Abstract
The identification of m6A demethylases and high-throughput sequencing analysis of methylated transcriptome corroborated m6A RNA epigenetic modification as a dynamic regulation process, and reignited its investigation in the past few years. Many basic concepts of cytogenetics have been revolutionized by the growing understanding of the fundamental role of m6A in RNA splicing, degradation and translation. In this review, we summarize typical features of methylated transcriptome in mammals, and highlight the ‘writers’, ‘erasers’ and ‘readers’ of m6A RNA modification. Moreover, we emphasize recent advances of biological functions of m6A and conceive the possible roles of m6A in the regulation of immune response and related diseases.
Keywords: m6A, mRNA, methylation
1. Introduction
RNA serves as an inevitable connecting link for genetic information passing from DNA to protein. The intimate relationship between mRNA and protein makes it accredited to present mRNA data for gene expression when protein levels are difficult to address. However, cellular protein levels are not necessarily correlated with mRNA levels [1,2], suggesting that post-transcriptional mRNA regulation plays an important role in gene expression. Indeed, more than 100 types of chemical modification have been identified in cellular RNA (including rRNA, tRNA, snRNA, mRNA and long-non-coding RNA) in recent decades [3,4], among the most prevalent internal mRNA/lncRNA modifications of which is N6-methyladenosine (m6A). Discovered in the 1970s [5–8], m6A has been observed in a wide range of eukaryotes, ranging from yeast, Arabidopsis thaliana, Drosophila to mammals, and is also found in the RNA of viruses [9–11]. However, owing to a lack of knowledge of m6A demethylating enzymes and the short life of most RNA species (median mammalian RNA half-lives are approx. 5 h [12,13]), m6A modifications had long been considered to be static and unalterable. The inability to identify m6A-containing mRNAs also hindered investigation of the biological roles of this chemical modification.
In 2011, the discovery of fat mass and obesity associated protein (FTO) as the first genuine m6A demethylase revived interest in mRNA/lncRNA methylation [14], because it defined m6A RNA modification as a dynamic process and its disturbance probably correlated to human diseases. Two independent studies developed an m6A RNA immunoprecipitation approach followed by high-throughput sequencing (MeRIP-seq) in 2012 that defined the methylated transcriptome in mammals [15,16]. These results demonstrated for the first time that m6A was a prevalent mRNA modification, and reignited the investigation on m6A ‘writers’, ‘erasers’, ‘readers’ and their physiology functions. Recent studies have already shown that dysregulation of this modification may contributes to obesity, brain development abnormalities and other diseases [17–22], thus emphasizing the importance of m6A RNA modification.
In this review, we discuss recent studies that profiled the features of methylated transcriptome. We also highlight the dynamic regulation of m6A RNA modification by adenosine methyltransferases (‘writers’) and demethylases (‘erasers’). Furthermore, we emphasize recent advances on the role of m6A RNA modification in biological processes and human diseases. Finally, we give some perspective for further investigation and conceive the possible role of m6A in the regulation of innate and adaptive immunity.
2. Widespread m6A mRNA/lncRNA modification
In the 1970s, several groups found that polyadenylated RNA from mammalian cells contained the most abundant chemical modification, m6A [5–8]. However, poly(A) RNA could have arisen from mitochondrial RNA, tRNA and rRNA, because these RNAs contains poly (A) tracts [23–25]. Besides, very few defined internal mRNAs were shown to contain m6A, which led to doubts whether m6A was indeed a prevalent modification in mRNA and whether this modification played any important role in biological processes. In 2012, two studies rested these doubts using MeRIP-seq techniques showing that thousands of mRNAs and lncRNAs contained m6A [15,16], unequivocally demonstrating that m6A is a widespread modification in mRNA.
These two studies and results from other groups published recently presented a striking finding that m6A residues were enriched in 5′ untranslated regions (UTRs), around stop codons and in 3′ UTRs adjacent to stop codons in mammalian mRNAs [15,16,26,27]; in Arabidopsis thaliana, m6A is also enriched around the start codons [28]. Many mRNA binding proteins bind to the 3′ UTR [29], which is the most structured portion of mRNAs [30,31]. m6A was reported to marginally reduce the stability of A : U base pairing [32]; methylated transcripts in meiotic yeast were less structured [33]; and a recent study found that m6A-dependent RNA structural switches regulated RNA–protein interactions to affect the abundance as well as alternative splicing of target mRNAs (see below) [34]. These results indicated that the methylation regions' specificity of m6A was intimately correlated with their unique regulatory functions.
The question is how this region-specific methylation is targeted. Bioinformatic analysis of MeRIP-Seq data using the motif discovery algorithm finding informative regulatory elements (FIRE) identified the predominant consensus motifs of m6A: G [G/A] m6ACU and related variants ([AC]GAC[GU], GGAC, [AU][CG]G[AG]AC and UGAC), and almost 90% of all m6A peaks contain at least one of the motifs [16]. This consensus motif is extremely similar to the identified sequence obtained from mutational studies and substrate preference of methyltransferase enzyme in vitro in the 1970s: [G/A/U] [G/A] m6AC [U/A/C] [35–39]. Other methylation motifs were also identified, but were much less prominent [15,16]. These data suggest that the adenosine methyltransferases and demethylases may also constitute a limited repertoire with predominant and a few less abundant elements.
Another interesting phenomenon was that only a minor part of mRNA transcripts were m6A modified. This was not due to the lack of consensus motifs in some mRNAs, as GAC motif is commonly found approximately every 64 nucleotides in RNA. In fact, the majority of m6A consensus motifs were not methylated, and more importantly, there may be only some copies of an mRNA transcript that were modified [15,16,18,26,27]. These results potentiate the concept that m6A mRNA modification as a dynamic process. However, some undefined sequences around these consensus motifs that could regulate the methylation status may exist, and specific structures of certain mRNAs may also explain the relatively low percentage of m6A-modified mRNAs. Thus, the development of techniques to map the m6A sites at single-nucleotide resolution would help to address these questions. IP-based cross-linking-assisted approaches were developed by several groups for the mapping of mammalian mRNAs [40–42], and high-resolution mapping of yeast m6A was also achieved [33].
3. m6a writers—adenosine methyltransferases
3.1. METTL3
A multiprotein methyltransferase complex was shown to mediate m6A mRNA methylation [43–45], and METTL3 was earlier identified as a S-adenosyl-l-methionine (SAM)-binding component of this complex [45] and could exhibit catalytic functions by itself [46]. Knockdown METTL3 reduced m6A peaks in mRNAs from mouse embryonic stem cells, Hela cells and HepG2 cells [15,26,27,47]. These results defined METTL3 as a methyltransferase for m6A RNA modification. Genetic ablation of METTL3 in blastocysts generated by mating of METTL3+/− mice led to almost complete depletion of m6A on mRNAs, further emphasizing the critical role of METTL3 in m6A modification [18]. Also, METTL3 is highly conserved in eukaryotes, and homologues in yeast, plant and Drosophila have also been identified [48–50]. Both nuclear and cytoplasmic localization of METTL3 were observed [27,33], suggesting that mRNA methylation could occur in both nucleus and cytoplasm, which is consistent with early studies showing that cytosolic extracts also possessed methyltransferase activity [51].
3.2. METTL14
METTL14 was a close homologue of METTL3 [52]. Purified METTL14 could also specifically methylate the consensus GAC motifs by itself [46,47], and knockdown of METTL14 could also lead to decreases of m6A content in mRNAs. Further studies revealed that these two components form a complex in cells and the methylation activity of this complex was much more efficient than separated parts [46,47].
3.3. WTAP
Wilms tumour 1-associated protein (WTAP) was known to be involved in mRNA splicing [53]. The important role of WTAP in m6A methylation was first established in yeast and Arabidopsis thaliana by studying its homologues Mum2 and FIP37, respectively, which were found to associate with METTL3 and were required for efficient methylation of mRNA [48,54]. Recent researches revealed that mammalian WTAP also interacts with the METTL3–METTL14 core complex [46,55]. Although WTAP alone did not show any methyltransferase activity in vitro, knockdown of WTAP strikingly reduced the m6A peaks in cellular mRNAs, even more significantly than knockdown of METTL3 or METTL14 [46]. Further research uncovered that one of the possible mechanisms of regulating methylation by WTAP was to facilitate METTL3–METTL14 translocation to nuclear speckles [55]. Furthermore, a recent study identified WTAP-dependent and -independent m6A modification sites characters in multiple dynamic systems. WTAP-dependent sites were located at internal positions and topologically static, whereas WTAP-independent sites contributed as part of the cap structure at the transcription start bases [56]. These results suggested different regulation patterns of mA6 methylation in a region-dependent way.
3.4. KIAA1429
As mentioned previously, the m6A methyltransferase complex is a multicomponent extract, suggesting other candidates may be involved in this process. A recent study revealed 13 candidates associating with known methyltransferase components by a proteomic approach. siRNA depletion experiments revealed that one of the candidates, KIAA1429, was required for the full methylation programme in mammals [56].
4. m6a erasers—demethylases
4.1. FTO
The discovery of FTO as the first m6A mRNA demethylase entrenched the conception of m6A as reversible modification [14]. This study found that both DNA and RNA were substrates of FTO-mediated demethylation, and knockdown of FTO increased m6A peaks while over expression experiments reduced them [14]. Later investigations revealed the oxidation of m6A by FTO and discovered two new intermediate modifications, 6-hydroxymethyladenosine and 6-formyladenosine [57]. The correlation of FTO dysregulation with obesity, brain malformations and growth retardation was also reported, and suggested m6A may have important regulatory functions in these diseases [22,58–60].
4.2. ALKBH5
FTO is a member of the ALKB family. Another member of this family, ALKBH5, was also identified as a demethylase, as knockdown of this protein in human cell lines yielded higher m6A mRNA peaks [61]. ALKBH5 catalytic reaction directly removes the methyl group from m6A-methylated adenosine instead of oxidative demethylation, which was different from FTO [61]. Alkbh5-knockout mice showed a marked increase of apoptotic cells in the testes, indicating a defect in spermatogenesis [61]. Later studies found that, in addition to mRNAs, other types of nuclear RNAs were also substrates of ALBKH5 [62].
5. m6a readers—binding proteins
Each component of an organism, eukaryotic or prokaryotic, coordinates with each other to construct a concerted system, and modulating these components would lead to subsequent biological consequences. It is conceivable that m6A mRNA modification performs its function through two main approaches: fine-tuning the structure of the methylated transcripts to block or induce protein–RNA interactions, or being directly recognized by m6A binding proteins to induce subsequent reactions.
5.1. HNRNPC and HNRNPA2B1
Biochemical approaches have verified the structural alternations in m6A-modified RNAs, favouring the transition from paired to unpaired RNA [63]. Recent publications uncovered that m6A destabilized the stacking properties of the region around its opposing U-tracts in the hairpin-stem of RNA transcripts, which made the U-tracts more single-stranded or accessible, thus enhancing its binding with heterogeneous nuclear ribonucleoprotein C (HNRNPC) [34,64]. HNRNPC is an abundant nuclear RNA binding protein known to be involved in pre-mRNA processing [65–69], and further research revealed that the modulation of HNRNPC–RNA binding by m6A affected the abundance and alternative splicing of target transcripts [34]. Another example of this structure alternation mediating regulating protein–RNA interaction was HuR; the m6A modification affected its ability to bind to different RNA probes in vitro [47].
FIRE analysis revealed highly significant enrichment of the RGAC element among the binding sites of another member of the HNRNP family, HNRNPA2B1, suggesting HNRNPA2B1 as an m6A reader candidate. Further research showed that HNRNPA2B1 cross-linking-induced deletions performed 20-fold higher overlaps with m6A peaks compared with background deletions, strongly supporting HNRNPA2B1 as an m6A reader by directly binding to a subset of m6A consensus sequences. Furthermore, HNRNPA2B1 interacts with the DGCR8 protein, a component of the pri-miRNA microprocessor complex, and facilitates the processing of pri-miRNAs [70].
5.2. YTHDF2 and YTHDF1
Several mammalian proteins were identified as selective m6A binding proteins. YTHDF1–3 were reported to possess much higher binding affinity to methylated probe compared with the unmethylated one [15,71]. All of these three members of YTH domain family showed preferential binding with m6A-containing mRNAs in vitro. Knockdown experiments suggested that YTHDF2 binding affected the cognate mRNA degradation process as these mRNA targets showed decreased half-lives. Further investigation found that binding with YTHDF2 resulted in mRNA localization to mRNA decay sites such as processing bodies (P-bodies) for accelerated degradation [71]. Another report showed that YTHDF2 preserves 5′UTR methylation of stress-induced transcripts by limiting the m6A ‘eraser’ FTO from demethylation, and the increased 5′UTR methylation in the form of m6A promotes cap-independent translation initiation [72]. These results suggested diverse roles of YTHDF2 under different circumstances.
Recently, in vivo binding of m6A by YTHDF1 was also demonstrated. Knockdown of YTHDF1 reduced ribosome occupancy and decreased translation of m6A-modified mRNAs. Further investigation revealed that YTHDF1 interacts with initiation factors to promote translation [73]. These results presented a novel mechanism of translation regulation by m6A modification in mRNA.
5.3. eIF3
Most recently, Meyer et al. [74] reported that eukaryotic initiation factor 3 (eIF3), a component of 43S translation preinitiation complex, directly binds with 5′ UTR m6A. Cross-linking of eIF3 to the m6A-containing RNA probe was substantially increased compared with the A-containing probe, and eIF3 preferably bind to Gm6AC nucleotides. Further research revealed that eIF3-binding sites were primarily localized to 5′ UTRs of mRNAs, which was important in regulating translation initiation. Restraining m6A modification by FTO overexpression substantially depleted mRNAs that contain a high stoichiometry m6A site within their 5′ UTR in the eIF3-bound fraction, indicating that eIF3 interacts with mRNAs in an m6A-dependent manner in cells. Moreover, the researchers found that the binding of eIF3 to 5′ UTR m6A was independent of YTHDF1, which was reported to interact with eIF3, thus supporting the idea that eIF3 was able to directly bind m6A [74].
6. m6A and miRNAs
m6A mRNA modification were enriched in 5′ UTRs, around stop codon and in the proximal region of 3′ UTRs, whereas miRNAs-targeted sites at the 5′ end and 3′ end of 3′UTRs suggested a potential link between m6A modification and miRNA targeting sites [15,16,26,27]. A recent study also showed that m6A peaks were enriched at miRNAs target sites [27]. Further research verified this hypothesis using Dicer knockdown and overexpression approaches, which showed that m6A abundance was positively correlated with Dicer level, which mediates miRNA maturation. ASF (a nuclear speckle marker) staining revealed that knockdown of Dicer, which presents in both the nucleus and cytoplasm [75], resulted in disrupted localization of METTL3 to nuclear speckles without affecting METTL3 abundance. Consistently, experiments by knockdown, overexpression and mutation of certain miRNAs showed that miRNAs regulated the m6A methyltransferase activity of METTL3 by modulating its binding to mRNAs in a sequence-dependent manner [27].
More recently, m6A modification was also identified on primary microRNAs (pri-miRNAs). Researchers found that METTL3 methylates pri-miRNAs, marking them for recognition and processing by DGCR8. METTL3 depletion reduced the binding of DGCR8 to pri-miRNAs, and resulted in the global reduction of mature miRNAs and concomitant accumulation of unprocessed pri-miRNAs [76]. Moreover, the ‘reader’ that recognized the m6A modification on pri-miRNAs (i.e. HNRNPA2B1) was also identified. HNRNPA2B1 interacts with DGCR8 to promote pri-miRNA processing [70]. These results revealed tight regulations among m6A modification, miRNA biogenesis and function.
7. m6a function—effects on mRNA fate and biological consequences
During the past 3–4 years, the breakthrough of developing transcriptome-wide profiling of m6A led to the feature of crucial regulatory roles of m6A modification in a wide range of fundamental cellular processes, including gene expression, meiosis, stemness and circadian rhythm. The writers, erasers and readers were also found to have intimate relevance to certain diseases, such as obesity, infertility and growth retardation. Considering the prevalent distribution of m6A modification in mRNAs and lncRNAs, it would not be surprising to uncover more specific regulatory roles of m6A along with identification of more m6A readers.
7.1. Effects on mRNA fate—splicing, degradation and translation
The localization of METTL3, METTL14, WTAP and ALKBH5 were mainly found in nuclear speckles, and FTO was also found partially co-localized with nuclear speckles, a well-known site for pre-mRNA processing [77–81]. These phenomena raised the prospect of a regulatory role of m6A in mRNA splicing. Knockdown of WTAP or METTL3 indeed generated different mRNA isoforms, and WTAP was a known splicing factor [53,55]. Also, ALKBH5 was shown to affect the rate of splicing [61]. All of these results supported the hypothesis that m6A may be involved in mRNA splicing. A recent study demonstrated that m6A-mediated mRNA structure remodelling affected binding to HNRNPC, which was an abundant nuclear RNA binding protein responsible for pre-mRNA processing, and alternative splicing [34]. Indeed, knockdown of Mettl3/14 co-regulated the expression of 5251 genes with HNRNPC knockdown in HEK293T cells, and 890 of these genes were in high confidence in containing m6A-mediated structure remodelling switch. Further research indicated that this remodelling tended to regulate splicing events at nearby exons. The regulatory role of m6A in mRNA splicing was also reported in the study of FTO-depleted 3T3-L1 pre-adipocytes. The researchers found that enhanced m6A level in response to FTO depletion promotes RNA binding ability of splicing regulatory protein SRSF2, leading to increased inclusion of target exons [17]. These data provide strong evidence on a mechanistic relationship between the presence of m6A and splicing events.
Cellular mRNAs possess fast turnover with a median half-life of about 5 h. The dynamic mRNA synthesis and degradation render cells liable to make rapid adjustment in response to environmental changes via newly degraded nucleotides for de novo synthesis. Thus, the identification and regulation of certain mRNAs for degradation is vitally important. Knockdown of METTL3 or METTL14 in mouse embryonic stem cells modestly increased the stability of target mRNAs, suggesting that m6A modification induces mRNA instability [18,47]. Further studies revealed that the binding activities of HuR, a known mRNA stabilizer, were impaired by m6A modification adjacent to the binding sites in vitro [47]. The regulatory role of m6A on mRNA degradation was verified by the discovery that binding with YTHDF2 promoted thousands of cellular mRNA degradation via translocation to decay sites [71] (figure 1). Another m6A reader—HNRNPC—may also regulate mRNA degradation, because knockdown of HNRNPC also affected the abundance of target transcripts [34]. Knockdown of an m6A eraser—ALKBH5—increased poly(A) mRNAs in the nucleus [61], suggesting that ALKBH5 and its demethylation activity may affect mRNA export from nucleus to cytoplasm, or nascent mRNA synthesis.
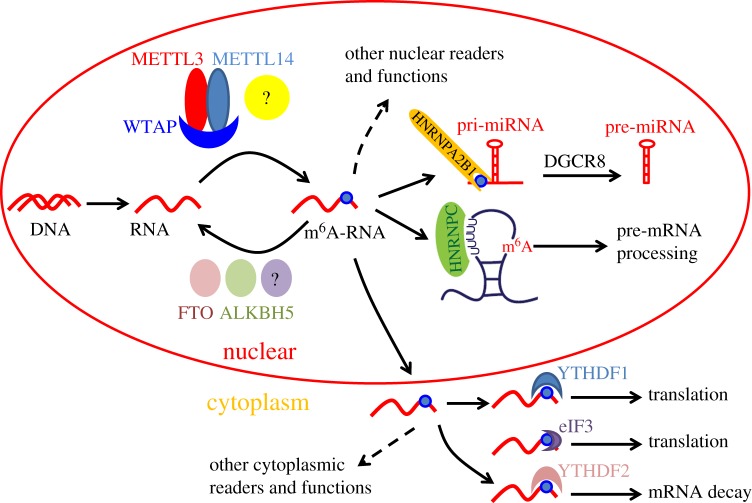
Figure 1.
Dynamic m6A RNA modifications and mediated functions. m6A mRNA methylation is mediated by a multiprotein complex that includes METTL3, METTL14 and WTAP, whereas demethylases, such as FTO and ALKBH5, erase m6A. Recognition of m6A by HNRNPC in the nucleus mediates alternative splicing of pre-mRNA, and HNRNPA2B1 promotes pri-miRNA processing to pre-miRNA. In cytoplasm, binding of m6A sites with different readers mediates divergent functions. YTHDF1 binds m6A-modified mRNAs through interactions with initiation factors and ribosomes to increase translational output, and eIF3 can also directly bind to 5′UTR m6A to initiate translation, whereas m6A recognition by YTHDF2 leads to mRNA decay. More nuclear and cytoplasmic readers need to be defined to illuminate the functions of m6A in mRNA export, translation and storage.
Organisms perform their biological functions mainly through proteins. Because mRNAs are the direct templates for protein synthesis, the enrichment of m6A in exons and around the stop codon regions makes it conceivable that m6A may also regulate translation. In a recent study performed in mouse embryonic stem cells (mESCs) and embryoid bodies (EBs), METTL3 ablation modestly yet significantly increased translation efficiency, indicating a regulatory role of m6A in translation [18]. More recently, another m6A reader, YTHDF1, was reported to interact with initiation factors and ribosomes to increase translational output [73], presenting direct evidence for translational regulation functions of m6A. One of the translation initiation factors, eIF3, was also reported to directly bind 5′ UTR m6A, which was sufficient to recruit the 43S complex to initiate translation in the absence of the cap-binding factor eIF4E [74] (figure 1). Furthermore, the researchers also found that diverse cellular stresses induced a transcriptome-wide redistribution of m6A, resulting in increased numbers of mRNAs with 5′ UTR m6A, which thus presented a concept of dynamic m6A events in response to stress. The identification of more m6A readers will help to better elucidate the translation process.
7.2. Biological consequences of m6A—dysregulation in cellular processes and diseases
7.2.1. Stemness—mammalian embryonic stem cell fate transition
Several groups have reported the prevalent m6A mRNA modification in mammalian embryonic stem cells and a similar region distribution with somatic cells [18,26,27,47]. However, the regulatory role of m6A modification in cell fate transition in ESCs was conflictive among these studies. The earliest reports showed that knockdown of METTL3 or METTL14 via shRNA interfering led to decreased proliferation rate of mESCs; RT-qPCR of pluripotency factors displayed reduction in knockdown cells, whereas developmental regulators were increased. Enrichment of developmental factors rather than pluripotency-related genes were also found in METTL3 and METTL14 targets, and m6A methylation destabilized these transcripts via damping the binding with HuR. Based on these observations, a logical deduction was made that m6A methylation was required to keep mESCs at ground state [47]. Another group overexpressed METTL3 in mouse embryonic fibroblasts (MEFs) and found a significant increase in m6A abundance, enhanced expression of key pluripotent factors and improvement of reprogramming efficiency. Reverse results were also found in METTL3 knockdown and methionine adenosyltransferase inhibitor-treated cells, indicating that m6A was required for MEF reprogramming to pluripotency [27].
Contrary to these results, genetic knockout or shRNA knockdown of Mettl3 demonstrated improved self-renewal in mESCs reported in a recent study [26]. The same group also found impaired differentiation towards cardiomyocytes and the neural lineage in vitro in Mettl3 KO mESCs which retained high levels of pluripotency regulator Nanog expression. The in vivo teratoma generation experiments also showed poorly differentiated cells in teratomas derived from KO ESCs with higher staining of NANOG and the proliferation marker KI67 [26]. These results suggested that m6A suppresses self-renewal and promotes differentiation.
The conflict was rested with the generation of Mettl3 KO ESCs by mating of Mettl3+/− mice. Mettl3 KO embryonic blastocysts failed to adequately repress pluripotent genes, and differentiated into mature neurons in vitro and poorly differentiated in teratomas in vivo, and also hampered priming from naive pluripotent state towards an epiblast-like state [18]. These results indicated that depletion of m6A modification blocked differentiation in ESCs and led to a hypernaive pluripotenct state. Further research adopted an siRNA interfering approach to knockdown METTL3 in mESCs in both naive pluripotent state and primed EpiSC state. Quantitative PCR results showed upregulation of both pluripotent regulators and developmental factors upon knockdown of METTL3 in mESCs in either state. However, the basal transcript levels of pluripotency genes are abundant, whereas lineage factors are extremely low under naive state. When progressing towards the primed state, the pluripotency genes were downregulated and lineage commitment makers became abundantly expressed. Thus, the obliteration of METTL3 potentiated the already high pluripotency genes in the naive condition to create a hypernaive pluripotent state, but mounted the dominating developmental factors in the primed state and tipped the balance towards differentiation [18]. Most recently, Aguillo et al. [82] showed that ZFP217 sequesters METTL3 and diminishes METTL3 binding with RNAs to restrain m6A modification, and that low m6A levels in ESC-related transcripts enable pluripotency and reprogramming.
These results demonstrated that m6A modification determined the fate transition in mESCs. Also, m6A methylomes in human and mouse ESCs were shown to be highly conserved [26], and a recent research showed an important role of METTL3 homologue in the development of Arabidopsis embryo [83], which suggested a conserved role of m6A in ESC development.
7.2.2. Obesity—FTO in adipogenesis
Genome-wide association studies linked common variants of FTO gene with childhood and adult obesity in 2007 [58–60]. Loss-of-function mutation in the FTO gene is responsible for a recessive lethal syndrome, including postnatal growth retardation, microcephaly and cardiac defects [22]. Studies by inactivation or overexpression of FTO in mice suggested that FTO tended to promote obesity and metabolic syndrome by driving obesity-prone behaviours such as increased food intake [84–87], consistent with its highest expression level in the brain [88]. The finding that FTO-mediated m6A demethylation controls exonic splicing of adipogenic regulatory factor RUNX1T1 emphasized the regulatory role of FTO in adipogenesis [17]. Another group reported that obesity variants within the FTO gene formed a long-range connection with IRX3 [89], which was located downstream from FTO, and deficiency in this gene resulted in 25–30% body weight loss [90], thus questioning a direct role for FTO in obesity.
m6A mRNA modification has also been shown to exert regulatory functions in apoptosis, circadian rhythm and meiosis, and aberrant m6A mRNA modifications are correlated to a variety of human diseases, including cancer, infertility and hepatitis, which has been reviewed elsewhere [19–21,91,92].
8. Possible role of m6A in immune response
The immune system serves as the security guard of the human body, and plays a most important role in clearance of pathogens, either endogenous or exogenous. The dysfunction of the immune system is involved in almost all known human diseases, including cancers, infection diseases, inflammation diseases, allergies, metabolism syndromes and autoimmune diseases. The immune system is composed of two parts—the innate and adaptive immune system. The innate immune reactions are rapid and non-specific, whereas the adaptive response needs antigen presentation, clone expansion and differentiation to perform antigen-specific reactions [93,94]. The abundance of antigens in the environment and the quick turnover of apoptotic internal cells demands rapid adjustment abilities of immune cells. Indeed, upon antigen recognition, innate immune cells and memorial adaptive cells are capable of releasing a robust amount of cytokines in as little as 2 h [95], which is called ‘cytokine storm’ and is unlikely to be driven from de novo gene transcription. As discussed above, the fast turnover of mRNAs is an energy-cost-effective process in responding to environmental changes compared with proteins. Because m6A plays critical roles in mRNA splicing, degradation and translation, it is conceivable that it may also play an important role in immune reactions. In fact, m6A has been shown to protect RNA from recognition by TLR3 and TLR7 as invasive species for degradation [96,97]. Also, one of the erasers of m6A, ALKBH5, has been shown to be highly expressed in the spleen and lung, organs enriched in immune cells and with frequent immune reactions [61]. Performing experimental immune disease models using FTO, Alkbh5 and Mettl3 knockout mice may help elucidate the role of m6A in immune response.
Acknowledgements
We thank Caroline Lieber for manuscript assistance.
Competing interests
We declare we have no competing interests.
Funding
Our research was supported in part by the Howard Hughes Medical Institute (R.A.F.) and by the Key International Collaboration Grant from the National Natural Science Foundation of China 31420103901 (Z.Y.). H.-B.L. is supported by NIH T32 2T32DK007356.
References
- 1.Khan Z, Ford MJ, Cusanovich DA, Mitrano A, Pritchard JK, Gilad Y. 2013. Primate transcript and protein expression levels evolve under compensatory selection pressures. Science 342, 1100–1104. (doi:10.1126/science.1242379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L, Candille SI, Choi Y, Xie D, Jiang L, Li-Pook-Than J, Tang H, Snyder M. 2013. Variation and genetic control of protein abundance in humans. Nature 499, 79–82. (doi:10.1038/nature12223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motorin Y, Helm M. 2011. RNA nucleotide methylation. Wiley Interdiscip. Rev. RNA 2, 611–631. (doi:10.1002/wrna.79) [DOI] [PubMed] [Google Scholar]
- 4.Machnicka MA, et al. 2013. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Res. 41, D262–D267. (doi:10.1093/nar/gks1007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desrosiers R, Friderici K, Rottman F. 1974. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl Acad. Sci. USA 71, 3971–3975. (doi:10.1073/pnas.71.10.3971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuichi Y, Morgan M, Shatkin AJ, Jelinek W, Salditt-Georgieff M, Darnell JE. 1975. Methylated, blocked 5 termini in HeLa cell mRNA. Proc. Natl Acad. Sci. USA 72, 1904–1908. (doi:10.1073/pnas.72.5.1904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams JM, Cory S. 1975. Modified nucleosides and bizarre 5′-termini in mouse myeloma mRNA. Nature 255, 28–33. (doi:10.1038/255028a0) [DOI] [PubMed] [Google Scholar]
- 8.Wei CM, Gershowitz A, Moss B. 1975. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell 4, 379–386. (doi:10.1016/0092-8674(75)90158-0) [DOI] [PubMed] [Google Scholar]
- 9.Wei CM, Moss B. 1975. Methylated nucleotides block 5′-terminus of vaccinia virus messenger RNA. Proc. Natl Acad. Sci. USA 72, 318–322. (doi:10.1073/pnas.72.1.318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krug RM, Morgan MA, Shatkin AJ. 1976. Influenza viral mRNA contains internal N6-methyladenosine and 5′-terminal 7-methylguanosine in cap structures. J. Virol. 20, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rottman FM, Desrosiers RC, Friderici K. 1976. Nucleotide methylation patterns in eukaryotic mRNA. Prog. Nucleic Acid Res. Mol. Biol. 19, 21–38. (doi:10.1016/S0079-6603(08)60906-X) [DOI] [PubMed] [Google Scholar]
- 12.Rabani M, et al. 2011. Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nat. Biotechnol. 29, 436–442. (doi:10.1038/nbt.1861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwanhausser B, Busse D, Li N, Li Na, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. 2011. Global quantification of mammalian gene expression control. Nature 473, 337–342. (doi:10.1038/nature10098) [DOI] [PubMed] [Google Scholar]
- 14.Jia G, et al. 2011. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887. (doi:10.1038/nchembio.687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominissini D, et al. 2012. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. (doi:10.1038/nature11112) [DOI] [PubMed] [Google Scholar]
- 16.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. 2012. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646. (doi:10.1016/j.cell.2012.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X, et al. 2014. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 24, 1403–1419. (doi:10.1038/cr.2014.151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geula S, et al. 2015. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347, 1002–1006. (doi:10.1126/science.1261417) [DOI] [PubMed] [Google Scholar]
- 19.Liu N, Pan T. 2015. RNA epigenetics. Transl. Res. 165, 28–35. (doi:10.1016/j.trsl.2014.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klungland A, Dahl JA. 2014. Dynamic RNA modifications in disease. Curr. Opin. Genet. Dev. 26, 47–52. (doi:10.1016/j.gde.2014.05.006) [DOI] [PubMed] [Google Scholar]
- 21.Blanco S, Frye M. 2014. Role of RNA methyltransferases in tissue renewal and pathology. Curr. Opin. Cell Biol. 31, 1–7. (doi:10.1016/j.ceb.2014.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boissel S, et al. 2009. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am. J. Hum. Genet. 85, 106–111. (doi:10.1016/j.ajhg.2009.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perlman S, Abelson HT, Penman S. 1973. Mitochondrial protein synthesis: RNA with the properties of Eukaryotic messenger RNA. Proc. Natl Acad. Sci. USA 70, 350–353. (doi:10.1073/pnas.70.2.350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaike T, Suzuki T, Ueda T. 2008. Polyadenylation in mammalian mitochondria: insights from recent studies. Biochim. Biophys. Acta 1779, 266–269. (doi:10.1016/j.bbagrm.2008.02.001) [DOI] [PubMed] [Google Scholar]
- 25.Slomovic S. 2006. Polyadenylation of ribosomal RNA in human cells. Nucleic Acids Res. 34, 2966–2975. (doi:10.1093/nar/gkl357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batista PJ, et al. 2014. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719. (doi:10.1016/j.stem.2014.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T, et al. 2015. m6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell 16, 289–301. (doi:10.1016/j.stem.2015.01.016) [DOI] [PubMed] [Google Scholar]
- 28.Luo GZ, et al. 2014. Unique features of the m6A methylome in Arabidopsis thaliana. Nat. Commun. 5, 5630 (doi:10.1038/ncomms6630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baltz AG, et al. 2012. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell 46, 674–690. (doi:10.1016/j.molcel.2012.05.021) [DOI] [PubMed] [Google Scholar]
- 30.Gosai SJ, et al. 2015. Global analysis of the RNA-protein interaction and RNA secondary structure landscapes of the Arabidopsis nucleus. Mol. Cell 57, 376–388. (doi:10.1016/j.molcel.2014.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, et al. 2012. Global analysis of RNA secondary structure in two metazoans. Cell Rep. 1, 69–82. (doi:10.1016/j.celrep.2011.10.002) [DOI] [PubMed] [Google Scholar]
- 32.Kierzek E, Kierzek R. 2003. The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res. 31, 4472–4480. (doi:10.1093/nar/gkg633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz S, et al. 2013. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 155, 1409–1421. (doi:10.1016/j.cell.2013.10.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. 2015. N6-methyladenosine-dependent RNA structural switches regulate RNA–protein interactions. Nature 518, 560–564. (doi:10.1038/nature14234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimock K, Stoltzfus CM. 1977. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry 16, 471–478. (doi:10.1021/bi00622a021) [DOI] [PubMed] [Google Scholar]
- 36.Canaani D, Kahana C, Lavi S, Groner Y. 1979. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 6, 2879–2899. (doi:10.1093/nar/6.8.2879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei CM, Gershowitz A, Moss B. 1976. 5′-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry 15, 397–401. (doi:10.1021/bi00647a024) [DOI] [PubMed] [Google Scholar]
- 38.Wei CM, Moss B. 1977. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 16, 1672–1676. (doi:10.1021/bi00627a023) [DOI] [PubMed] [Google Scholar]
- 39.Schibler U, Kelley DE, Perry RP. 1977. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J. Mol. Biol. 115, 695–714. (doi:10.1016/0022-2836(77)90110-3) [DOI] [PubMed] [Google Scholar]
- 40.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. 2015. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 12, 767–772. (doi:10.1038/nmeth.3453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ke S, et al. 2015. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 29, 2037–2053. (doi:10.1101/gad.269415.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen K, et al. 2015. High-resolution N6-methyladenosine (m6A) map using photo-crosslinking-assisted m6A sequencing. Angew. Chem. Int. Ed. Engl. 54, 1587–1590. (doi:10.1002/anie.201410647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narayan P, Rottman FM. 1988. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science 242, 1159–1162. (doi:10.1126/science.3187541) [DOI] [PubMed] [Google Scholar]
- 44.Bokar JA, et al. 1994. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J. Biol. Chem. 269, 17 697–17 704. [PubMed] [Google Scholar]
- 45.Bokar JA, Shambaugh ME, Polayes D et al. 1997. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3, 1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, et al. 2014. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95. (doi:10.1038/nchembio.1432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. 2014. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 16, 191–198. (doi:10.1038/ncb2902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, Fray RG. 2008. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 20, 1278–1288. (doi:10.1105/tpc.108.058883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clancy MJ. 2002. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 30, 4509–4518. (doi:10.1093/nar/gkf573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hongay CF, Orr-Weaver TL. 2011. Drosophila inducer of meiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc. Natl Acad. Sci. USA 108, 14 855–14 860. (doi:10.1073/pnas.1111577108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harper JE, Miceli SM, Roberts RJ, Manley JL. 1990. Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Res. 18, 5735–5741. (doi:10.1093/nar/18.19.5735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bujnicki JM, Feder M, Radlinska M, Blumenthal RM. 2002. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA:m6A methyltransferase. J. Mol. Evol. 55, 431–444. (doi:10.1007/s00239-002-2339-8) [DOI] [PubMed] [Google Scholar]
- 53.Little NA, Hastie ND, Davies RC. 2000. Identification of WTAP, a novel Wilms’ tumour 1-associating protein. Hum. Mol. Genet. 9, 2231–2239. (doi:10.1093/oxfordjournals.hmg.a018914) [DOI] [PubMed] [Google Scholar]
- 54.Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. 2012. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 8, e1002732 (doi:10.1371/journal.pgen.1002732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ping XL, et al. 2014. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 24, 177–189. (doi:10.1038/cr.2014.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz S, et al. 2014. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 8, 284–296. (doi:10.1016/j.celrep.2014.05.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu Y, et al. 2013. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 4, 1798 (doi:10.1038/ncomms2822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dina C, et al. 2007. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 39, 724–726. (doi:10.1038/ng2048) [DOI] [PubMed] [Google Scholar]
- 59.Frayling TM, et al. 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894. (doi:10.1126/science.1141634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scuteri A, et al. 2007. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 3, e115 (doi:10.1371/journal.pgen.0030115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng G, et al. 2013. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29. (doi:10.1016/j.molcel.2012.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng G, Dahl JA, Niu Y, Fu Ye, Klungland A, Yang Y-G, He C. 2013. Sprouts of RNA epigenetics: the discovery of mammalian RNA demethylases. RNA Biol. 10, 915–918. (doi:10.4161/rna.24711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spitale RC. 2015. Structural imprints in vivo decode RNA regulatory mechanisms. Nature 519, 486–490. (doi:10.1038/nature14263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou KI, et al. 2015. N-methyladenosine modification in a long noncoding RNA hairpin predisposes its conformation to protein binding. J. Mol. Biol. 428, 822–833. (doi:10.1016/j.jmb.2015.08.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Konig J, Zarnack K, Rot G, Curk T, Kayikci M, Zupan B, Turner DJ, Luscombe NM, Ule J. 2010. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 17, 909–915. (doi:10.1038/nsmb.1838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCloskey A, Taniguchi I, Shinmyozu K, Ohno M. 2012. hnRNP C tetramer measures RNA length to classify RNA polymerase II transcripts for export. Science 335, 1643–1646. (doi:10.1126/science.1218469) [DOI] [PubMed] [Google Scholar]
- 67.Rajagopalan LE, Westmark CJ, Jarzembowski JA, Malter JS. 1998. hnRNP C increases amyloid precursor protein (APP) production by stabilizing APP mRNA. Nucleic Acids Res. 26, 3418–3423. (doi:10.1093/nar/26.14.3418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zarnack K, et al. 2013. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell 152, 453–466. (doi:10.1016/j.cell.2012.12.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cienikova Z, Damberger FF, Hall J, Allain FHT, Maris C. 2014. Structural and mechanistic insights into poly(uridine) tract recognition by the hnRNP C RNA recognition motif. J. Am. Chem. Soc. 136, 14 536–14 544. (doi:10.1021/ja507690d) [DOI] [PubMed] [Google Scholar]
- 70.Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. 2015. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell 162, 1299–1308. (doi:10.1016/j.cell.2015.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X, et al. 2014. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120. (doi:10.1038/nature12730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou J, et al. 2015. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594. (doi:10.1038/nature15377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, et al. 2015. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399. (doi:10.1016/j.cell.2015.05.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian S-B, Jaffrey SR. 2015. 5′ UTR m6A promotes cap-independent translation. Cell 163, 999–1010. (doi:10.1016/j.cell.2015.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White E, Schlackow M, Kamieniarz-Gdula K, Proudfoot NJ, Gullerova M. 2014. Human nuclear Dicer restricts the deleterious accumulation of endogenous double-stranded RNA. Nat. Struct. Mol. Biol. 21, 552–559. (doi:10.1038/nsmb.2827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. 2015. N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485. (doi:10.1038/nature14281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spector DL. 1993. Nuclear organization of pre-mRNA processing. Curr. Opin. Cell Biol. 5, 442–447. (doi:10.1016/0955-0674(93)90009-F) [DOI] [PubMed] [Google Scholar]
- 78.Lamond AI, Spector DL. 2003. Nuclear speckles: a model for nuclear organelles. Nat. Rev. Mol. Cell Biol. 4, 605–612. (doi:10.1038/nrm1172) [DOI] [PubMed] [Google Scholar]
- 79.Sleeman JE, Trinkle-Mulcahy L. 2014. Nuclear bodies: new insights into assembly/dynamics and disease relevance. Curr. Opin. Cell Biol. 28, 76–83. (doi:10.1016/j.ceb.2014.03.004) [DOI] [PubMed] [Google Scholar]
- 80.Mao YS, Zhang B, Spector DL. 2011. Biogenesis and function of nuclear bodies. Trends Genet. 27, 295–306. (doi:10.1016/j.tig.2011.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao R, Bodnar MS, Spector DL. 2009. Nuclear neighborhoods and gene expression. Curr. Opin. Genet. Dev. 19, 172–179. (doi:10.1016/j.gde.2009.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aguilo F, et al. 2015. Coordination of m6A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell 17, 689–704. (doi:10.1016/j.stem.2015.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bodi Z, Zhong S, Mehra S, Song Jie, Graham N, Li H, May S, Fray RG. 2012. Adenosine methylation in Arabidopsis mRNA is associated with the 3′ end and reduced levels cause developmental defects. Front. Plant Sci. 3, 48 (doi:10.3389/fpls.2012.00048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, Rüther U. 2009. Inactivation of the Fto gene protects from obesity. Nature 458, 894–898. (doi:10.1038/nature07848) [DOI] [PubMed] [Google Scholar]
- 85.Church C, et al. 2010. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 42, 1086–1092. (doi:10.1038/ng.713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hess ME, et al. 2013. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 16, 1042–1048. (doi:10.1038/nn.3449) [DOI] [PubMed] [Google Scholar]
- 87.Gulati P, et al. 2013. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc. Natl Acad. Sci. USA 110, 2557–2562. (doi:10.1073/pnas.1222796110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerken T, et al. 2007. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318, 1469–1472. (doi:10.1126/science.1151710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smemo S, et al. 2014. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507, 371–375. (doi:10.1038/nature13138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ragvin A, et al. 2010. Long-range gene regulation links genomic type 2 diabetes and obesity risk regions to HHEX, SOX4, and IRX3. Proc. Natl Acad. Sci. USA 107, 775–780. (doi:10.1073/pnas.0911591107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu Y, Dominissini D, Rechavi G, He C. 2014. Gene expression regulation mediated through reversible m6A RNA methylation. Nat. Rev. Genet. 15, 293–306. (doi:10.1038/nrg3724) [DOI] [PubMed] [Google Scholar]
- 92.Meyer KD, Jaffrey SR. 2014. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 15, 313–326. (doi:10.1038/nrm3785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gasteiger G, Rudensky AY. 2014. Interactions between innate and adaptive lymphocytes. Nat. Rev. Immunol. 14, 631–639. (doi:10.1038/nri3726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwasaki A, Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353. (doi:10.1038/ni.3123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Artis D, Spits H. 2015. The biology of innate lymphoid cells. Nature 517, 293–301. (doi:10.1038/nature14189) [DOI] [PubMed] [Google Scholar]
- 96.Kariko K, Buckstein M, Ni H, Weissman D. 2005. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175. (doi:10.1016/j.immuni.2005.06.008) [DOI] [PubMed] [Google Scholar]
- 97.Kawai T, Akira S. 2008. Toll-like receptor and RIG-I-like receptor signaling. Ann. N.Y. Acad. Sci. 1143, 1–20. (doi:10.1196/annals.1443.020) [DOI] [PubMed] [Google Scholar]