Abstract
Mural cells (MCs) consisting of vascular smooth muscle cells and pericytes cover the endothelial cells (ECs) to regulate vascular stability and homeostasis. Here, we clarified the mechanism by which MCs develop and cover ECs by generating transgenic zebrafish lines that allow live imaging of MCs and by lineage tracing in vivo. To cover cranial vessels, MCs derived from either neural crest cells or mesoderm emerged around the preformed EC tubes, proliferated and migrated along EC tubes. During their migration, the MCs moved forward by extending their processes along the inter-EC junctions, suggesting a role for inter-EC junctions as a scaffold for MC migration. In the trunk vasculature, MCs derived from mesoderm covered the ventral side of the dorsal aorta (DA), but not the posterior cardinal vein. Furthermore, the MCs migrating from the DA or emerging around intersegmental vessels (ISVs) preferentially covered arterial ISVs rather than venous ISVs, indicating that MCs mostly cover arteries during vascular development. Thus, live imaging and lineage tracing enabled us to clarify precisely how MCs cover the EC tubes and to identify the origins of MCs.
KEY WORDS: Mural cells, Pericytes, Vascular smooth muscle cells, Zebrafish, Pdgfrb
Highlighted article: Live imaging of mural cell dynamics in zebrafish shows how the mural cells develop and cover the endothelial cells during vascular development.
INTRODUCTION
The vasculature consists of two principle cell types: endothelial cells (ECs) and mural cells (MCs). ECs line the inner surface of the blood vessels, whereas the MCs cover the abluminal surface of the ECs. MCs are further classified into two types: vascular smooth muscle cells (VSMCs) and pericytes (Gaengel et al., 2009). The former consist of multi-layered cells and ensheath ECs, whereas the latter are solitary cells associated with small diameter blood vessels, such as arterioles, venules and capillaries. MCs play an essential role in vascular stability and homeostasis (Armulik et al., 2011). Therefore, loss or detachment of MCs leads to various vascular diseases (French et al., 2014; Garg et al., 2014; Joutel et al., 1996).
MCs originate from distinct developmental precursors such as mesoderm-derived mesenchymal cells and neural crest cells (Majesky, 2007; Wasteson et al., 2008; Winkler et al., 2011). These precursors are thought to be recruited to the vessel wall and to differentiate into MCs, which subsequently proliferate and migrate to cover entire vessels (Armulik et al., 2011; Hellstrom et al., 1999). However, when and how the progenitor cells differentiate into MCs and cover the blood vessels during vascular development remains largely unknown, because no clear methods for analyzing MCs in living animals have been developed. To date, although zebrafish transgenic (Tg) lines, such as Tg(sm22a-b:GFP) and Tg(acta2:EGFP), have been developed to visualize MCs in vivo (Seiler et al., 2010; Whitesell et al., 2014), only limited parts of vasculature were covered by perivascular enhanced green fluorescent protein (EGFP)-expressing cells in these Tg zebrafish lines. Thus, these fish lines may not faithfully report all MCs in vivo.
The platelet-derived growth factor (PDGF)-B/PDGF receptor β (PDGFRβ) signaling pathway plays a crucial role in the regulation of MC coverage of blood vessels (Hellstrom et al., 1999; Olson and Soriano, 2011; Wang et al., 2014). PDGF-B secreted from ECs binds to PDGFRβ receptors expressed on the MCs, thereby promoting recruitment of MCs and their subsequent proliferation and migration. Recently, Wang et al. successfully visualized pericytes covering cerebral vessels in the zebrafish brain by performing fluorescence in situ hybridization (ISH) for pdgfrb (Wang et al., 2014). However, this method does not allow the analysis of MC dynamics in vivo.
Here, we have succeeded in visualizing the MCs in living zebrafish by developing Tg lines in which EGFP, mCherry or the Gal4FF driver, an engineered transcriptional activator consisting of the DNA-binding domain from Gal4 fused to two transcription activation modules from VP16 (Asakawa et al., 2008), was expressed under the control of pdgfrb promoter. Exploiting these Tg lines, we demonstrated how MCs develop and cover ECs in the cranial and trunk vessels and identified the origins of MCs covering the cranial and trunk vasculature.
RESULTS
Development of Tg zebrafish lines for live imaging of MCs
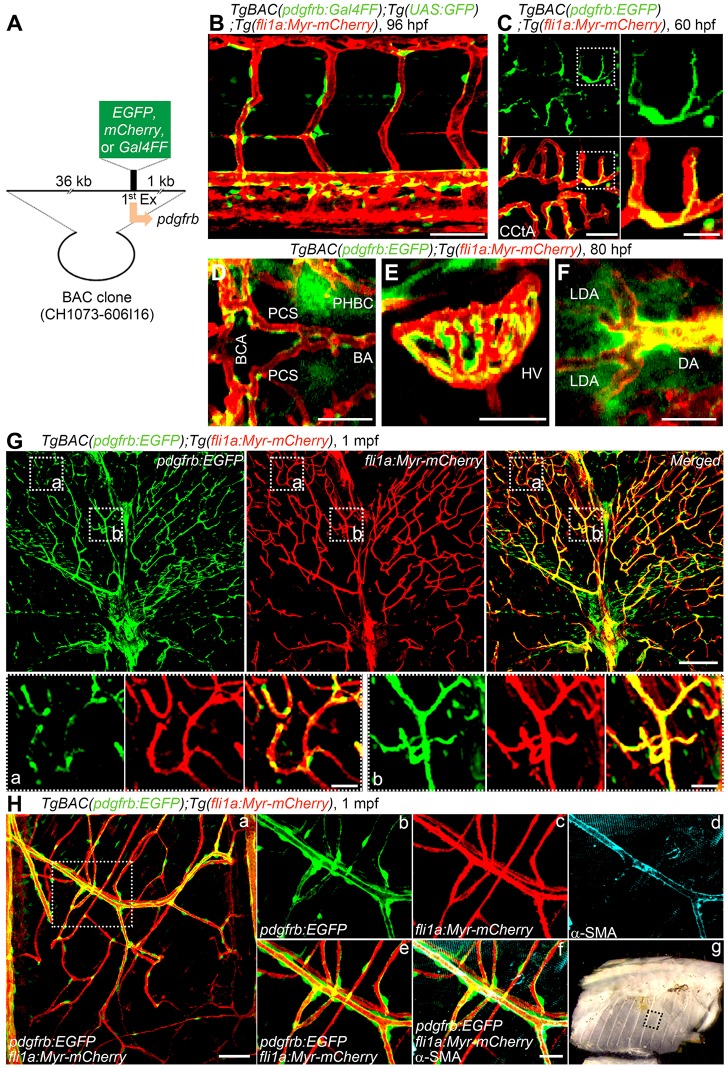
The Pdgfrb promoter is activated in MCs of mice (Foo et al., 2006). To visualize MCs using living animals, we developed TgBAC(pdgfrb:EGFP), TgBAC(pdgfrb:mCherry) and TgBAC(pdgfrb:Gal4FF);Tg(UAS:GFP) zebrafish lines, in which EGFP, mCherry or the Gal4FF driver was expressed under control of pdgfrb promoter, respectively (Fig. 1A). To simultaneously visualize ECs and MCs, the first and the third lines were crossed with Tg(fli1a:myryistoylation-signal tagged (Myr)-mCherry) fish. The second line was crossed with Tg(fli1a:Myr-EGFP). The expression pattern of fluorescent proteins in these reporter lines was similar to the previously reported expression pattern of pdgfrb mRNA (Wang et al., 2014; French et al., 2014; Wiens et al., 2010). In the TgBAC(pdgfrb:EGFP) embryos, EGFP started to be expressed around the 8-somite stage in the cranial neural crests in which pdgfrb mRNA is expressed (French et al., 2014) (Fig. S1A,B; Movies 1 and 2). EGFP expression was induced in the base of the brain from 17 h post-fertilization (hpf) (Fig. S1A,B; Movies 1 and 2). In the trunk of the TgBAC(pdgfrb:EGFP) and TgBAC(pdgfrb:mCherry) embryos, fluorescence signal was observed in the floor plate and hypochord at 24 hpf (Fig. S1C). At late stages, the dorsal aorta (DA), intersegmental vessels (ISVs) and dorsal longitudinal anastomotic vessels were surrounded by EGFP-positive cells in the trunk region of TgBAC(pdgfrb:Gal4FF);Tg(UAS:GFP) larvae (Fig. 1B). In the head region of TgBAC(pdgfrb:EGFP) larvae, EGFP-positive cells covered the vessels, such as the central artery (CtA), basal communicating artery (BCA), posterior communicating segment (PCS), basilar artery (BA), primordial hindbrain channel (PHBC) and hyaloid vessels (HVs) (Fig. 1C-E). In addition, EGFP-positive cells were accumulated in the anterior region of the DA, including the lateral DA where Transgelin-positive MCs also exist (Fig. 1F) (Santoro et al., 2009). Similarly, perivascular cells in the cranial and trunk vessels were visualized by mCherry in the TgBAC(pdgfrb:mCherry);Tg(fli1a:Myr-EGFP) larvae (Fig. S1D,E). These results indicate that fluorescent proteins successfully label MCs in our reporter lines. Indeed, RT-PCR analyses revealed that EGFP-positive cells isolated from TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) larvae expressed not only pdgfrb but also other MC marker genes, such as chondroitin sulfate proteoglycan 4 (cspg4) and actin, alpha 2, smooth muscle, aorta (acta2) (Fig. S1F).
Fig. 1.
Generation of Tg zebrafish lines for live imaging of MCs. (A) A schematic structure of the BAC clone (CH1073-606I16) used to generate Tg zebrafish lines for live imaging of MCs. cDNA encoding either EGFP, mCherry or Gal4FF was inserted at the start codon of pdgfrb gene. (B) Confocal stack fluorescence image of trunk vasculature in a 96 hpf TgBAC(pdgfrb:Gal4FF);Tg(UAS:GFP);Tg(fli1a:Myr-mCherry) larva. Lateral view, anterior to the left. Merged image of pdgfrb:Gal4FF;UAS:GFP (green) and fli1a:Myr-mCherry (red). (C-F) Confocal images of hindbrain vasculature (C,D), hyaloid vessels (E) and anterior region of dorsal aorta (F) in the TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) larvae at 60 hpf (C) and 80 hpf (D-F). Dorsal view, anterior to the left. Merged images of pdgfrb:EGFP (green) and fli1a:Myr-mCherry (red). In C, the boxed areas are enlarged to the right. (G) Confocal images of trunk vasculature in a 1 mpf TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) juvenile. Cross-sectional views (200 μm thick) through the caudal region as depicted in Fig. S1H are shown. Upper left, pdgfrb:EGFP (green); upper center, fli1a:Myr-mCherry (red); upper right, merged image. The boxed areas labeled a and b are enlarged below. (H) Confocal images of blood vessels in the intercostal muscle of a 1 mpf TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) juvenile. Pleural tissue as indicated by the box shown in g was cut out and immunostained with anti-α-SMA antibody to visualize VSMCs. The merged image of pdgfrb:EGFP (green) and fli1a:Myr-mCherry (red) is shown on the left (a). The boxed area in a is enlarged to the right: pdgfrb:EGFP (b), fli1a:Myr-mCherry (c), α-SMA (d), merge of pdgfrb:EGFP (green) and fli1a:Myr-mCherry (red) (e) and merge of pdgfrb:EGFP (green), fli1a:Myr-mCherry (red) and α-SMA (blue) (f). (g) Brightfield image of the thorax showing the region where the image shown in a was taken. BA, basilar artery; BCA, basal communicating artery; CCtA, cerebellar central artery; DA, dorsal aorta; LDA, lateral DA; HV, hyaloid vessel; PCS, posterior communicating segment; PHBC, primordial hindbrain channel. Scale bars: 20 μm (enlarged images in C and H; D-F); 50 μm (B,C); 100 μm (G,H).
We also visualized VSMCs by generating the TgBAC(tagln:EGFP) zebrafish line, in which EGFP is expressed under the control of smooth muscle-specific transgelin promoter (Robin et al., 2013). Larvae of this Tg fish exhibited EGFP signal in the floor plate, swim bladder, gut and rostral notochord (Fig. S1G). In addition, EGFP-positive cells were detected in the ventral part of the DA, but not in the cranial vessels (data not shown), as previously observed in the Tg(sm22a-b:GFP) zebrafish line (Seiler et al., 2010). These findings indicate that the TgBAC(tagln:EGFP) line labels VSMCs in vivo.
Live imaging of blood vessel MCs in juvenile zebrafish
Next, we investigated whether MCs can be labeled by EGFP in the juvenile TgBAC(pdgfrb:EGFP) zebrafish. At 1 month post-fertilization (mpf), most blood vessels in the trunk were covered by EGFP-positive cells (Fig. 1G; Fig. S1H). Blood vessels with a diameter >5-10 μm were continuously ensheathed by EGFP-positive cells and were also stained with antibody for the VSMC marker α-SMA (Acta2), indicating that EGFP-positive cells were VSMCs in the TgBAC(pdgfrb:EGFP) zebrafish (Fig. 1G,H). Consistently, these thick vessels were also EGFP-positive in the TgBAC(tagln:EGFP) zebrafish line (Fig. S1I). By contrast, the capillaries with a diameter <5 μm were irregularly and discontinuously covered by EGFP-positive cells in the TgBAC(pdgfrb:EGFP) fish, but not in the TgBAC(tagln:EGFP) line (Fig. 1G,H; Fig. S1I), suggesting that EGFP-positive cells covering the capillaries are pericytes. Thus, our TgBAC(pdgfrb:EGFP) line precisely monitors pericytes and VSMCs.
Live imaging of MC coverage of cranial vessels
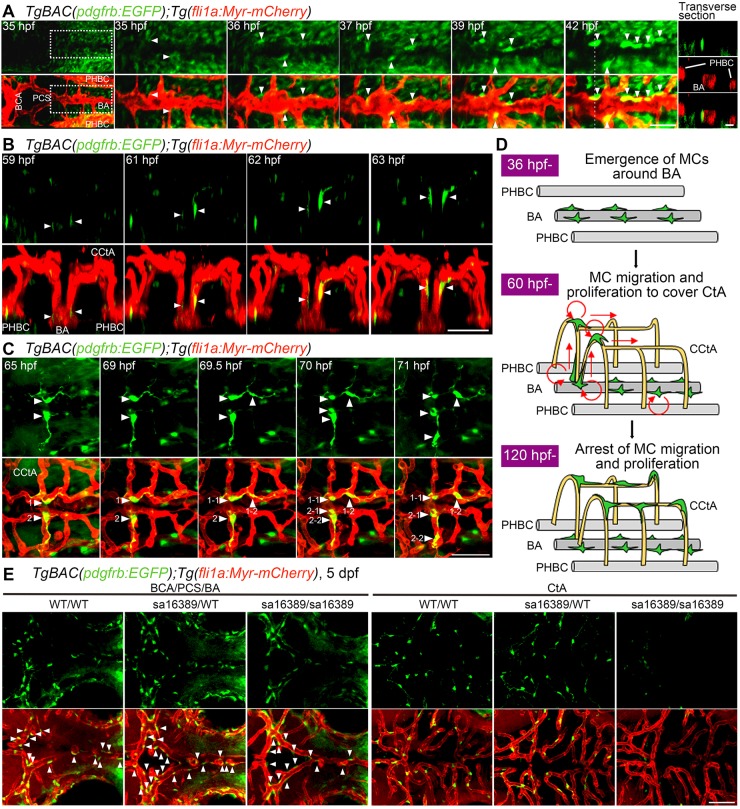
To investigate how cranial vessels become covered by MCs, we time-lapse imaged the TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) embryos, focusing on the MCs covering the CtA, which consists of cerebellar CtA (CCtA), anterior mesencephalic CtA (AMCtA), middle mesencephalic CtA (MMCtA) and posterior mesencephalic CtA (PMCtA). The CCtA are formed by ECs sprouting from the PHBC. The sprouting CCtA ECs from the PHBC first migrate dorsally to penetrate the hindbrain parenchyma, then turn ventrally and connect to the BA at 40-48 hpf (Fujita et al., 2011). During CCtA formation, weakly EGFP-positive cells existed in the cerebral base at the level of the BA (Fig. 2A). The location of the EGFP-positive cells implied that they might be anterior endodermal cells. However, they were not identical to sox17 promoter-active endodermal cells (Fig. S2A). Among the weakly EGFP-positive cells, cells located in the vicinity of the BA started to emit strong green fluorescence and then made tight contact with the BA (Fig. 2A). At ∼60 hpf, strongly EGFP-positive cells covering the BA started to migrate along the CCtA and frequently proliferated to cover the ECs throughout the CCtA (Fig. 2B,C; Movies 3-5). These results indicate that the CCtA is covered by MCs that originally emerge around the BA (Fig. 2D).
Fig. 2.
Live imaging of MC coverage in cranial vessels. (A-C) Time-lapse confocal images of the hindbrain vasculature in TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) larvae at 35-42 hfp (A), 59-63 hpf (B) and 65-71 hpf (C). Upper, pdgfrb:EGFP; lower, merged images of pdgfrb:EGFP (green) and fli1a:Myr-mCherry (red). (A) Dorsal view, anterior to the left. The central panels are enlarged and subsequent time-lapse images of the boxed areas in the leftmost column. The transverse sectional views of the areas indicated by dashed lines on the 42 hpf image are shown in the rightmost column. Top, pdgfrb:EGFP (green); middle (rightmost column only), fli1a:Myr-mCherry (red); bottom, merged image. Note that the cells located in the vicinity of the BA (arrowheads) gradually emitted strong EGFP signal and tightly contacted ECs. (B) 3D-rendered confocal images of EGFP-positive cells (green) migrating along the CCtAs. Lateral view, dorsal to the top and anterior to the front. Note that EGFP-positive cells located around the BA (arrowheads) dorsally migrated along the CCtAs. (C) Dorsal view, anterior to the left. Arrowheads with numbers indicate individual EGFP-positive cells spreading on the CCtAs. Note that EGFP-positive cells spreading on the CCtA (1 and 2) divided into two daughter cells (1-1/1-2 and 2-1/2-2). (D) Schematic of how CCtAs become covered by MCs. MCs develop around the BA and migrate towards the CCtAs. During their migration, the MCs actively proliferate to cover the CCtAs. (E) Confocal images of hindbrain vasculature of pdgfrb wild-type (WT/WT), heterozygous (sa16389/WT) and homozygous (sa16389/sa16389) larvae in the TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) background at 5 dpf. Dorsal view, anterior to the left. The vessels in the cerebral base, such as BCA, PCA and BA (BCA/PCS/BA), and the CtA, are shown in the left and right columns, respectively. Upper, pdgfrb:EGFP; lower, merged images of pdgfrb:EGFP (green) and fli1a:Myr-mCherry (red). Arrowheads indicate MCs emerged around the BCA, PCS and BA. Scale bars: 20 μm (transverse sectional image in A); 50 μm (A,B,C,E).
MC differentiation around the BA implies an instructive role for the BA in MC development. Consistently, kdr/kdrl-double morphants, which failed to develop the BA, exhibited a lack of strongly EGFP-positive cells in the midline of the hindbrain (Fig. S2B). Although EC function is influenced by blood flow, loss of blood flow did not affect MC emergence around the BA but did prevent their subsequent recruitment into the CtA (Fig. S2C). These results suggest that ECs regulate MC development independently of blood flow, whereas MC recruitment might be promoted by a flow-dependent EC-derived signal.
We also investigated how MCs are recruited to the AMCtA, MMCtA and PMCtA. These CtA vessels extend from the vessels located in the cerebral base, such as the BCA, PCS and choroidal vascular plexus (CVP), and subsequently penetrate into the brain parenchyma (Isogai et al., 2003). Weakly EGFP-positive cells located in close proximity to the BCA, PCS and CVP started to emit strong green fluorescence and were recruited to the AMCtA, MMCtA and PMCtA (Fig. S2D; Movies 4 and 5). By 120 hpf, most of the CtA vessels became surrounded by strongly EGFP-positive cells that no longer proliferated and migrated (data not shown). Collectively, these results suggest that the CtA vessels are covered by MCs that originally differentiated around the vessels located in the cerebral base.
Pdgfrβ signaling is involved in recruitment and proliferation of MCs during embryogenesis (Hellstrom et al., 1999; Olson and Soriano, 2011; Wang et al., 2014). Thus, we analyzed pdgfrbsa16389/sa16389 mutant larvae, which lack functional Pdgfrβ to clarify the role of Pdgfrβ in MC coverage of cranial vessels (Fig. 2E; Fig. S2E). The pdgfrb mutant larvae developed cranial vessels normally and exhibited MC coverage of PCS, BCA and BA located in the cerebral base. However, they lost MC coverage of the CtA. Similarly, the number of MCs covering the CtA was reduced by treatment with AG1296, a Pdgfrβ inhibitor (Fig. S2F). These results suggest that Pdgfrβ signaling is dispensable for MC differentiation around the vessels located in the cerebral base, but indispensable for their subsequent recruitment into the CtA.
MC migration along inter-EC junctions
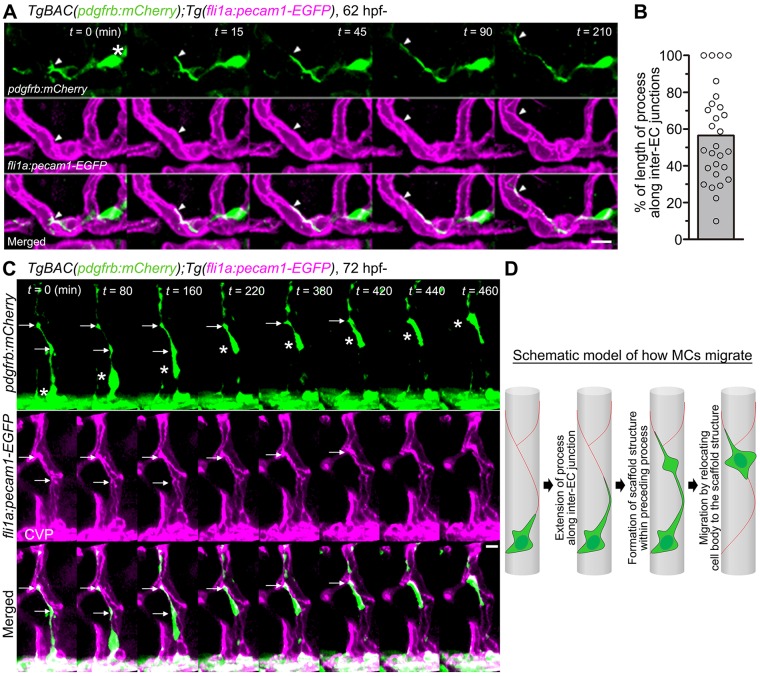
We further investigated how MCs migrate along the EC tube to cover the CtA by utilizing the Tg(fli1a:pecam1-EGFP) line, in which inter-EC junctions are visualized by Pecam1-EGFP. Time-lapse imaging revealed that MCs preferentially extended their processes along inter-EC junctions during their migration along the CtA (Fig. 3A; Movie 6). The tip of the extending process attached to the inter-EC junction in ∼70% of the cases (Fig. S3A). In addition, ∼60% of the total length of the processes was aligned along the inter-ECs junctions (Fig. 3B; Fig. S3B; Movie 7). Importantly, some parts of the processes spread widely on the inter-EC junctions as if the junctions became scaffold structures for MC migration (Fig. 3C). Consistently, of the 30 MCs migrating along the CtA that we analyzed, 90% moved forward by sequentially relocating their cell bodies to the scaffold structures formed within the preceding processes (Fig. 3C; Movie 8). When the MCs migrated across the unicellular tube, they extended their processes around the EC tube even in the absence of contact with the inter-EC junctions (Fig. S3C; Movie 9). However, once the tip of the processes reached the inter-EC junctions, the MCs further extended their processes along the junctions and established the scaffold structures within these processes (Fig. S3C; Movie 9). These results suggest that during MC coverage of CtA, MCs extend their processes along inter-EC junctions and establish the scaffold structures required within the processes to move forward (Fig. 3D).
Fig. 3.
Migration of MCs along inter-EC junctions. (A) Time-lapse confocal images of an MC migrating along the CtA in the hindbrain of a TgBAC(pdgfrb:mCherry);Tg(fli1a:pecam1-EGFP) larva. 3D-rendered confocal images at 62 hpf (leftmost column) and their subsequent time-lapse images with the elapsed time (min) at the top right. Top, pdgfrb:mCherry (green); middle, fli1a:pecam1-EGFP (magenta); bottom, merged images. Arrowheads and asterisk indicate the tip of the MC process and the cell body, respectively. Note that the MC extended a process along the Pecam1-EGFP-labeled inter-EC junctions. (B) Alignment of MC processes along inter-EC junctions, as observed in A, expressed as a percentage of the total length (n=28). Bar and circles indicate the average and the values of individual processes, respectively. (C) Time-lapse confocal images of an MC migrating along the CtA, as in A. 3D-rendered confocal images at 72 hpf (leftmost column) and their subsequent time-lapse images with the elapsed time (min) at the top right. Note that the MC moved forward by sequentially relocating its cell body (asterisks) to the punctate structures formed within the preceding processes (arrows). CVP, choroidal vascular plexus. (D) Schematic of how MCs migrate along the EC tube to cover the CtA. Red lines indicate the inter-EC junctions, green cells represent MCs. Scale bars: 10 μm (A,C).
Live imaging of MC coverage of axial vessels in the trunk
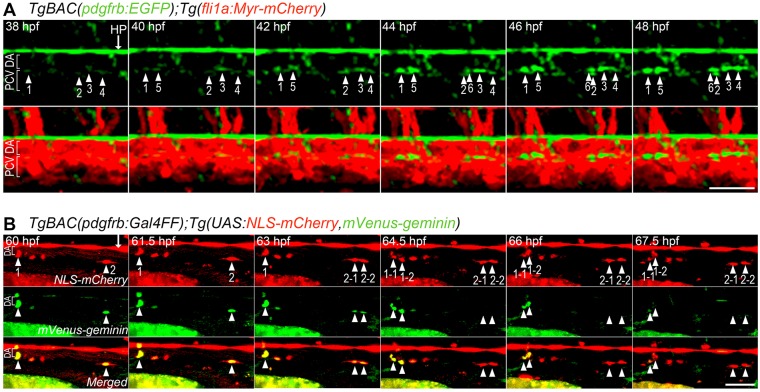
Next, we analyzed MC coverage of trunk axial vessels by time-lapse imaging of the TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) fish. EGFP-positive cells started to emerge in the ventral part of the DA at 36 hpf, whereas the posterior cardinal vein (PCV) was not covered by EGFP-positive cells until at least 7 dpf (Fig. 4A; data not shown). Unfortunately, we could not analyze MC emergence in the dorsal side of the DA because of EGFP expression in the hypochord, which is located just along the dorsal side of the DA (Fig. 4A; Fig. S1C). However, we confirmed that EGFP-positive cells in the ventral part of the DA did not originate from the hypochord. EGFP-positive cells emerged at the ventral side of the DA, gradually emitted strong fluorescence and underwent cell division frequently (Fig. 4A; Movie 10). These EGFP-positive cells dorsally extended multiple processes, irrespective of the presence of inter-EC junctions, to ensheath the DA wall (Fig. S4A). Proliferation of MCs at the ventral part of the DA was confirmed by utilizing the Fucci (fluorescent ubiquitination-based cell cycle indicator) system (Sakaue-Sawano et al., 2008). We generated a Tg zebrafish line in which NLS-mCherry and mVenus-geminin, a Fucci biosensor for visualizing cells in S/G2/M phases, were simultaneously expressed in pdgfrb promoter-activated cells. The mCherry/mVenus double-positive cells at the ventral side of the DA apparently divided into two daughter cells, which subsequently lost mVenus fluorescence, but retained mCherry fluorescence, although these fluorescent proteins tended to be expressed in a mosaic fashion (Fig. 4B). These findings indicate that MCs cover the DA by differentiation at the ventral part of the DA and by their subsequent proliferation.
Fig. 4.
Live imaging of MC coverage of axial vessels in the trunk. (A) Time-lapse confocal images of an axial vessel in the trunk of TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) embryos (38-48 hpf). Upper, pdgfrb:EGFP; lower, merged images of pdgfrb:EGFP (green) and fli1a:Myr-mCherry (red). Arrowheads with numbers indicate individual EGFP-positive cells emerging at the ventral part of the DA. Arrow indicates hypochord (HP). (B) Time-lapse confocal images of a trunk axial vessel in TgBAC(pdgfrb:Gal4FF);Tg(UAS:NLS-mCherry,mVenus-geminin) embryo (60-67.5 hpf). Top, NLS-mCherry (red); middle, mVenus-geminin (the cells in the S/G2/M phase of the cell cycle) (green); bottom, merged images. Arrowheads with numbers indicate individual mCherry/mVenus double-positive cells located in the ventral part of DA. Note that mCherry/mVenus double-positive cells (1 and 2) divided into two daughter cells (1-1/1-2 and 2-1/2-2), which subsequently lost mVenus fluorescence. Arrow indicates hypochord. Lateral view, anterior to the left. Scale bars: 50 μm.
We analyzed MC coverage of the DA by utilizing the TgBAC(tagln:EGFP) zebrafish line to visualize VSMCs. In the TgBAC(pdgfrb:Gal4FF);Tg(UAS:RFP);TgBAC(tagln:EGFP) larvae at 7 dpf, EGFP/RFP double-positive cells were observed in the ventral part of the DA (Fig. S4B,C), indicating that the MCs emerging on the ventral side of the DA were pdgfrb- and tagln-double positive VSMCs. These results were consistent with those from a previous report using a Tg(acta2:EGFP) line that marks smooth muscle cells (Whitesell et al., 2014). However, EGFP-positive cells at the dorsal side of the DA did not exhibit RFP expression (Fig. S4B), suggesting that VSMCs at the dorsal part do not express pdgfrb. Thus, distinct types of VSMCs might be present on the dorsal and ventral sides of the DA.
Live imaging of MC coverage of ISVs
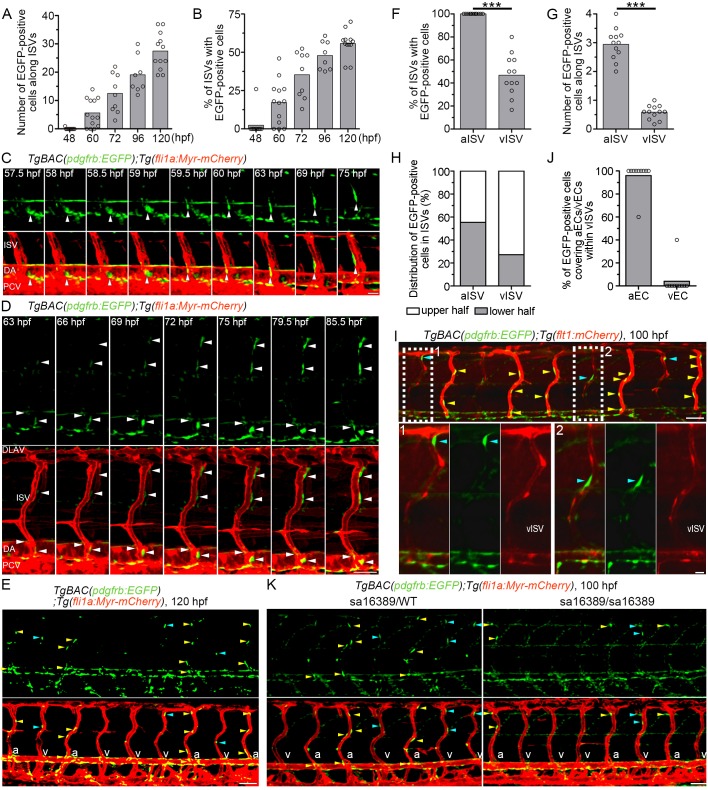
We then explored when and how ISVs become covered by MCs by analyzing TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) zebrafish. The emergence of EGFP-positive cells around the ISVs started from 48 to 60 hpf (Fig. 5A,B). The number of EGFP-positive cells in the ISVs and the number of ISVs covered by EGFP-positive cells gradually increased until at least 120 hpf (Fig. 5A,B). EGFP-positive cells tended to extend their processes along the inter-EC junctions, as observed in the CtA (Fig. S4D,E). However, alignment of MC processes along the inter-EC junctions in ISVs was less frequent than in CtA vessels, suggesting a diversity of MCs in different vascular beds. Time-lapse imaging analyses suggested two distinct modes of MC coverage of ISVs. One mechanism depended on recruitment of MCs that originally emerged in the ventral part of the DA. Some EGFP-positive cells located in the ventral side of the DA migrated dorsally to cover the ISVs (Fig. 5C; Movie 10). The other mechanism involved de novo formation of MCs around the ISVs. Indeed, the cells located in close proximity to the ISVs started to emit green fluorescence and contacted the ECs in the ISVs (Fig. 5D; Movie 10). Furthermore, the EGFP-positive cells around the ISVs frequently proliferated; therefore, it is possible that they contribute to the MC coverage of ISVs (Movie 10). These results indicate that MCs cover the ISVs in two ways, one relying on the recruitment of MCs from the DA and the other involving de novo differentiation of MCs in the ISVs followed by their proliferation.
Fig. 5.
Live imaging of MC coverage of ISVs. (A,B) The number of EGFP-positive cells covering mCherry-labeled ISVs (A) and the percentage of ISVs covered by more than one EGFP-positive cell (B) in the left side of TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) embryos or larvae at the stages indicated below. (n≥8). (C) Time-lapse confocal images of the trunk vasculature in a TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) embryo (57.5-75 hpf). Upper, pdgfrb:EGFP; lower, merged image of pdgfrb:EGFP (green) and fli1a:Myr-mCherry (red). Arrowheads indicate an EGFP-positive cell that initially located in the ventral part of DA and subsequently migrated towards the ISV. (D) Time-lapse confocal images of the trunk vasculature in a TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) embryo (63-85.5 hpf), as in C. Arrowheads indicate EGFP-positive cells covering the ISVs. Note that the cells around the ISVs gradually emitted a stronger EGFP signal. (E) Confocal images of trunk vasculature in a TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) larva at 120 hpf. Upper, pdgfrb:EGFP; lower, merged image of pdgfrb:EGFP (green) and fli1a:Myr-mCherry (red). Yellow and blue arrowheads indicate the EGFP-positive cells covering arterial ISVs (aISVs) and those covering venous ISVs (vISVs), respectively. ‘a’ and ‘v’ in the merged image indicate aISVs and vISVs, respectively. (F) Percentage of aISVs/vISVs covered by more than one EGFP-positive cell, as observed in E (n≥12). (G) The number of EGFP-positive cells covering aISVs/vISVs, as observed in E (n≥12). (H) Percentage of EGFP-positive cells covering the upper and lower half of aISVs/vISVs, as observed in E (n=12). (I) Confocal images of trunk vasculature in a TgBAC(pdgfrb:EGFP);Tg(flt1:mCherry) larva at 100 hpf. Fluorescence signal derived from flt1:mCherry labels arterial ECs (Bussmann et al., 2010). The merged image of pdgfrb:EGFP (green) and flt1:mCherry (red) is shown at the top. The boxed areas labeled 1 and 2 are enlarged at the bottom, showing the merged image of pdgfrb:EGFP and flt1:mCherry (left), pdgfrb:EGFP (center) and flt1:mCherry (right). Yellow and blue arrowheads indicate the EGFP-positive cells covering aISVs and those covering vISVs, respectively. Note that MCs adhered to the flt1:mCherry-positive arterial ECs within vISVs. (J) Percentage of EGFP-positive cells adhering to arterial ECs (aEC) and those adhering to venous ECs (vECs) within vISVs, as observed in I (n=10). (K) Confocal images of trunk vasculature of the pdgfrb heterozygous (sa16389/WT) and homozygous (sa16389/sa16389) larvae in the TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) background at 100 hpf are shown, as in E. In A,B,F,G and J, bars and circles indicate averages and individual values, respectively. ***P<0.001. Lateral view, anterior to the left. DLAV, dorsal longitudinal anastomotic vessel; ISV, intersegmental vessel; DA, dorsal aorta; PCV, posterior cardinal vein; aISV, arterial ISV; vISV, venous ISV. Scale bars: 20 μm (C, enlarged images in I); 50 μm (D,E,I,K).
The number of MCs covering ISVs increased during development (Fig. 5A). Nevertheless, ∼40% of the ISVs remained uncovered by MCs at 120 hpf (Fig. 5B), prompting us to hypothesize that MC coverage of ISVs might be affected by the difference between arteries and veins. To address this hypothesis, we counted separately the number of EGFP-positive cells in arterial ISVs (aISVs) and in venous ISVs (vISVs) in 120 hpf TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) larvae (Fig. 5E-G). Most aISVs, but only 50% vISVs, were covered by EGFP-positive cells (Fig. 5F). The number of EGFP-positive cells around the aISVs was significantly greater than that around the vISVs (Fig. 5G). These results indicate that MCs preferentially cover aISVs compared with vISVs. However, despite this preferential coverage of aISVs with MCs, ∼50% vISVs was covered by MCs (Fig. 5F). In addition, we noticed that MCs covering the vISVs tended to be located in the dorsal part (Fig. 5E,H). As ECs located in the dorsal part of the vISVs are thought to be derived from the DA (Isogai et al., 2003), these ECs might maintain the arterial identity even though they exist in venous vessels. If this is the case, MCs might contact arterial ECs within the vISVs. Therefore, we analyzed TgBAC(pdgfrb:EGFP);Tg(flt1:mCherry) larvae, in which arterial ECs are visualized by mCherry fluorescence (Kwon et al., 2013). The dorsal part of the vISVs was weakly but clearly labeled by mCherry fluorescence, suggesting that ECs located in the dorsal region of vISVs retain arterial identity (Fig. 5I). Importantly, EGFP-positive cells preferentially adhered to flt1 promoter-activated arterial ECs compared with venous ECs within the vISVs (Fig. 5I,J). Taken together with the evidence that MCs covered the DA, but not the PCV (Fig. 4A), these findings indicate that arterial ECs are preferentially covered by MCs during vascular development.
The preferential coverage of arterial ECs with MCs prompted us to hypothesize that arterial ECs might promote MC development. To test this hypothesis, we analyzed kdrl and kdr morphants that exhibited defective formation of the DA and aISVs, and found that MC development in the trunk was severely impaired in the absence of the DA and aISVs (Fig. S5A). These results suggest that arterial, but not venous, ECs play an instructive role in MC development. As functional characteristics of arteries and veins partly depend on differential levels of shear stress and pressure exerted by blood flow, blood flow might be involved in MC development. However, MCs covering the DA and ISVs were observed even in tnnt2a morphants that lack blood flow (Fig. S5B). Thus, arterial ECs might promote MC development in the trunk in a blood flow-independent manner.
We investigated further the role of Pdgfrβ signaling in MC development in the trunk vasculature. Neither pdgfrbsa16389/sa16389 mutant nor AG1296-treated larvae showed apparent defects in trunk vessels (Fig. 5K; Fig. S5C,D). MCs covering the DA and ISVs were detected even in the pdgfrbsa16389/sa16389 mutant and AG1296-treated larvae, although the number of MCs was lower compared with pdgfrbsa16389/WT and control larvae, respectively (Fig. 5K; Fig. S5C,D). These findings indicate that Pdgfrβ-mediated signaling is not essential for the development of MCs covering the trunk vessels but might regulate their subsequent proliferation and recruitment.
Lineage tracing for identification of MC origins in zebrafish
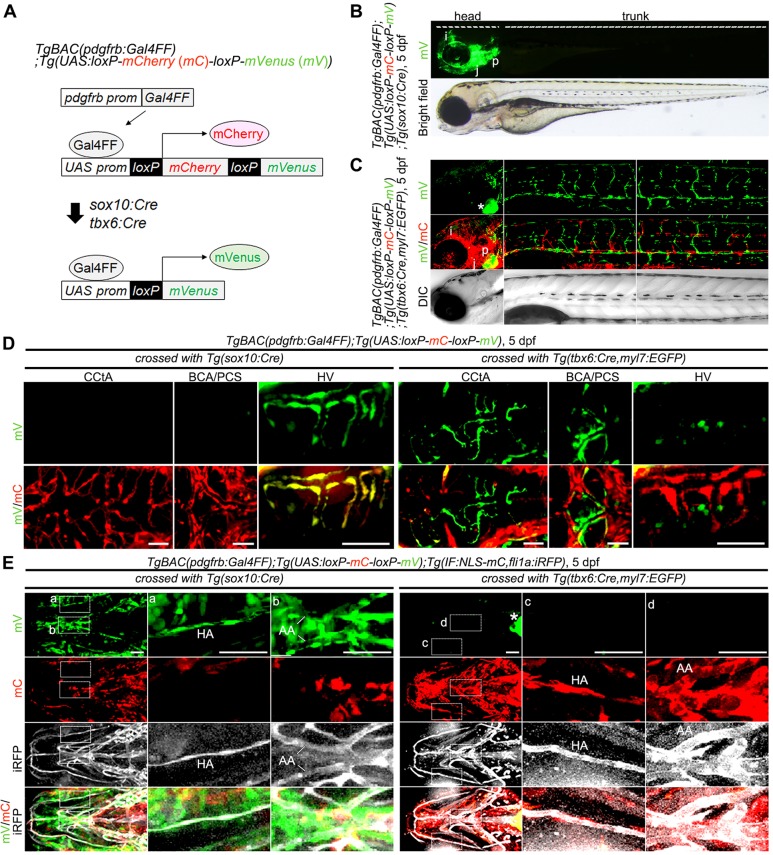
MCs originate from neural crest cells and mesoderm (Majesky, 2007; Wasteson et al., 2008; Winkler et al., 2011). To analyze the origins of MCs in the zebrafish vasculature, we established TgBAC(pdgfrb:Gal4FF);(UAS:loxP-mCherry-loxP-mVenus) (pdgfrb reporter) fish and crossed with Tg(sox10:Cre) (Rodrigues et al., 2012) or Tg(tbx6:Cre,myl7:EGFP) (Lee et al., 2013) lines for labeling the neural crest- or mesoderm-derived MCs with mVenus expression, respectively (Fig. 6A). Indeed, pdgfrb reporter larvae crossed with Tg(sox10:Cre) line exhibited mVenus fluorescence in the pdgfrb promoter-active neural crest-derived tissues, including iris, jaw and pharyngeal arch (Mongera et al., 2013; Rodrigues et al., 2012), suggesting that this lineage-tracing system works (Fig. 6B).
Fig. 6.
Lineage tracing for identification of MC origins. (A) Schematic of the protocol used for lineage-tracing analysis. TgBAC(pdgfrb:Gal4FF);Tg(UAS:loxP-mCherry (mC)-loxP-mVenus (mV)) zebrafish (pdgfrb reporter) express mCherry in the pdgfrb-positive cells (top). In the pdgfrb reporter fish crossed with Tg(sox10:Cre) or Tg(tbx6:Cre,myl7:EGFP) line, the sox10-positive neural crest-derived MCs or tbx6-positive mesoderm-derived MCs, respectively, are labeled with mVenus expression because of Cre-mediated excision of mCherry gene flanked by loxP (bottom). (B) Lateral view of a TgBAC(pdgfrb:Gal4FF);Tg(UAS:loxP-mC-loxP-mV);Tg(sox10:Cre) larva at 5 dpf. Upper, mVenus; lower, brightfield image. (C) Confocal images of head (left column) and trunk (center and right columns) regions in a TgBAC(pdgfrb:Gal4FF);Tg(UAS:loxP-mC-loxP-mV);Tg(tbx6:Cre,myl7:EGFP) larva at 5 dpf. Upper, mVenus; middle, merged images of mVenus (green) and mCherry (red); lower, differential interference contrast (DIC) images. (D) Confocal images of head regions in a 5 dpf TgBAC(pdgfrb:Gal4FF);Tg(UAS:loxP-mC-loxP-mV) larva crossed with Tg(sox10:Cre) (left three columns) or Tg(tbx6:Cre,myl7:EGFP) (right three columns) fish lines. Dorsal view, anterior to the left. Images of CCtA, BCA/PCS and HV are shown in the left, center and right columns, respectively. Upper, mVenus; lower, the merged images of mVenus (green) and mCherry (red). Note that mVenus-labeled cells indicate sox10-positive neural crest-derived MCs (left three columns) or tbx6-positive mesoderm-derived MCs (right three columns). Scale bars: 50 μm (CCtA, HV); 20 μm (BCA/PCS). (E) Confocal images of pharyngeal regions in 5 dpf TgBAC(pdgfrb:Gal4FF);Tg(UAS:loxP-mC-loxP-mV);Tg(IF:NLS-mCherry,fli1a:iRFP) larvae crossed with Tg(sox10:Cre) (left three columns) or Tg(tbx6:Cre,myl7:EGFP) (right three columns) fish lines. The larvae expressing iRFP670 under the control of fli1a promoter were identified by intestinal fatty acid binding protein (IF; also known as fabp2) promoter-driven expression of NLS-mCherry in the intestine. Ventral view, anterior to the left. Boxed areas showing hypobranchial artery (HA) (a,c) and aortic arches (AA) (b,d) are enlarged to the right of the original images. Top row, mVenus; second row, mCherry; third row, fli1a:iRFP (iRFP); bottom row, merged images of mVenus (green), mCherry (red) and iRFP (white). Scale bars: 50 μm. Asterisks in C and E indicate heart visualized by myl7:EGFP. mC, mCherry; mV, mVenus; i, iris; j, jaw; p, pharyngeal arch.
First, we determined the origin of MCs in the trunk vasculature. Most MCs in the trunk vasculature were labeled with mVenus expression in the pdgfrb reporter crossed with the Tg(tbx6:Cre,myl7:EGFP) line, but not in that crossed with the Tg(sox10:Cre) line (Fig. 6B,C). To identify the MC origin in the trunk vasculature, we analyzed foxd3/tfap2a double morphants, which exhibit defects in neural crest formation, and tbx6/hand2 double morphants, in which the tissues derived from paraxial and lateral plate mesoderm fail to develop (Lee et al., 2009; Nikaido et al., 2002; Santoro et al., 2009; Wang et al., 2011). MC emergence in the DA and ISVs was severely impaired in the tbx6/hand2 double morphant larvae but occurred normally in the foxd3/tfap2a-double morphant larvae (Fig. S6A,B). These results suggest that MCs in the trunk vessels are derived from the paraxial and lateral plate mesoderm.
Recently, Wang et al. have reported that zebrafish brain vessels contain neural crest-derived pericytes (Wang et al., 2014). However, it remains unknown whether all or specific types of brain vessels are covered by the neural crest-derived pericytes. Therefore, we addressed this question by analyzing our pdgfrb reporter line. When crossed with the Tg(sox10:Cre) line, pdgfrb reporter fish exhibited mVenus fluorescence in the anterior part of MMCtAs and in the CVP at the larval stage and in the forebrain at the juvenile stage (Fig. S7A,B; data not shown). Together with evidence that the MCs originally emerging around the CVP migrated to MMCtAs (Movie 4), these results suggest that neural crest-derived MCs develop around the CVP and migrate to cover the MMCtAs. Consistently, depletion of foxd3/tfap2a led to the reduction of pdgfrb-positive MCs in the CVP (Fig. S7C).
By contrast, the mVenus-positive MCs were hardly observed in the hindbrain vessels of the pdgfrb reporter line crossed with the Tg(sox10:Cre) line (Fig. 6D; Fig. S7A,B). In addition, MCs emerged around the BCA, PCS and BA even in the foxd3/tfap2a double morphant larvae, although the MC coverage of CtA was significantly impaired in these morphants (Fig. S7D-F). These results suggest that neural crest cells do not contribute to MC development in the base of the hindbrain but might be required for their subsequent recruitment into the CtA.
Thus, we investigated the contribution of mesodermal cells to the MC coverage of hindbrain vasculature and found that some of the pdgfrb reporter larvae crossed with the Tg(tbx6:Cre,myl7:EGFP) line showed mVenus-positive MCs in the hindbrain vessels (Fig. 6D; Fig. S7G,H). Because activity of the tbx6 promoter used to express Cre recombinase is relatively low in the dorsal mesoderm, we also utilized the no tail a (ntla; ta – Zebrafish Information Network) promoter, which is active in the dorsal mesoderm (Goering et al., 2003; Lee et al., 2013). In pdgfrb reporter larvae injected with a plasmid (ntla:Cre-2A-mCherry), mVenus-positive MCs were detected not only in the trunk vessels but also in the hindbrain vessels (Fig. S7I), suggesting a mesodermal origin of MCs in the hindbrain vessels. We also analyzed tbx6/hand2 double morphant larvae to confirm the mesodermal origin of MCs, but they exhibited severe defects in hindbrain vessels (Fig. S7J). However, tbx6 morphants lacking paraxial mesoderm-derived tissues showed a decreased number of MCs covering the hindbrain vessels without apparent vascular defects (Fig. S7K-M). These results indicate that MCs in hindbrain vessels are mainly derived from the mesoderm. In addition, lineage-tracing studies and analyses of foxd3/tfap2a and tbx6/hand2 double morphants revealed the neural crest origin of MCs in the hyaloid vessels (Fig. 6D; Fig. S8A,B).
Finally, we analyzed the origin of MCs covering vessels in the pharyngeal region. mVenus-positive MCs were detected in the hypobranchial artery (HA) and aortic arches (AAs) of pdgfrb reporter larvae crossed with the Tg(sox10:Cre) line, but not of those crossed with the Tg(tbx6:Cre) line (Fig. 6E). Consistently, foxd3/tfap2a double morphant and foxd3 morphant larvae showed a decreased number of MCs covering the HA and AAs (Fig. S8C,D). These results indicate that the HA and AAs are covered by neural crest-derived MCs.
DISCUSSION
In this study, we succeeded for the first time in analyzing MC dynamics in living animals at single-cell resolution by generating zebrafish Tg lines that express fluorescent proteins and the Gal4FF driver under control of the pdgfrb promoter. By exploiting our Tg lines, we showed that MCs terminally differentiate around vessels, then actively proliferate and migrate along EC tubes, thereby covering entire vessels. In addition, our lineage-tracing analyses revealed that MCs in the trunk vasculature are derived from the mesoderm, whereas those in the head region have either neural crest or mesodermal origin.
Signaling from the arterial ECs might regulate terminal differentiation of MCs. MCs mainly covered arterial blood vessels, at least, until the larval stage. Similarly, preferential MC coverage of arterial vessels has been reported in mouse embryos (Hellstrom et al., 1999). Why is it mainly the arterial blood vessels that are surrounded by MCs? Our data showed that, although weakly EGFP-positive cells settled around both arterial and venous vessels in Tg(pdgfrb:EGFP) embryos, only the cells in close vicinity of arterial vessels differentiated into strongly EGFP-positive MCs. In addition, MC development was impaired by the defective formation of blood vessels. These findings suggest that signaling from arterial ECs, but not from venous ECs, promotes MC differentiation. Consistent with this, Jagged-1, which is preferentially expressed in the arterial vessels, promotes MC differentiation through activation of Notch 3 receptors (Armulik et al., 2011; Liu et al., 2009; Villa et al., 2001).
Arterial ECs might also promote expansion of differentiated MCs surrounding the arteries. EC-derived PDGF-B is known to induce proliferation and migration of PDGFRβ-expressing MCs during embryogenesis in mice (Hellstrom et al., 1999). Consistent with this, we observed that suppression of Pdgfrβ signaling inhibited MC coverage of CtA vessels, which depends on proliferation and migration of MCs originally emerging around the vessels in the cerebral base. Importantly, as endothelial expression of PDGF-B is restricted to the arteries and capillaries in the early stages of mouse development (Hellstrom et al., 1999), arterial EC-derived PDGF-B might regulate MC coverage of arterial vessels by inducing proliferation and migration of differentiated MCs.
Coverage of the DA with VSMCs occurs through a complex mechanism. Consistent with our lineage-tracing analyses, previous studies in zebrafish, quail and mice have also suggested the mesodermal origin of VSMCs in the DA (Santoro et al., 2009; Wasteson et al., 2008; Wiegreffe et al., 2007). However, VSMCs in the DA might have a heterogeneous cellular origin. At 7 dpf, tagln-positive cells in the ventral side of the DA were pdgfrb-positive, whereas those in the dorsal side did not express pdgfrb, suggesting that VSMCs in the dorsal and ventral sides of the DA have distinct origins. Consistent with this, it has been reported that lateral plate mesoderm-derived VSMCs cover the ventral side of the descending DA in the early stage of aortic development in mice, whereas the VSMCs covering the dorsal side are derived from the somites (Wasteson et al., 2008).
In zebrafish, MCs covering the cranial vessels originated from both mesoderm and neural crest cells. To date, contribution of neural crest cells to brain MCs has been demonstrated in vertebrates including mice, bird and zebrafish (Etchevers et al., 2001; Heglind et al., 2005; Korn et al., 2002; Trost et al., 2013; Wang et al., 2014; Yamanishi et al., 2012). In agreement with this, our analyses indicated a neural crest origin of MCs in the anterior part of the brain and in the hyaloid vessels. However, our data also revealed a mesodermal origin of MCs in hindbrain vessels. Two groups have previously analyzed the origins of MCs in avian brain and have reported discrepant results. Consistent with our observation, Etchevers et al. have reported that MCs in the forebrain are derived from neural crest cells, whereas those of mesodermal origin cover the vessels in the posterior part of brain (Etchevers et al., 2001). By contrast, Korn et al. have reported that neuroectoderm can give rise to MCs in all the vessels in the brain (Korn et al., 2002). Thus, further careful examinations are required to identify the presence of mesoderm-derived MCs in the brain.
MCs migrate along inter-EC junctions during their coverage of blood vessels. During MC coverage of the CtA, MCs extend their processes along inter-EC junctions and subsequently relocate their cell bodies to the scaffold structures formed within the proceeding processes in order to move forward. It is known that substrate stiffness largely influences cell migration. Indeed, recent studies have shown that many different types of cells, including VSMCs, move towards stiffer substrates (Isenberg et al., 2009). Therefore, inter-EC junctions might provide the rigid scaffold required for efficient MC migration, because inter-EC junctions are supported by the actin cytoskeleton (Ando et al., 2013; Phng et al., 2015). Alternatively, N-cadherin (Cadherin 2) might regulate MC migration along inter-EC junctions. In ECs, vascular endothelial-cadherin (Cadherin 5) mediates EC-EC junctions, whereas N-cadherin is thought to regulate cell adhesion between MCs and ECs. However, a previous study has also reported that N-cadherin partially localizes to inter-EC junctions in addition to its plasma membrane localization (Luo and Radice, 2005). Therefore, N-cadherin expressed by MCs might interact with N-cadherin localized at the inter-EC junctions to mediate MC migration along the inter-EC junctions. However, further studies are needed to clarify the mechanism by which MCs migrate along inter-EC junctions and its biological significance.
Here, we have successfully uncovered the mechanism underlying MC coverage of blood vessels by establishing novel zebrafish lines for in vivo visualization of MCs. Thus, Tg lines that we have developed in this study will substantially contribute to our understanding of MC biology.
MATERIALS AND METHODS
Zebrafish husbandry
Zebrafish (Danio rerio) were maintained as previously described (Fukuhara et al., 2014). Embryos and larvae were staged by hpf at 28°C (Kimmel et al., 1995).
Plasmid construction and BAC recombineering
Construction of the Tol2 vectors used to generate transgenic zebrafish lines is described in supplementary Materials and Methods. BAC clones encoding EGFP, mCherry or Gal4FF under pdgfrb promoter were prepared as described in supplementary Materials and Methods according to the method described in a previous report (Bussmann and Schulte-Merker, 2011).
Transgenic and mutant zebrafish lines
The Tol2 transposon system was used to generate transgenic zebrafish lines as described in supplementary Materials and Methods. pdgfrbsa16389 mutant zebrafish were obtained from the European Zebrafish Resource Center.
Image acquisition and processing
Embryos and larvae were anesthetized and mounted in 1% low-melting agarose on a 35-mm diameter glass-based dish (Asahi Techno Glass, Shizuoka, Japan), as previously described (Fukuhara et al., 2014).
Confocal images were obtained using a FluoView FV1000 or FV1200 confocal upright microscope (Olympus, Tokyo, Japan) equipped with a water-immersion 20× (XLUMPlanFL, 1.0 NA) lens. The 473 nm, 559 nm and 633 nm laser lines were employed. Obtained confocal images were processed using Olympus Fluoview (FV10-ASW) or FluoRender (http://www.fluorender.org). Images processed with FluoRender were shown in FluoRender composite mode in which deeper objections became visible.
Fluorescence and brightfield images were taken with a fluorescence stereozoom microscope (SZX12, Olympus).
FACS and RT-PCR
EGFP-negative/mCherry negative, EGFP-positive/mCherry negative and EGFP-negative/mCherry-positive cells were isolated from TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) larvae by FACS and subjected to RT-PCR analyses as described in supplementary Materials and Methods.
Immunohistochemistry
TgBAC(pdgfrb:EGFP);Tg(fli1a:Myr-mCherry) and TgBAC(tagln:EGFP);Tg(fli1a:Myr-mCherry) juvenile zebrafish were subjected to immunohistochemistry with anti-α-SMA antibody at 1:300 as described in supplementary Materials and Methods.
Quantification
To quantify MC processes following the inter-EC junctions, we measured the total length of each process extended by MCs (X) and the length of the corresponding process that aligned along the Pecam1-EGFP-labeled inter-EC junctions (Y). Alignment of MC processes along the inter-EC junctions was expressed as a percentage of total length (X/Y×100).
Injections of morpholino oligonucleotides
For morpholino oligonucleotide (MO)-mediated knockdown, embryos were injected at one-cell stage with the MOs indicated in supplementary Materials and Methods.
Statistical analysis
Data are expressed as means±s.e.m. Statistical significance was determined by a Student's t-test for paired samples or one-way analysis of variance with Turkey's test for multiple comparisons. Data were considered statistically significant if P-values <0.05.
Acknowledgements
We thank S. Schulte-Merker for providing information and plasmids for BAC recombineering; K. Kawakami for the Tol2 system and the Tg(UAS:GFP) and Tg(UAS:RFP) lines; T. J. Carney for the Tg(tbx6:Cre,myl7:EGFP)sq6 line; G. Felsenfeld for the pJC13-1; and A. Sakakibara for the iRFP670. We are grateful to M. Sone, T. Babazono, W. Koeda, K. Hiratomi and E. Okamoto for technical assistance. This work was made possible in part by software funded by the NIH: FluoRender: An Imaging Tool for Visualization and Analysis of Confocal Data as Applied to Zebrafish Research [R01-GM098151-01].
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
K.A., S.F. and N.M. conceived, designed the research and wrote the manuscript. K.A. performed the experiments and analyzed the results. N.I. conceived the research. H.N. and H.F. supported the experiments. R.N.K. developed Tg(sox10:Cre) line.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research on Innovative Areas ‘Fluorescence Live Imaging’ [No. 22113009 to S.F.] and ‘Neuro-Vascular Wiring’ [No. 22122003 to N.M.] from The Ministry of Education, Culture, Sports, Science, and Technology, Japan; by Grants-in-Aid for Young Scientists (Start-up) [No. 26893336 to K.A.], for Scientific Research (B) [No. 25293050 to S.F. and No. 24370084 to N.M.], and for Exploratory Research [No. 26670107 to S.F.] from the Japan Society for the Promotion of Science; and by grants from the Ministry of Health, Labour, and Welfare of Japan [KHC1103 to N.M.]; the Core Research for Evolutional Science and Technology (CREST) program of the Japan Agency for Medical Research and Development (AMED) [15652238 to N.M.]; Precursory Research for Innovative Medical Care (PRIME), AMED [15665521 to S.F.]; the Takeda Science Foundation (S.F., N.M.); the Naito Foundation (S.F.); Mochida Memorial Foundation for Medical and Pharmaceutical Research (S.F.); the Daiichi Sankyo Foundation of Life Science (S.F.); and the Medical Research Council [MR/J001457/1 to R.N.K.]. Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.132654/-/DC1
References
- Ando K., Fukuhara S., Moriya T., Obara Y., Nakahata N. and Mochizuki N. (2013). Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J. Cell Biol. 202, 901-916. 10.1083/jcb.201301115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A., Genove G. and Betsholtz C. (2011). Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193-215. 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Asakawa K., Suster M. L., Mizusawa K., Nagayoshi S., Kotani T., Urasaki A., Kishimoto Y., Hibi M. and Kawakami K. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 105, 1255-1260. 10.1073/pnas.0704963105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J. and Schulte-Merker S. (2011). Rapid BAC selection for tol2-mediated transgenesis in zebrafish. Development 138, 4327-4332. 10.1242/dev.068080 [DOI] [PubMed] [Google Scholar]
- Bussmann J., Bos F. L., Urasaki A., Kawakami K., Duckers H. J. and Schulte-Merker S. (2010). Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137, 2653-2657. 10.1242/dev.048207 [DOI] [PubMed] [Google Scholar]
- Etchevers H. C., Vincent C., Le Douarin N. M. and Couly G. F. (2001). The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128, 1059-1068. [DOI] [PubMed] [Google Scholar]
- Foo S. S., Turner C. J., Adams S., Compagni A., Aubyn D., Kogata N., Lindblom P., Shani M., Zicha D. and Adams R. H. (2006). Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161-173. 10.1016/j.cell.2005.10.034 [DOI] [PubMed] [Google Scholar]
- French C. R., Seshadri S., Destefano A. L., Fornage M., Arnold C. R., Gage P. J., Skarie J. M., Dobyns W. B., Millen K. J., Liu T. et al. (2014). Mutation of FOXC1 and PITX2 induces cerebral small-vessel disease. J. Clin. Invest. 124, 4877-4881. 10.1172/JCI75109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Cha Y. R., Pham V. N., Sakurai A., Roman B. L., Gutkind J. S. and Weinstein B. M. (2011). Assembly and patterning of the vascular network of the vertebrate hindbrain. Development 138, 1705-1715. 10.1242/dev.058776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Zhang J., Yuge S., Ando K., Wakayama Y., Sakaue-Sawano A., Miyawaki A. and Mochizuki N. (2014). Visualizing the cell-cycle progression of endothelial cells in zebrafish. Dev. Biol. 393, 10-23. 10.1016/j.ydbio.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Gaengel K., Genove G., Armulik A. and Betsholtz C. (2009). Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 630-638. 10.1161/ATVBAHA.107.161521 [DOI] [PubMed] [Google Scholar]
- Garg N., Khunger M., Gupta A. and Kumar N. (2014). Optimal management of hereditary hemorrhagic telangiectasia. J. Blood Med. 5, 191-206. 10.2147/JBM.S45295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering L. M., Hoshijima K., Hug B., Bisgrove B., Kispert A. and Grunwald D. J. (2003). An interacting network of T-box genes directs gene expression and fate in the zebrafish mesoderm. Proc. Natl. Acad. Sci. USA 100, 9410-9415. 10.1073/pnas.1633548100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heglind M., Cederberg A., Aquino J., Lucas G., Ernfors P. and Enerback S. (2005). Lack of the central nervous system- and neural crest-expressed forkhead gene Foxs1 affects motor function and body weight. Mol. Cell. Biol. 25, 5616-5625. 10.1128/MCB.25.13.5616-5625.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M., Kalen M., Lindahl P., Abramsson A. and Betsholtz C. (1999). Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047-3055. [DOI] [PubMed] [Google Scholar]
- Isenberg B. C., Dimilla P. A., Walker M., Kim S. and Wong J. Y. (2009). Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys. J. 97, 1313-1322. 10.1016/j.bpj.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai S., Lawson N. D., Torrealday S., Horiguchi M. and Weinstein B. M. (2003). Angiogenic network formation in the developing vertebrate trunk. Development 130, 5281-5290. 10.1242/dev.00733 [DOI] [PubMed] [Google Scholar]
- Joutel A., Corpechot C., Ducros A., Vahedi K., Chabriat H., Mouton P., Alamowitch S., Domenga V., Cecillion M., Marechal E. et al. (1996). Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383, 707-710. 10.1038/383707a0 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. and Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Korn J., Christ B. and Kurz H. (2002). Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J. Comp. Neurol. 442, 78-88. 10.1002/cne.1423 [DOI] [PubMed] [Google Scholar]
- Kwon H.-B., Fukuhara S., Asakawa K., Ando K., Kashiwada T., Kawakami K., Hibi M., Kwon Y.-G., Kim K.-W., Alitalo K. et al. (2013). The parallel growth of motoneuron axons with the dorsal aorta depends on Vegfc/Vegfr3 signaling in zebrafish. Development 140, 4081-4090. 10.1242/dev.091702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-C., Tseng W.-A., Lo F.-Y., Liu T.-M. and Tsai H.-J. (2009). FoxD5 mediates anterior-posterior polarity through upstream modulator Fgf signaling during zebrafish somitogenesis. Dev. Biol. 336, 232-245. 10.1016/j.ydbio.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Lee R. T. H., Knapik E. W., Thiery J. P. and Carney T. J. (2013). An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development 140, 2923-2932. 10.1242/dev.093534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Kennard S. and Lilly B. (2009). NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ. Res. 104, 466-475. 10.1161/CIRCRESAHA.108.184846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y. and Radice G. L. (2005). N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J. Cell Biol. 169, 29-34. 10.1083/jcb.200411127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W. (2007). Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 27, 1248-1258. 10.1161/ATVBAHA.107.141069 [DOI] [PubMed] [Google Scholar]
- Mongera A., Singh A. P., Levesque M. P., Chen Y.-Y., Konstantinidis P. and Nusslein-Volhard C. (2013). Genetic lineage labeling in zebrafish uncovers novel neural crest contributions to the head, including gill pillar cells. Development 140, 916-925. 10.1242/dev.091066 [DOI] [PubMed] [Google Scholar]
- Nikaido M., Kawakami A., Sawada A., Furutani-Seiki M., Takeda H. and Araki K. (2002). Tbx24, encoding a T-box protein, is mutated in the zebrafish somite-segmentation mutant fused somites. Nat. Genet. 31, 195-199. 10.1038/ng899 [DOI] [PubMed] [Google Scholar]
- Olson L. E. and Soriano P. (2011). PDGFRbeta signaling regulates mural cell plasticity and inhibits fat development. Dev. Cell 20, 815-826. 10.1016/j.devcel.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng L.-K., Gebala V., Bentley K., Philippides A., Wacker A., Mathivet T., Sauteur L., Stanchi F., Belting H.-G., Affolter M., et al. (2015). Formin-mediated actin polymerization at endothelial junctions is required for vessel lumen formation and stabilization. Dev. Cell 32, 123-132. 10.1016/j.devcel.2014.11.017 [DOI] [PubMed] [Google Scholar]
- Robin Y.-M., Penel N., Perot G., Neuville A., Velasco V., Ranchere-Vince D., Terrier P. and Coindre J.-M. (2013). Transgelin is a novel marker of smooth muscle differentiation that improves diagnostic accuracy of leiomyosarcomas: a comparative immunohistochemical reappraisal of myogenic markers in 900 soft tissue tumors. Mod. Pathol. 26, 502-510. 10.1038/modpathol.2012.192 [DOI] [PubMed] [Google Scholar]
- Rodrigues F. S. L. M., Doughton G., Yang B. and Kelsh R. N. (2012). A novel transgenic line using the Cre-lox system to allow permanent lineage-labeling of the zebrafish neural crest. Genesis 50, 750-757. 10.1002/dvg.22033 [DOI] [PubMed] [Google Scholar]
- Sakaue-Sawano A., Kurokawa H., Morimura T., Hanyu A., Hama H., Osawa H., Kashiwagi S., Fukami K., Miyata T., Miyoshi H. et al. (2008). Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487-498. 10.1016/j.cell.2007.12.033 [DOI] [PubMed] [Google Scholar]
- Santoro M. M., Pesce G. and Stainier D. Y. (2009). Characterization of vascular mural cells during zebrafish development. Mech. Dev. 126, 638-649. 10.1016/j.mod.2009.06.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C., Abrams J. and Pack M. (2010). Characterization of zebrafish intestinal smooth muscle development using a novel sm22alpha-b promoter. Dev. Dyn. 239, 2806-2812. 10.1002/dvdy.22420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost A., Schroedl F., Lange S., Rivera F. J., Tempfer H., Korntner S., Stolt C. C., Wegner M., Bogner B., Kaser-Eichberger A. et al. (2013). Neural crest origin of retinal and choroidal pericytes. Invest. Ophthalmol. Vis. Sci. 54, 7910-7921. 10.1167/iovs.13-12946 [DOI] [PubMed] [Google Scholar]
- Villa N., Walker L., Lindsell C. E., Gasson J., Iruela-Arispe M. L. and Weinmaster G. (2001). Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech. Dev. 108, 161-164. 10.1016/S0925-4773(01)00469-5 [DOI] [PubMed] [Google Scholar]
- Wang W.-D., Melville D. B., Montero-Balaguer M., Hatzopoulos A. K. and Knapik E. W. (2011). Tfap2a and Foxd3 regulate early steps in the development of the neural crest progenitor population. Dev. Biol. 360, 173-185. 10.1016/j.ydbio.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Pan L., Moens C. B. and Appel B. (2014). Notch3 establishes brain vascular integrity by regulating pericyte number. Development 141, 307-317. 10.1242/dev.096107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson P., Johansson B. R., Jukkola T., Breuer S., Akyurek L. M., Partanen J. and Lindahl P. (2008). Developmental origin of smooth muscle cells in the descending aorta in mice. Development 135, 1823-1832. 10.1242/dev.020958 [DOI] [PubMed] [Google Scholar]
- Whitesell T. R., Kennedy R. M., Carter A. D., Rollins E.-L., Georgijevic S., Santoro M. M. and Childs S. J. (2014). An alpha-smooth muscle actin (acta2/alphasma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLoS ONE 9, e90590 10.1371/journal.pone.0090590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegreffe C., Christ B., Huang R. and Scaal M. (2007). Sclerotomal origin of smooth muscle cells in the wall of the avian dorsal aorta. Dev. Dyn. 236, 2578-2585. 10.1002/dvdy.21279 [DOI] [PubMed] [Google Scholar]
- Wiens K. M., Lee H. L., Shimada H., Metcalf A. E., Chao M. Y. and Lien C.-L. (2010). Platelet-derived growth factor receptor beta is critical for zebrafish intersegmental vessel formation. PLoS ONE 5, e11324 10.1371/journal.pone.0011324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E. A., Bell R. D. and Zlokovic B. V. (2011). Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398-1405. 10.1038/nn.2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi E., Takahashi M., Saga Y. and Osumi N. (2012). Penetration and differentiation of cephalic neural crest-derived cells in the developing mouse telencephalon. Dev. Growth Differ. 54, 785-800. 10.1111/dgd.12007 [DOI] [PubMed] [Google Scholar]