Abstract
Trichopus zeylanicus Gaertn, (Trichopodaceae) is also known as “Arogyappacha” meaning the greener of health by tribal inhabitants (Kani tribes). This plant used as health tonic and rejuvenator. The whole plant material of Trichopus zeylanicus is defatted and successively extracted with methanol. The alkaloid fraction of Trichopus zeylanicus was obtained from methanol extract. Up to the dose of 2000 mg/kg b.w. per orally alkaloid fraction of Trichopus zeylanicus did not show any mortality or toxicity. Immunomodulatory activity of alkaloid fraction of Trichopus zeylanicus Gaertn was evaluated using various in vivo models including neutrophil adhesion test, delayed type hypersensitivity reaction, and effect on hematological parameter like, total white blood cell's, red blood cell's and hemoglobin and cyclophosphamide induce immunosupression. Sheep red blood cells were used to immunized the animals. The percentage of neutrophils adhesion to the nylon fiber was dose dependently increased in alkaloid fraction of Trichopus zeylanicus75, 150 and 300 mg/kg, p.o treated groups (50.57, 52.99 and 54.21%), respectively compared to control group. A dose dependent potentiating of delayed type hypersensitivity reaction induced by sheep red blood cells was also observed from the alkaloid fraction of Trichopus zeylanicus. On chronic administration of alkaloid fraction of Trichopus zeylanicus (75, 150 and 300 mg/kg. p.o.) caused significant (P<0.001) increased in hematological parameter like, total white blood cell's, red blood cell's and hemoglobin. Alkaloid fraction of Trichopus zeylanicus also prevented the myelosupression in mice treated cyclophosphamide (30 mg/kg, p.o.). The result of present investigation suggested that alkaloid fraction of Trichopus zeylanicus stimulate defense system by modulating several immunological parameters.
Keywords: Trichopus zeylanicus, immunomodulatory activity, alkaloid fraction, cyclophosphamide
An era of antibiotics chemotherapy shows resistance to number of strains of microorganisms during disease treatment, so it is need to look for herbal immunomodulatory agents to treat infections. There is growing interest in identifying and characterizing natural compounds with immunomodulatory activity. Compounds like alkaloids, phenols, polysaccharides, lectins, peptide, flavonoids and tannin have been reported for its immunomodulatory activity[1].
Trichopus zeylanicus commonly known as “Arogyapachha” i.e. greener of health. It is a wild plant, a rare genus, small glabrous herb growing in Agasthyar hilly forest of Kerala, India and use of this plant as a health tonic and rejuvenator. This information is based on ethno-medico-botanical investigation[2]. Trichopus zeylanicus was reported for its adaptogenic[3] and immunomodulatory[3], aphrodisiac[4], hepatoprotective[5], antistress[6,7,8], antioxidant activities[9]. However, there is no experimental evidence presently available in literature with regards to its alkaloid fraction shows immunomodulatory activity. Hence, the present study has been carried out to investigate its immunomodulatory activity using alkaloid fraction from Trichopus zeylanicus Gaertn.
The plant Trichopus zeylanicus Gaertn, (Family: Trichopodaceae) subspecies travancorius was collected from Agasthyar hills of Kerala in the month of September 2008, and authenticated by taxonomist of NIPER nursery, Mohali, Chandigarh, India. A voucher specimen No.NIP-159 has been deposited at that institute.
The whole plant material of Trichopus zeylanicus (3.0 kg) was dried. It was ground to coarse powder and defatted with petroleum ether then it was successively extracted with 90% methanol at room temperature for three times. The methanol extract was concentrated by using rotary evaporator. The (350 g) of methanol extract was further analyzed by various phytochemical tests for carbohydrates, proteins, saponin, glycosides, alkaloids, flavonoids, steroids and triterpenoids[10]. The methanol extract shows positive test for saponins, alkaloids, flavonoids, steroids, triterpenoids, carbohydrates and proteins. The (110 g) methanol extract was then moistened with 25% ammonia solution and allow to stand overnight then the extract was concentrated on rotary evaporator.
The brownish solid mass (100 g) of crude methanol extract was dissolved in 10% acetic acid (500 ml). After shaking and filtering, the acidic solution was washed with chloroform (500 ml), its pH adjusted to 9 by addition of 25% ammonia, and rewashed with chloroform (1.2 l). The latter extract was dried over anhydrous disodium sulphate (Na2 SO4) and after removal of the solvent crude alkaloid fraction (10 g, 1% w/w). This fraction is referred as alkaloid fraction of Trichopus zeylanicus (AFTZ), it was subjected to TLC using precoated silica gel G plates (F254) using a mixture of n-propanol:formic acid:water (9:0.1:0.9) as eluent and Dragendorff's reagent for detection of alkaloids[10,11].
Cyclophosphamide (CY) was obtained from Khandelwal Lab. Ltd, Mumbai. Freshly prepared leishman's stain (Loba Chemie, Mumbai), Alsever's solution, WBC's (Loba Chemie, Mumbai) and RBC's diluting fluid (Research Lab, Mumbai). 0.1N HCl for hemoglobin estimation were used. For dosing, the drug was dissolved in saline isotonic saline (SIS). The sheep red blood cells (SRBC) were used as antigenic material to immunize the animal. The SRBC were obtained from slaughter house and it was collected in sterile Alsever's solution. During the experimentation, adequate amount of SRBC were washed three times in large volumes of pyrogen free, sterile saline and adjusted to 0.5×109 cells/ml.
AFTZ (75, 150 and 300 mg/kg, p.o.) were prepared in 0.1% sodium carboxymethyl cellulose (CMC) was used for experimental studies. The vehicle alone served as control. For dosing, the CY (30 mg/kg, i.p.) was dissolved in SIS.
Male Swiss Albino mice (25-30 g) were selected for the study of immunomodulatory activity. The animals were obtained from the Department of Pharmacology, JKK Nattaraja College of Pharmacy, Komarapalyam. The animals were housed in well ventilated colony cages in the departmental animal house at (25±2°, 12:12 h L:D (Light and Dark) cycle). The animals were fed with standard rodent pellet diet and water ad libitum. All the experimental procedure and protocols used in the study were approved by IAEC of JKK Nattaraja College of Pharmacy, Komarapalyam, and as per CPCSEA (887/ac/05/CPCSEA) guidelines.
Acute toxicity assay was performed as per OECD guidelines 423 (limit test). Six female Wistar albino rats (three animals in each step) were randomly selected. The animals were kept fasting for overnight providing only water. The test drug was administered orally at one dose level of 2000 mg/kg b.w. after that rat were observed continuously for the first 4 h and then periodically up to 24 h for toxic symptoms and mortality[12].
Albino mice of either sex were divided into four groups designated as I-IV each group containing six mice. The control Group I received 0.1% sodium CMC whereas, Group II, III and IV animals were pretreated with AFTZ (75, 150, and 300 mg/kg, p.o.), respectively. Blood samples were collected from mice (after 14 days of drug treatment) by retro-orbital puncture in EDTA containing vials and subjected to total leukocyte cells (TLC) count and differential leukocyte cells (DLC) count. After initial count, the blood samples were incubated with 80 mg/ml of nylon fiber at 37° for 15 min[13]. The incubated blood samples were again analyzed for DLC counts. The percentage of neutrophil adhesion was calculated using following formula. Neutrophil adhesion (%)=[(NU-NT/NU×100], where NU=neutrophil count in untreated blood, NT=neutrophil count of fiber treated blood.
The cell mediated immune response was assessed by DTH reaction. Six animals per group (control and treated) were immunized by injecting 20 µl of 0.5×109 SRBC, s.c. into right foot pad. After 14 days of the drug treatment the thickness left foot pad was measured using Plethysmometer. At the same day, challenged by 20 µl of 0.025×109 SRBC s.c. into left foot pad. After 24 h, 48 h thickness of left foot pad of each animal were measured. The difference between the paw volume of left foot pad before and after challenge was expressed as a measure of DTH[14,15].
Albino mice of either sex were divided into four groups designated as I-IV each group containing six mice. The control Group I received 0.1% sodium CMC. Mice were divided into four groups of six each. Group II, III and IV animals received AFTZ (75, 150, and 300 mg/kg, p.o.) for 15 days, respectively. On day 16, blood was collected from retro-orbital plexuses of the individual animals and total WBC, RBC, and Hb were determined[16].
Albino mice were divided into five groups designated as I-V each group containing six mice. The control Group I received 0.1% sodium CMC. Groups II was administered with only CY at the dose of (30 mg/kg, i.p.) while Group III, IV and V received AFTZ at doses of (75, 150, and 300 mg/kg, p.o.) administered for 15 days prior to administration of CY. Groups II to V received CY at a dose of (30 mg/kg, p.o.) for the next 3 days. On day 19, blood was collected from retro-orbital plexuses of individual animals and analyzed for hematological determination[16].
Values were expressed as mean±SEM (n=6). Statistical analysis was performed using one-way ANOVA (Graph pad prism version 6.0) followed by Dunnets post-hoc test and values of P<0.05 were considered to be statistically significant.
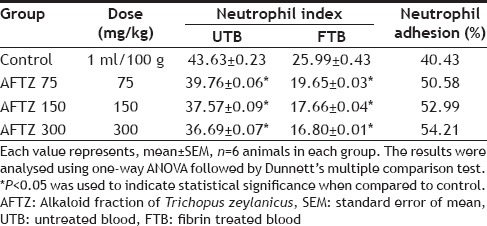
TLC plates was observed in day light the brownish-red colour spot with Rf value 0.4-0.45. The tested extract did not exhibit any toxicity symptoms and mortality in all groups when given orally at a dose 2000 mg/kg b.w. All the fractions were safe up to the dose of 2000 mg/kg b.w., hence three different doses (75,150 and 300 mg/kg b.w., p.o.) were selected for pharmacological study. In neutrophil adhesion test, as presented in Table 1, pretreatment of AFTZ (75,150 and 300 mg/kg; p.o.) elicted a significant increased in neutrophil adhesion to nylon fibers, which correlates the increase in percentage of neutrophils. Percentage of neutrophils was increased more significantly (P<0.001) as compared to control group. The percentage of neutrophil adhesion rate in control group animal was noted to be 40.43%, where as in AFTZ treated groups it was found with 50.58, 52.99 and 54.21%, respectively.
TABLE 1.
EFFECT OF ALKALOID FRACTION OF TRICHOPUS ZEYLANICUS ON NEUTROPHIL ADHESION TEST
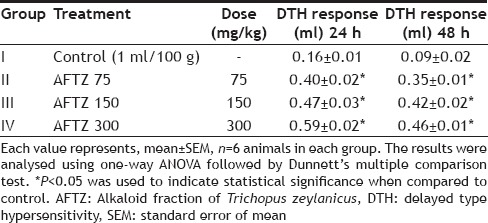
The cell mediated immune response of AFTZ was assessed by DTH reaction, i.e. foot pad reaction. As shown in Table 2, AFTZ (75, 150 and 300 mg/kg; p.o.) produced a significant, dose related increased in DTH reaction in mice i.e. inhibition of foot paw edema. At 48 h inhibition of foot paw edema was observed more significantly as compared to control group. DTH reaction in mice in response to cell dependent antigen revealed the stimulatory effect of AFTZ on T cells.
TABLE 2.
EFFECT OF ALKALOID FRACTION OF TRICHOPUS ZEYLANICUS ON DELAYED TYPE HYPERSENSITIVITY
A dose dependent effect of AFTZ on the peripheral blood count like WBC, RBC and Hb in control and treated animals is presented in fig. 1. Administration of AFTZ at various doses (75, 150, and 300 mg/kg, p.o.) for 15 days significant increase in WBC's (11.34±0.03, 11.60±0.02, and 12.18±0.01 thousand/mm3), respectively. RBC's count at AFTZ 75 mg/kg showed 4.417±0.008 whereas for AFTZ 150 and 300 mg/kg showed significantly higher (P<0.001, 4.55±0.01, 4.67±0.02 millions/mm3) RBC's count as compared to control (4.42±0.02 millions/mm3) group. In case of Hb concentration (g%) at AFTZ 75 mg/kg dose showed 14.73±0.12, and at a dose of AFTZ 150 and 300 mg/kg showed significantly higher hemoglobin (15.29±0.06 and 15.94±0.08 g%) concentration respectively as compared to control group (14.25±0.16). The increased in hematological count signify AFTZ possessed immunomodulation property (fig. 1).
Fig. 1.
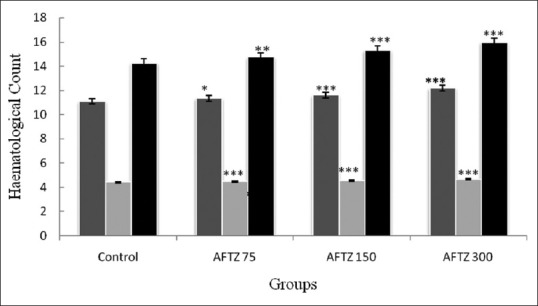
Effect of chronic administration alkaloid fraction of Trichopus zeylanicus on peripheral blood count.
Each value represents mean±SEM, n=6 animals in each group. The results were analysed using One-way ANOVA followed by Dunnett's multiple comparison test. *P < 0.05, were used to indicate statistical significance when compared to control. AFTZ=alkaloid fraction of Trichopus zeylanicus.  WBC counts (thousands per cmm),
WBC counts (thousands per cmm), RBC counts (millions per mm3), ▀ Haemoglobin concentration (g %).
RBC counts (millions per mm3), ▀ Haemoglobin concentration (g %).
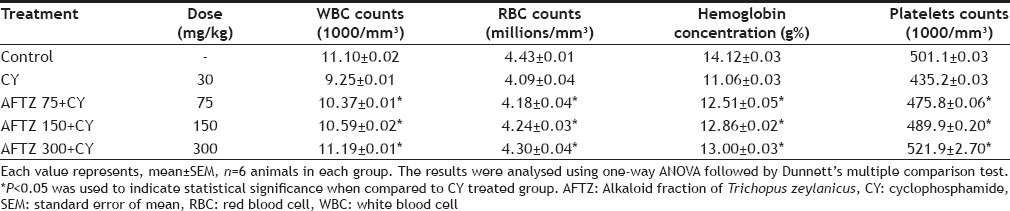
CY at the dose of (30 mg/kg; p.o.) caused a significant reduction in the WBC's, RBC's, Hb, and platelets concentration. Combined treatment of CY and AFTZ (75,150, and 300 mg/kg; p.o.) resulted in restoration of bone marrow activity as compared with cyclophosphamide treatment alone. All hematological parameters are significantly increased (Table 3). Recovery period observation indicated that CY induced immunosupression was not restored to normal, even after discontinuous use of CY. It was observed that mice treated with AFTZ showed more normal activity as compared to CY treated groups.
TABLE 3.
EFFECT OF ALKALOID FRACTION OF TRICHOPUS ZEYLANICUS ON BLOOD CELLS OF MICE TREATED CYCLOPHOSPHAMIDE FOR 15 DAYS
The results obtained in this study, claimed the traditional and medicinal purpose of Trichopus zeylanicus. In the present investigation, the immunomodulatory activity of AFTZ played an important role in indigenous system on Indian medicine was studied.
Ayurveda emphasizes the use of plant-based medicines and treatments. Various plants well-known in the Indian Ayurvedic system of medicine demonstrate prosperity of pharmacological properties. The Ayurvedic system of medicine is one of the oldest systems of medicine and includes various ethnopharmacological activities such as immunostimulation, tonic, neurostimulation, antiageing, antibacterial, antiviral, antirheumatic, anticancer, adaptogenic, etc.
An entire section of the Materia Medica of Ayurveda is devoted to “Rasayana”, drugs reputed to enhance body resistance. Listed as a class in the texts of traditional Indian Medicine literature, Rasayana consists of a number of plants reputed to promote physical and mental health, improve defense mechanisms of the body and enhance longevity. These attributes are similar to the modern concept of adaptogenic agents, which are known to afford protection of the human physiological system against diverse stressors. A number of medicinal plants as Rasayanas have been claimed to possess immunomodulatory activity, such as Withania somnifera, Tinospora cordifolia etc and others[17].
The present studied plant has shown immunomodulatory activity might be due to the presence of alkaloid like compounds. Alkaloids like tetradrine, berbamine and daurince contains bis-isoquonoline nucleus, these are responsible for their immunomodulatory activity[17,18]. On the basis of reported immunomodulatory activity of various alkaloids, the present investigation of AFTZ is responsible for restoration of immunity in studied immunomodulatory methods.
In the present study, AFTZ showed significant immunomodulatory activity. AFTZ may produce immunity against foreign body using various mechanisms. In neutrophil adhesion test, the neutrophil, an end cell unable to divide and with limited capacity for protein synthesis is nevertheless capable of wide range of responses in particular chemotaxisis, phagocytosis and exocytosis and both by extra and intra cellular killing[19]. In the present study, AFTZ evoked significant increase in percent neutrophils. Boundary of neutrophil from the blood stream require a firm adhesion which is mediated through the interaction of the B2 integrins stored in cell granule and up regulated for firm adhesion[20]. In present study AFTZ evolved significant increased in percent neutrophil this may help to increase immunity against infection and adhesion of neutrophil to nylon fibers was increased in AFTZ treated groups as compared to control.
DTH is a part of the process of graft rejection, tumor immunity, and, most important, immunity to many intracellular infectious microorganisms, especially those causing chronic diseases such as tuberculosis[21]. DTH reaction is characterized by an immunoinflammatory reaction in which TH1 and macrophages play an important role. DTH reaction needs specific antigenic substance which will release cytokines by activation with T-lymphocyte. In present investigation SRBC was used as the antigenic substance which elicits hypersensitivity reaction in mice. Number of evidence suggest the DTH reaction is important in host defense against parasite and bacteria that can live and multiply intracellularly[22]. Treatment with AFTZ enhanced DTH reaction, which is reflected from increased foot pad thickness compared to control group suggesting increased infiltration of macrophage to the inflammatory site. It was found that AFTZ dose-dependently potentiated the DTH reaction induced by SRBC.
Chronic administration of AFTZ significantly increased the total WBC's count, RBCs count, and Hb count. A high degree of cell proliferation renders the bone marrow a sensitive target particularly to cytotoxic drugs. Bone marrow is the organ most affected during immunosupression therapy with this class of drug. Loss of stem cells and inability of the bone marrow to regenerate new blood cells results in thrombocytopenia and leucopenia[23]. On administration of CY at a dose of (30 mg/kg, p.o.) showed significantly lowered the total WBC count, while AFTZ was found to significantly increase the total WBC count and platelet counts, indicates that the AFTZ can stimulate bone marrow activity.
The present investigation suggests that AFTZ may play an important role in stimulation of both humoral and cell mediated immunity. The AFTZ helps to reduce the side effects of drug induced myelosupression, such evidence support for balancing and adaptogenic activity of AFTZ. Thus result obtained, it can be concluded that Trichopus zeylanicus could be served as an effective immunomodulatory activity. Further studies on the mechanism of action AFTZ in order to establish its therapeutic efficacy in autoimmune disorder.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Acknowledgements:
Authors are thankful to Dr. Pushpangadan P and Dr. Saudagar RB, for providing information regarding the plant Trichopus zeylanicus and also thankful Dr. Lalit and Prof Vaibhav for providing necessary information for isolation of alkaloid fraction and biological activity.
Footnotes
Bachhav and Sambathkumar: Immunomodulatory Activity of Trichopus zeylanicus Gaertn
REFERENCES
- 1.Shivaprasad HN, Kharya MD, Rana AC, Mohan S. Preliminary immunomodulatory activities of aqueous extract of Terminalia chebula. Pharm Biol. 2006;44:32–4. [Google Scholar]
- 2.Pushpangadan P, Rajasekharan S, Ratheshkumar PK, Jawahar CR, Nair VV, Lakshmi N, et al. 'Arogyappacha' (Trichopus zeylanicus gaerin), the 'Ginseng' of Kani tribes of Agashyar hills (Kerala) for ever green health and vitality. Anc Sci Life. 1988;8:13–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Singh B, Gupta DK, Chandan BK. Adaptogenic activity of a glyco-peptido-lipid fraction from the alcoholic extract of Trichopus zeylanicus Gaertn. Phytomedicine. 2001;8:283–91. doi: 10.1078/0944-7113-00038. [DOI] [PubMed] [Google Scholar]
- 4.Subramoniam A, Madhavachandran V, Rajasekharan S, Pushpangadan P. Aphrodisiac property of Trichopus zeylanicus extract in male mice. J Ethnopharmacol. 1997;57:21–7. doi: 10.1016/s0378-8741(97)00040-8. [DOI] [PubMed] [Google Scholar]
- 5.Subramoniam A, Evans DA, Rajasekharan S, Pushpangadan P. Hepatoprotective activity of Trichopus zeylanicus extract against paracetamol-induced hepatic damage in rats. Indian J Exp Biol. 1998;36:385–9. [PubMed] [Google Scholar]
- 6.Subramoniam A, Evans DA, Valsaraj R, Rajasekharan S, Pushpangadan P. Inhibition of antigen-induced degranulation of sensitized mast cells by Trichopus zeylanicus in mice and rats. J Ethnopharmacol. 1999;68:137–43. doi: 10.1016/s0378-8741(99)00077-x. [DOI] [PubMed] [Google Scholar]
- 7.Subramoniam A, Evans DA, Rajasekharan S, Pushpangadan P. Effect of Trichopus zeylanicus leaf extract on the energy metabolism in mice during exercise and at rest. Indian J Pharmacol. 2002;34:32–7. [Google Scholar]
- 8.Tharakan B, Dhanasekaran M, Brown-Borg HM, Manyam BV. Trichopus zeylanicus combats fatigue without amphetamine-mimetic activity. Phytother Res. 2006;20:165–8. doi: 10.1002/ptr.1773. [DOI] [PubMed] [Google Scholar]
- 9.Tharakan B, Dhanasekaran M, Manyam BV. Antioxidant and DNA protecting properties of anti-fatigue herb Trichopus zeylanicus. Phytother Res. 2005;19:669–73. doi: 10.1002/ptr.1725. [DOI] [PubMed] [Google Scholar]
- 10.Khandelwal KR. Practical Pharmacognosy. 19th ed. Pune, India: Nirali Publication; 2008. [Google Scholar]
- 11.Harborne JB. Phytochemical Methods – A Guide to Modern Techniques of Plant Analysis. 3rd ed. London: Chapman and Hall; 1998. pp. 203–11. [Google Scholar]
- 12.OECD Guidelines for Testing of Chemicals, Guideline No. 423. [Last cited on 2001 Dec 17]. Available from: http://www.oecd.org/chemicalsafety/riskassessment/1948370.pdf .
- 13.Wilkinson PC. Neutrophil adhesion test. In: Vane JK, Ferreria SH, editors. Handbook of Experimental Pharmacology. 1st ed. Vol. I. Berlin: Springer Verlag; 1978. p. 109. [Google Scholar]
- 14.Lagrange PH, Mackaness GB, Miller TE. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974;139:1529–39. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee D, Khatua TN, Venkatesh P, Saha BP, Mukherjee PK. Immunomodulatory potential of rhizome and seed extracts of Nelumbo nucifera Gaertn. J Ethnopharmacol. 2010;128:490–4. doi: 10.1016/j.jep.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwardhan B. Studies on the immunomodulatory effects of ashwagandha. J Ethnopharmacol. 1996;50:69–76. doi: 10.1016/0378-8741(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 17.Panossian A, Wikman G, Wagner H. Plant adaptogens. III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine. 1999;6:287–300. doi: 10.1016/S0944-7113(99)80023-3. [DOI] [PubMed] [Google Scholar]
- 18.Srisilam K, Sumalatha DV, Thilagam E, Veeresham C. Immunomodulator from higher plants. Indian J Nat Prod. 2000;20:3–15. [Google Scholar]
- 19.Elgert KD. Immunology: Understanding the Immune System. New York: Wiley; 1996. pp. 306–8. [Google Scholar]
- 20.Dale MM, Foreman JC. Text Book of Immunopharmacology. London: Blackwell Scientific; 1984. [Google Scholar]
- 21.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 22.Descotes J. An Introduction to Immunotoxicology. London: Taylor and Francis; 1999. pp. 235–6. [Google Scholar]
- 23.Agrawal SS, Singh VK. Immunomodulators: A review of studies on Indian medicinal plants and synthetic peptides. Part: I medicinal plants. Proc Indian Natl Sci Acad. 1999;65:179–84. [Google Scholar]


