Abstract
Accumulating evidence underscored the utility of ketamine in treating severely treatment-resistant depressed patients. Here, we investigated the relationship between the rapid antidepressant effects of ketamine and hippocampal volume, a biomarker of antidepressants treatment outcome. Sixteen medication-free major depressive disorder (MDD) patients received a single subanesthetic dose infusion of ketamine (0.5 mg/kg over 40 minutes). Depression severity was assessed pretreatment and at 24h post-treatment with the Montgomery-Asberg Depression rating scale (MADRS). Prior to treatment, patients underwent magnetic resonance imaging (MRI) to estimate hippocampal volume, and viable MRI data was obtained in 13 patients. Delta MADRS (post minus pretreatment) was significantly correlated with the pretreatment volumes of the left hippocampus (r = 0.66, p = 0.01), but not the right hippocampus (r = 0.49, p = 0.09). The correlation between delta MADRS and the left hippocampus remained high (r > 0.6, p = 0.13) after controlling for several demographic and clinical variables, although p value increased due to the reduced degree of freedom (df = 5). Ketamine exerts enhanced antidepressant effects in patients with relatively smaller hippocampus, a patient population that has been repeatedly shown to be refractory to traditional antidepressants.
Keywords: ketamine, antidepressant, hippocampus, major depressive disorder, MDD
Introduction
Treatment resistance is a critical issue in the management of major depressive disorder (MDD); for example, the STAR*D study reported that fewer than 50% of MDD patients respond to 3-month treatment with a monoaminergic reuptake inhibitor (Trivedi et al., 2006). Identification of biological markers of treatment outcome will provide insight into the underlying pathology of treatment resistance and can potentially guide the development of novel therapeutics. In this brief report, we investigated the relationship between the rapid antidepressant effects of ketamine and hippocampal volume, a biomarker that was previously associated with treatment outcome in MDD patients treated with traditional antidepressants.
Convergent evidence has demonstrated aberrant glutamatergic function in mood and anxiety disorders. In animal models of depression, studies have shown reduced glutamate metabolism, abnormal glutamate release, reduced post-synaptic glutamate receptors, and glutamate uptake deficits (Sanacora et al., 2011). These glutamatergic abnormalities are believed to precipitate excitotoxicity and structural changes leading to hippocampal volume reduction (Drevets et al., 2008). In human studies, smaller hippocampal volume was found in treatment-resistant depressed patients and has been consistently associated with poor response to traditional antidepressants (Vakili et al., 2000; Hsieh et al., 2002; Frodl et al., 2004; Frodl et al., 2008; Kronmuller et al., 2008; MacQueen et al., 2008; MacQueen and Frodl, 2011). However, the relationship between hippocampal volume and the novel glutamate-based antidepressant ketamine has not been previously reported.
This pilot study was conducted to determine whether smaller hippocampal volume was associated with the rapid antidepressant effects of the glutamate N-methyl- D-aspartate (NMDA) receptor antagonist, ketamine (Zarate et al., 2006; Murrough et al., 2013) in patients with treatment-resistant depression (TRD). Ketamine is an anesthetic that was found to exert rapid (within 4 hours) and potent (response rate 45–90%) antidepressant effects (Aan Het Rot et al., 2012). We addressed this question by conducting a trial of ketamine in patients with TRD and measuring pre-treatment hippocampal volumes. We hypothesized that relatively small hippocampal volumes in TRD patients would be associated with an enhanced antidepressant response to ketamine. Consistent with this hypothesis, we recently reported that riluzole – a glutamate modulating agent with antidepressant and anxiolytic properties – exerted enhanced therapeutic effects in patients with smaller hippocampal volume (Abdallah et al., 2012).
Methods and Materials
A subgroup of MDD patients enrolled in a randomized, double-blind controlled (ketamine vs. midazolam) clinical trial (ClinicalTrials.gov Identifier: NCT00768430) consented to participate in this neuroimaging study. Detailed procedures from the clinical trial are reported elsewhere (Murrough et al., 2013). All participants provided written informed consent, and an Institution Review Board at Baylor College of Medicine approved all procedures. All participants at Baylor site who consented for the neuroimaging component, did not have MR exclusion, and were able to be scheduled within the constraints of the timing of the parent trial were enrolled in the MRI study, blinded to their treatment assignment status. The parent trial randomly assigned patients under double-blind conditions to receive a single intravenous infusion of ketamine or midazolam in a 2:1 ratio. Twenty-four patients received a baseline high-resolution magnetic resonance imaging (MRI) scan within 24 hours prior to a single intravenous infusion of ketamine (0.5 mg/kg over 40 minutes; n = 16) or midazolam (0.045 mg/kg over 40 minutes; n = 8). Three participants in the ketamine group and 2 participants in the midazolam group had unsuccessful MRI scans due to motion artifact and were excluded. Adult patients (age 21–80) were medication-free for one week (four weeks for fluoxetine), had treatment resistance to at least three adequate antidepressant trials [according to Antidepressant Treatment History Form (ATHF) criteria] (Sackeim, 2001), and were currently in a major depressive episode according to DSM-IV TR criteria confirmed by a structured clinical interview (First et al., 1995). Major exclusion criteria included a lifetime history of a psychotic illness or bipolar disorder, alcohol or substance abuse/dependence in the previous 2 years, unstable medical illness, history of traumatic brain injury or neurological illness, taking contraindicated medications or MRI contraindications such as metallic implants or claustrophobia.
Depressive severity was assessed at baseline using the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) prior to study drug administration and then repeated 24 hours following infusion.
For this pilot neuroimaging study, all study procedures including MRI acquisition, treatment, and followups were conducted at only one site (the MEDVAMC/Baylor College of Medicine). The MRI acquisition was performed using a 3T Siemens Trio MR system (1 × 1 × 1 mm voxel, TR = 1200 ms, TE = 2.66 ms, flip angle = 12°, matrix size = 245 X 245, 192 1 mm slices). Hippocampal volumetric segmentation was performed as previously described (Abdallah et al., 2012). Briefly, the recon-all pipeline from Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) was used. This fully automated processing includes imaging segmentation and volumetric estimation of hippocampus and total brain segmentation volume (Fischl and Dale, 2000). Post-processing quality checking through visual inspection was carried out, however no manual intervention was required. Previous studies showed high agreement between FreeSurfer hippocampal segmentation and manual segmentation (Morey et al.; Sanchez-Benavides et al., 2010; Doring et al., 2011). For detailed description of the boundaries of FreeSurfer hippocampal segmentation as compared to manual tracing see Morey et al. (Morey et al., 2009).
IBM SPSS Statistics 19 (SPSS Inc.) program was used for the statistical analysis. Delta MADRS was computed by subtracting the baseline score from the 24-hour score. The adherence to the Gaussian distribution was tested prior to each analysis. Pearson’s product moment correlation was used to determine the relationship between pretreatment hippocampal volume and the treatment outcome. Additional analyses used partial correlation to control for potential confounds, as detailed in the Results section. All tests were two-tailed, with significance level set at p ≤ 0.05. To reduce the number of comparisons, we restricted our analysis of clinical outcome to the parent trial’s primary outcome (i.e. MADRS at 24 hours post treatment).
Results
The demographic and clinical characteristics of neuroimaging study participants are summarized in Table 1.
Table 1.
Demographic and Clinical Characteristics (n=13)
| Mean ± S.E.M. | Percent | |
|---|---|---|
| Age (years) | 46.6 ± 2.6 | |
| BMI (kg/m2) | 29.6 ± 1.3 | |
| Age at first major depressive episode (years) | 26.6 ± 3.0 | |
| Duration of illness (years) | 18.9 ± 2.5 | |
| Duration of index episode (years) | 10.7 ± 2.0 | |
| Number of adequate antidepressant trials | 5.5 ± 0.7 | |
| MADRS pre-treatment | 33.6 ± 1.4 | |
| MADRS post-treatment | 12.8 ± 2.6 | |
| Response rate | 77% | |
| Male | 61% | |
| White | 85% | |
| Hispanic ethnicity | 23% | |
| Right-handed | 85% | |
| History of psychiatric hospitalization | 46% | |
| Melancholic depression | 61% | |
| History of suicide attempts | 23% | |
| History of alcohol abuse/dependence | 31% | |
| History of substance abuse/dependence | 15% |
Abbreviations: BMI, Body Mass Index; MADRS, Montgomery-Åsberg Depression Rating Scale.
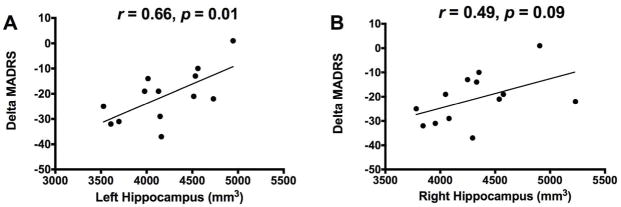
We found a significant positive association between delta MADRS and the estimated volume of the left hippocampus at baseline (r = 0.66, p = 0.01; Figure 1). But, there was no significant association between delta MADRS and the right hippocampal volume (r = 0.49, p = 0.09). The relationship between left hippocampus and delta MADRS maintains significance following a conservative Bonferroni correction for multiple comparisons (p < 0.025). In addition, the positive relationship between hippocampal volume and delta MADRS was also confirmed using bootstrap analysis (see Online Supplements). Secondary analyses were conducted to examine the effects of potential moderating factors and confounds. We found that the association between left hippocampus and delta MADRS remained high (r > 0.6, df = 5, p = 0.13) after controlling for total brain volume, handedness, age, gender, height, and race. To provide preliminary data regarding the relationship between hippocampal volume and the antidepressant effects of the GABA modulating agent midazolam, we correlated hippocampal volume with MADRS scores changes. There was no significant correlation between delta MADRS (24h post-treatment minus pre-treatment) and left (r = 0.73, n = 6, p = 0.10) or right hippocampal volume (r = 0.23, n = 6, p = 0.66).
Figure 1.
Correlation between response to ketamine and the left (A) or right (B) estimates of hippocampal volume. Delta MADRS = 24 h post-treatment minus pretreatment MADRS.
Discussion
This pilot study was designed to determine the relationship between hippocampal volume and the rapid antidepressant effects of ketamine in patients with TRD. We found a significant association between left hippocampal volume and rapid improvement in depression severity, such that patients with relatively smaller left hippocampus had a greater reduction in depression scores 24 hours following ketamine infusion. No statistically significant associations were found between baseline right hippocampal volume and response to ketamine.
A major focus of MDD research is to understand the mechanisms underlying the rapid-acting antidepressant effects of ketamine (Duman and Aghajanian, 2012). Investigating the relationship between ketamine treatment and the volumetric deficits observed in treatment-resistant mood disorders could provide insight into mechanisms underlying ketamine’s antidepressant effects. Animal studies have shown that repeated stress impairs the tripartite glutamate synapse, leading to increased extracellular glutamate and excitotoxicity (Sanacora et al., 2011). The excitotoxicity results in structural deficits – i.e. reduction of spine density, and dendritic shrinkage – precipitating an overall synaptic depression. A single injection of ketamine rapidly reverses these structural deficits within 24 hours of its administration in rodents, leading to an overall normalization of synaptic strength (Duman and Aghajanian, 2012). Ketamine has also been found to rapidly (within 30 minutes) increase hippocampal BDNF protein levels (Autry et al., 2011). The structural deficits observed using MRI in depressed patients (e.g. smaller hippocampus) are believed to reflect the microstructural changes of neuronal remodeling observed in animal models of depression (Drevets et al., 2008). Strongly supporting this hypothesis, recent preclinical work by Kassem et al. related the stress-induced anterior cingulate and hippocampal volume deficits as estimated by MRI to the reduction of spine density and dendritic length in the same brain regions (Kassem et al., 2013). Thus, we hypothesize that the rapid effects of ketamine on neurotrophic factors and synaptic plasticity rendered the drug particularly effective in patients with relatively greater structural deficits, as evidenced by smaller hippocampal volumes.
Of notice, there was a positive, yet statistically non-significant, association between left hippocampal volume and the antidepressant effects of the GABAergic agent midazolam. While the reader should interpret these preliminary data with extreme caution, it is an intriguing observation that, if confirmed in larger samples, it would raise an important question regarding the specificity of the ketamine finding and whether smaller hippocampal volume would predict enhanced antidepressant effects regardless of the treatment modality – i.e. glutamatergic, GABAergic, monoaminergic, or even placebo. We are not aware of studies reporting the relationship between hippocampal volume and placebo antidepressant effects. However, there is a relatively consistent literature associating smaller hippocampal volume with poor response to monoaminergic and traditional antidepressants (Vakili et al., 2000; Hsieh et al., 2002; Frodl et al., 2004; Frodl et al., 2008; Kronmuller et al., 2008; MacQueen et al., 2008; Sheline et al., 2012). In contrast, smaller hippocampal volume predicted enhanced response to ketamine (the current report) and riluzole (Abdallah et al., 2012). As predicted given the tight coupling between glutamate and GABA activities, both ketamine and riluzole have profound GABAergic effects (Banasr et al., 2010; Chowdhury et al., 2012). Thus, it is plausible that patients with smaller hippocampal volume are more likely to benefit from glutamate-/GABA-modulating agents, while those with larger hippocampal volume are better treated with monoaminergic antidepressants.
A limitation of this study is the small sample size, which may have constrained our ability to detect significant association between treatment effects and right hippocampal volume. However, if the observed hemispheric laterality was to be confirmed in future larger studies, it would be consistent with our prior work in non-human primates associating rearing stress with left, but not right, hippocampal deficits (Coplan et al., 2010; Jackowski et al., 2011). Another limitation is the lack of healthy control group, thus it is unknown whether the relatively smaller hippocampus in the current sample is abnormally small compared to the general healthy population. The strengths of this study include its relevance to the understanding of the neurobiology of TRD and its relevance to the development of response biomarkers for patient stratification and drug discovery. The use of fully automated segmentation methods further facilitates the implementation of these response biomarkers, if they prove to be of clinical value in larger future studies.
Supplementary Material
Acknowledgments
This work was supported by the Clinical Neuroscience Division of the VA National Center for PTSD, K23 MH-101498, NARSAD New Investigator Award, APF Early Academic Career Award (CGA), R01 MH-081870, NARSAD Independent Investigator Award, The Brown Foundation, Inc., and by resources and facilities at the Michael E. Debakey VA Medical Center (SJM).
Footnotes
Conflict of interest
CGA received research fund or consultation fee from Brain and Behavior Research Foundation (NARSAD), American Psychiatric Foundation, and Genentech. SJM received research funding or salary support over the last three years from the Banner Family Fund, Brain and Behavior Fund (NARSAD), The Brown Foundation, Inc., Bristol-Myers Squibb, Department of Veterans Affairs, Evotec, Johnson & Johnson, and the National Institute of Mental Health. He has received consulting fees or honoraria from Allergan, AstraZeneca, Cephalon, Corcept, Noven, Roche, and Takeda. He has received medication (Rilutek) from Sanofi-Aventis for a NIMH sponsored study. Dr. Mathew has been named as an inventor on a use-patent of ketamine for the treatment of depression. Dr. Mathew has relinquished his claim to any royalties and will not benefit financially if ketamine were approved for this use. JDC received grant support from NIMH, NYSTEM, GlaxoSmithKline, Pfizer, and Alexza Pharmaceuticals. He is on the Pfizer advisory board and gives talks for BMS, AstraZeneca, GSK, and Pfizer. No biomedical financial interests or potential conflicts of interest are reported for RS, AJ, PB, and JRS.
Role of the funding source:
The funding sources have no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
References
- Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Coplan JD, Jackowski A, Sato JR, Mao X, Shungu DC, Mathew SJ. A pilot study of hippocampal volume and N-acetylaspartate (NAA) as response biomarkers in riluzole-treated patients with GAD. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. LID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Mathew SJ, Abdallah CG, Mao X, Kral JG, Smith EL, Rosenblum LA, Perera TD, Dwork AJ, Hof PR, Gorman JM, Shungu DC. Early-life stress and neurometabolites of the hippocampus. Brain Res. 2010;1358:191–199. doi: 10.1016/j.brainres.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring TM, Kubo TT, Cruz LC, Jr, Juruena MF, Fainberg J, Domingues RC, Gasparetto EL. Evaluation of hippocampal volume based on MR imaging in patients with bipolar affective disorder applying manual and automatic segmentation techniques. J Magn Reson Imaging. 2011;33:565–572. doi: 10.1002/jmri.22473. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P. Biometric Research, New York State Psychiatric Institute; New York: 1995. Version 2.0. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Jager M, Smajstrlova I, Born C, Bottlender R, Palladino T, Reiser M, Moller HJ, Meisenzahl EM. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–430. [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, Jager M, Leinsinger G, Bottlender R, Reiser M, Moller HJ. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004;65:492–499. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, McQuoid DR, Levy RM, Payne ME, MacFall JR, Steffens DC. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry. 2002;17:519–525. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- Jackowski A, Perera TD, Abdallah CG, Garrido G, Tang CY, Martinez J, Mathew SJ, Gorman JM, Rosenblum LA, Smith EL, Dwork AJ, Shungu DC, Kaffman A, Gelernter J, Coplan JD, Kaufman J. Early-life stress, corpus callosum development, hippocampal volumetrics, and anxious behavior in male nonhuman primates. Psychiatry Res. 2011;192:37–44. doi: 10.1016/j.pscychresns.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, Hatton SN, Bennett MR. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Molecular neurobiology. 2013;47:645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- Kronmuller KT, Pantel J, Kohler S, Victor D, Giesel F, Magnotta VA, Mundt C, Essig M, Schroder J. Hippocampal volume and 2-year outcome in depression. Br J Psychiatry. 2008;192:472–473. doi: 10.1192/bjp.bp.107.040378. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biological Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Benavides G, Gomez-Anson B, Sainz A, Vives Y, Delfino M, Pena-Casanova J. Manual validation of FreeSurfer’s automated hippocampal segmentation in normal aging, mild cognitive impairment, and Alzheimer Disease subjects. Psychiatry Research. 2010;181:219–225. doi: 10.1016/j.pscychresns.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Disabato BM, Hranilovich J, Morris C, D’Angelo G, Pieper C, Toffanin T, Taylor WD, MacFall JR, Wilkins C, Barch DM, Welsh-Bohmer KA, Steffens DC, Krishnan RR, Doraiswamy PM. Treatment course with antidepressant therapy in late-life depression. Am J Psychiatry. 2012;169:1185–1193. doi: 10.1176/appi.ajp.2012.12010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.