Abstract
Plumeria (Apocynaceae) is a genus comprising mostly of lactiferous trees and deciduous shrubs. Plumeria obtusa L. is a widely available but pharmacologically lesser explored species of this genus. Thus, present research was undertaken to determine the phytochemical constituents and the antioxidant potential of the methanol extract and fractions of the plant. The antioxidant potential was determined using 1,1-diphenyl-2-picrylhydrazyl radical scavenging and inhibition of lipid peroxidation assay.
Keywords: Antioxidant studies, apocynaceae, DPPH assay, lipid peroxidation, Plumeria obtusa
Plumeria obtusa L. (Apocynaceae) commonly known as Gulechin, Graveyard Tree or Frangipanier[1,2] is a small, much-branched, evergreen or partly deciduous tree[3]. The plant has been used in the management of diabetes mellitus, asthma, gonorrhea and constipation and also as a contraceptive, expectorant and anthelmintic[4,5,6]. The present study was undertaken to determine the antioxidant activity of methanol leaf extract and various fractions of P. obtusa.
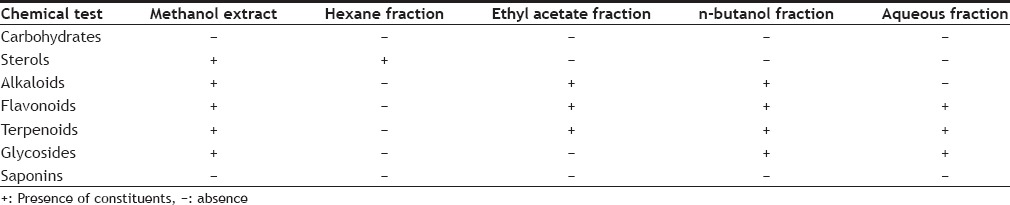
The leaves were collected from Lucknow and identified at Central Drug Research Institute (CDRI), Lucknow. They were shade dried and powdered. Six hundred grams of plant material was soaked in methanol (6 l×3 times) at room temperature for 48 h to get methanol extract. The methanol extract was concentrated using rotary evaporator to get a residue of about 100 g. Ten grams of crude methanol extract was used for evaluating biological activities and the rest for preparing fractions using solvents such as hexane, ethyl acetate, n-butanol and distilled water. All the fractions were concentrated individually using rotary evaporator. Preliminary phytochemical analysis of extract and fractions was done using standard procedures[7] and results are given in Table 1.
TABLE 1.
PHYTOCHEMICAL ANALYSIS OF METHANOL EXTRACT AND VARIOUS FRACTIONS OF PLUMERIA OBTUSA
The radical scavenging activity of crude methanol extract of P. obtusa and its fractions was studied by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay[8]. Briefly, to a methanol solution of DPPH (100 µM, 2.95 ml), 0.05 ml of test compounds dissolved in methanol was added at different concentration (200-1000 µg/ml). Reaction mixture was shaken and absorbance was measured at 517 nm using UV/Vis spectrophotometer at regular intervals of 30 s for 5 min, and the reading was taken till 20 min. Radical scavenging activity was expressed as % inhibition of DPPH radicals. Ascorbic acid was used as standard.
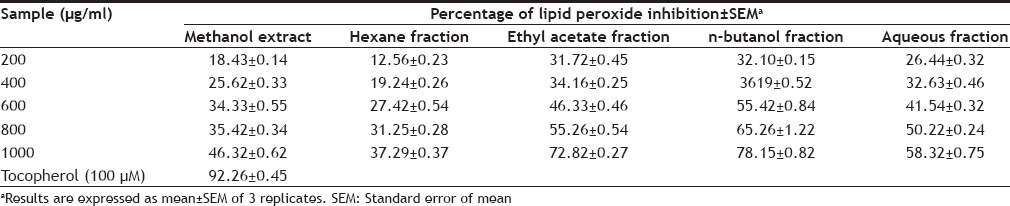
A modified thiobarbituric acid reactive species (TBARS) assay[9] was used to measure the lipid peroxide formed, using egg yolk homogenate as lipid rich media[10]. Egg homogenate (0.5 ml, 10% in distilled water v/v) and different concentration of P. obtusa leaf extract and fractions (200-1000 µg/ml) were allowed to react with 0.05 ml FeSO4(0.07 M) and then incubated for 30 min to induce lipid peroxidation. Subsequently 1.5 ml of 20% acetic acid (pH adjusted to 3.5 with NaOH) and 1.5 ml of 0.8% TBA (w/v) was (prepared in 1.1% sodium dodecyl sulfate and 0.5ml of 20% TCA) added, vortexed and then heated in a boiling water bath for 60 min. After cooling 5.0 ml of 1-butanol was added to each tube and centrifuged at 3000 rpm for 10 min. The absorbance of the organic upper layer was measured at 532 nm. The antioxidant activity was expressed as percentage inhibition of lipid peroxidation. Tocopherol was used as a reference standard. The leaf extract and fractions of P. obtusa have shown moderate antioxidant activity (Tables 2 and 3) based on DPPH and lipid peroxidation inhibition assays in dose dependent-manner.
TABLE 2.
IN VITRO 1,1-DIPHENYL-2-PICRYLHYDRAZYL RADICAL SCAVENGING ACTIVITY OF EXTRACT AND FRACTIONS
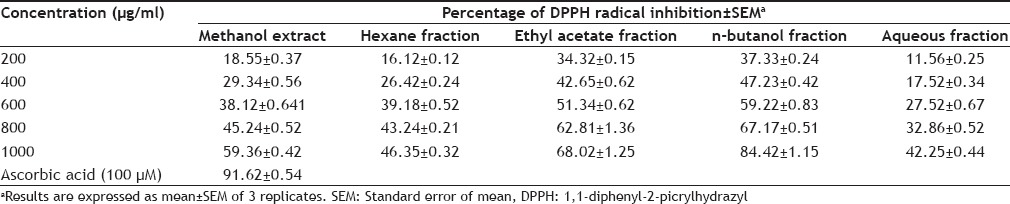
TABLE 3.
IN VITRO EFFECT OF EXTRACT AND FRACTIONS ON LIPID PEROXIDATION
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Footnotes
Dogra: In vitro Studies of Plumeria obtusa
REFERENCES
- 1.Krishnamurthi AS. The Wealth of India. New Delhi (India): Council of Scientific and Industrial Research; 1969. [Google Scholar]
- 2.Gandhi M, Singh Y. Brahma's Hair: The Mythology of Indian Plants. India: Rupa and Co.; 2000. [Google Scholar]
- 3.Cowen DV. Flowering Trees and Shrubs of India. 4th ed. India: Thacker and Co.; 1952. [Google Scholar]
- 4.Semenya S, Potgieter M, Erasmus L. Ethnobotanical survey of medicinal plants used by Bapedi healers to treat diabetes mellitus in the Limpopo Province, South Africa. J Ethnopharmacol. 2012;141:440–5. doi: 10.1016/j.jep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Haque MM, Choudhury MS, Hossain MS, Haque MA, Seraj S, Rahmatullah M. Ethnographic information and medicinal formulations of a Mro community of Gazalia Union in the Bandarbans district of Bangladesh. Am Eurasian J Sustain Agric. 2012;6:162–71. [Google Scholar]
- 6.Wetwitayaklung P, Phaechamud T, Limmatvapirat C, Keokitichai S. The study of antioxidant activities of edible flower extracts. Acta Hortic. 2008;786:185–92. [Google Scholar]
- 7.Farnsworth NR. Biological and phytochemical screening of plants. Indian J Pharm Sci. 1966;55:225–86. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- 8.Blois M. Antioxidant determinations by the use of stable free radical. Nature. 1958;26:1199. [Google Scholar]
- 9.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 10.Ruberto G, Baratta MT, Deans SG, Dorman HJ. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000;66:687–93. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]


