2232–2234. (doi:10.1007/s00125-014-3346-1) [DOI] [PubMed] [Google Scholar]
- 87.Gomez-Ambrosi J, Pascual E, Catalan V, Rodriguez A, Ramirez B, Silva C, Gil MJ, Salvador J, Fruhbeck G. 2014. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 99, E2004–E2009. (doi:10.1210/jc.2014-1568) [DOI] [PubMed] [Google Scholar]
- 88.Abu-Farha M, et al. 2016. Circulating angiopoietin-like protein 8 (betatrophin) association with HsCRP and metabolic syndrome. Cardiovasc. Diabetol. 15, 25 (doi:10.1186/s12933-016-0346-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calan M, Yilmaz O, Kume T, Unal Kocabas G, Yesil Senses P, Senses YM, Temur M, Gursoy Calan O. In press. Elevated circulating levels of betatrophin are associated with polycystic ovary syndrome. Endocrine (doi:10.1007/s12020-016-0875-z) [DOI] [PubMed] [Google Scholar]
- 90.Abu-Farha M, Sriraman D, Cherian P, AlKhairi I, Elkum N, Behbehani K, Abubaker J. 2016. Circulating ANGPTL8/betatrophin is increased in obesity and reduced after exercise training. PLoS ONE 11, e0147367 (doi:10.1371/journal.pone.0147367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pascual-Corrales E, et al. In press. Circulating ANGPTL8/betatrophin concentrations are increased after surgically induced weight loss, but not after diet-induced weight loss. Obes. Surg. (doi:10.1007/s11695-015-2026-7) [DOI] [PubMed] [Google Scholar]
- 92.Chen CC, Susanto H, Chuang WH, Liu TY, Wang CH. 2016. Higher serum betatrophin level in type 2 diabetes subjects is associated with urinary albumin excretion and renal function. Cardiovasc. Diabetol. 15, 3 (doi:10.1186/s12933-015-0326-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung HS, et al. 2016. Circulating angiopoietin-like protein 8 (ANGPTL8) and ANGPTL3 concentrations in relation to anthropometric and metabolic profiles in Korean children: a prospective cohort study. Cardiovasc. Diabetol. 15, 1 (doi:10.1186/s12933-015-0324-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barja-Fernandez S, et al. 2015. Circulating betatrophin levels are increased in anorexia and decreased in morbidly obese women. J. Clin. Endocrinol. Metab. 100, E1188–E1196. (doi:10.1210/JC.2015-1595) [DOI] [PubMed] [Google Scholar]
- 95.Guo K, Lu J, Yu H, Zhao F, Pan P, Zhang L, Chen H, Bao Y, Jia W. 2015. Serum betatrophin concentrations are significantly increased in overweight but not in obese or type 2 diabetic individuals. Obesity (Silver Spring). 23, 793–797. (doi:10.1002/oby.21038) [DOI] [PubMed] [Google Scholar]
- 96.Fenzl A, Itariu BK, Kosi L, Fritzer-Szekeres M, Kautzky-Willer A, Stulnig TM, Kiefer FW. 2014. Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia 57, 1204–1208. (doi:10.1007/s00125-014-3208-x) [DOI] [PubMed] [Google Scholar]
- 97.Eagle GR, Robinson DS. 1964. The ability of actinomycin D to increase the clearing-factor lipase activity of rat adipose tissue. Biochem. J. 93, 10C–11C. (doi:10.1042/bj0930010C) [DOI] [PubMed] [Google Scholar]
- 98.Bergo M, Wu G, Ruge T, Olivecrona T. 2002. Down-regulation of adipose tissue lipoprotein lipase during fasting requires that a gene, separate from the lipase gene, is switched on. J. Biol. Chem. 277, 11 927–11 932. (doi:10.1074/jbc.M200325200) [DOI] [PubMed] [Google Scholar]
- 99.Ben-Zeev O, Lusis AJ, LeBoeuf RC, Nikazy J, Schotz MC. 1983. Evidence for independent genetic regulation of heart and adipose lipoprotein lipase activity. J. Biol. Chem. 258, 13 632–13 636. [PubMed] [Google Scholar]
- 100.Wu G, Zhang L, Gupta J, Olivecrona G, Olivecrona T. 2007. A transcription-dependent mechanism, akin to that in adipose tissue, modulates lipoprotein lipase activity in rat heart. Am. J. Physiol. Endocrinol. Metab. 293, E908–E915. (doi:10.1152/ajpendo.00634.2006) [DOI] [PubMed] [Google Scholar]
- 101.Rader DJ. 2016. New therapeutic approaches to the treatment of dyslipidemia. Cell Metab. 23, 405–412. (doi:10.1016/j.cmet.2016.01.005) [DOI] [PubMed] [Google Scholar]
- 102.Sonnenburg WK, et al. 2009. GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J. Lipid Res. 50, 2421–2429. (doi:10.1194/jlr.M900145-JLR200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ben-Zvi D, Barrandon O, Hadley S, Blum B, Peterson QP, Melton DA. 2015. Angptl4 links alpha-cell proliferation following glucagon receptor inhibition with adipose tissue triglyceride metabolism. Proc. Natl Acad. Sci. USA 112, 15 498–15 503. (doi:10.1073/pnas.1513872112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Erol O, Ellidag HY, Ayik H, Ozel MK, Derbent AU, Yilmaz N. 2015. Evaluation of circulating betatrophin levels in gestational diabetes mellitus. Gynecol. Endocrinol. 31, 1–5. (doi:10.3109/09513590.2015.1056142) [DOI] [PubMed] [Google Scholar]
- 105.Mooradian AD. 2009. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 5, 150–159. (doi:10.1038/ncpendmet1066) [DOI] [PubMed] [Google Scholar]
- 106.Lek M, et al. In press. Analysis of protein-coding genetic variation in 60 706 humans. bioRxiv. (doi:10.1101/030338) [DOI] [PMC free article] [PubMed] [Google Scholar]