Abstract
Dosage form is a mean used for the delivery of drug to a living body. In order to get the desired effect the drug should be delivered to its site of action at such rate and concentration to achieve the maximum therapeutic effect and minimum adverse effect. Since oral route is still widely accepted route but having a common drawback of difficulty in swallowing of tablets and capsules. Therefore a lot of research has been done on novel drug delivery systems. This review is about oral dispersible tablets a novel approach in drug delivery systems that are now a day's more focused in formulation world, and laid a new path that, helped the patients to build their compliance level with the therapy, also reduced the cost and ease the administration especially in case of pediatrics and geriatrics. Quick absorption, rapid onset of action and reduction in drug loss properties are the basic advantages of this dosage form.
Keywords: Fast dissolving/disintegrating tablets, orodispersible tablets, GIT, bioavailability, first pass metabolism, superdisintegrants
Formulation of drugs into a presentable form is the basic requirement and need of today. Dosage form is a mean of drug delivery system, used for the application of drug to a living body. Various type of dosage forms are available such as tablets, syrups, suspensions, suppositories, injections, transdermal and patches having different type of drug delivery mechanisms. These classical/modern dosage forms have some advantages and disadvantages therefore the development of an ideal drug delivery system is a big challenge to the pharmacist in the presence scenario. In order to get the desired effect the drug should be delivered to its site of action at such rate and concentration to achieve the maximum therapeutic effect and minimum adverse effect. For the development of a suitable dosage form a thorough study about the physicochemical principles that governs a specific formulation of a drug should be subjected[1].
During establishing dosage form for a drug, it requires knowledge about each ingredient i.e. physical, chemical and biological properties along with the compatibility with the active drug, so that the product formed should be palatable, stable and efficacious[2]. Most drugs pass through the barrier by molecular diffusion, or through pores called pore diffusion. In pore diffusion the drug release rate is controlled by the crystal size, molecular size, pore size, pore structure and tortuosity of the polymers. In passive transport (Fick's first law) the drug moves from high concentration to the low concentration, while in active transport energy is required for the movement of drug from low to high concentration region through one or more transport mechanisms. It requires energy or carrier such as enzyme, protein[1].
GENERAL CONSIDERATIONS IN DOSAGE FORM DESIGN
Several physicochemical properties should be considered before going for new dosage form. The major aspects to be considered during formulation of a dosage form are mentioned as, the formulation qualifying the target parameters is considered as master formulation and any batch formulated must be on the specifications of master formula. Active agent can be incorporated in many dosage forms in such a way to achieve a convenient and efficacious drug delivery system for the treatment of diseases based on the route of administration. For oral use tablets and capsules are prepared for systemic effect as they can be easily handled by most of the patients, and if intended in emergency condition injectable form is applied for quick results. Other dosage forms include the patches and suppositories can be applied according to the patient condition[1].
NEED OF INNOVATIVE DRUG DELIVERY SYSTEM
The orally administered drug delivery is still considered as a standard system in pharmaceutics field and still considered safest, convenient and economical method of administration providing best route for patient compliance[3], however in case of tablet and capsule having a common drawback of difficulty in swallowing leading to poor compliance specially in geriatrics[4].
To improve compliance and making the administration convenient, design of new dosage forms gained significant importance. Conventional oral drug delivery present a drug with quick and full release that may go as such without producing the desired effect may be due to the presence of food, pH of the stomach, enzymatic degradation, change in GIT motility as so forth, giving not enough time to get absorbed[5,6]. Recently much light is being put on the area of designing drug delivery systems bearing organoleptic elegancy and maximum patient acceptability in pediatrics and geriatric groups[7,8,9]. A lot of innovative work is being done on drug delivery in which oral route is preferred because of ease of administration, cost effective therapy, self medication and noninvasive method leading to patient compliance to a higher level[10]. Tablet coating is one of the parameter in drug delivery designing applied to minimize the bad tasting and side effects while enhancing elegancy and drug bioavailability[11].
ORAL DISPERSIBLE TABLETS
Drinking water is mostly required for the oral administration of drugs, like tablet and capsules, in which some patients experience nuisance in swallowing bulky conventional dosage forms[12]. In order to prevent the dysphagia and improve patient compliance, orodispersible tablets are introduced as a substitute in oral DDS, designed to disintegrate in mouth without the aid of water. So they are useful in such conditions in which water is not available, or prohibited as before operation, in kinetosis, cough episodes due to neurological stimulation or chest infections. Different methods are adopted to manufacture the orodispersible tablets with the aim of giving fast disintegration to the dosage form as it gets in contact with saliva with good agreeable moth feeling[13]. These orodispersible tablets (ODT) can be administered to any patients having difficulty in swallowing. They are also recognized as mouth dissolvable, melt-in-mouth, fast dissolving, rapi-melts or porous tablets[14].
These are tablets which get dispersed or disintegrate when gets in a contact with saliva with the release of active drug[15,16], providing maximum drug bioavailability as compared to conventional dosage form[17]. This dispersible property is given by the addition of superdisintegrants to the dosage form, that releases the drug in mouth increasing the bioavailability[18]. Three different methods for the addition of disintegrants are used, they are intra granular (within the granules), extra granular (addition after granulation) and combination of both processes[19].
Te best time for an orodispersible tablet to get disperse is considered to be less than a minute[20,21]. Mostly the disintegration times varies from 5 to 30 seconds and are prepared applying; direct compression, solid dispersion, lyophilization or molding techniques. In all these methods direct compression is preferred because of its effortlessness, quick procedure and cost effectiveness[22]. ODTs are developed by the addition of super disintegrants like cross linked cellulose derivative; carboxymethyl cellulose, sodium starch glycolate, polyvinylpyrollidone, which gives burst disintegration when gets in contact with water or salivary secretions. Bioavailability of drugs may rise due to oral and pregastric absorption, reducing first pass metabolism in gastrointestinal tract[23].
Prerequisite of fast disintegrating tablets:
There are some prerequisite for fast disintegrating tablets which are mentioned as, Tablet must disintegrate and disperse in oral cavity without water intake. It can hold high drug quantity. It should be compatible with taste masking agents and excipients, and have optimum sensation effect. Leave minimum to no residue after administration. It should have optimum capacity to remain intact in formulation processes. It should be stable at the range of temperature and humidity. It should be adaptable and amenable to existing processing and packaging machinery. It should be manufactured at low cost[22,24].
Suitability of drugs for fast disintegrating tablets:
For developing FDT of a specific drug several factors should be kept forth while selecting drug, excipients and formulation method. These are as follows: Dugs to be used for sustained action are not suitable candidate for FDT. Drugs having very disagreeable taste are not suitable like clopidogrel. Patients suffering from Sjogren's syndrome and those with less saliva secretion and not suitable for FDT dosage form. Drugs of very short half life and requiring frequent dosing are not appropriate candidate. Patients on anticholinergic therapy are not suitable for FDT. Drugs showing altered pharmacokinetic behavior if formulated in such dosage form with respect to their conventional dosage form are not suitable, like selegiline, apomorphine and buspirone. Drugs producing considerable amounts of toxic metabolites on first pass metabolism and in GIT and having substantial absorption in oral and pregastric areas are good candidates. Drugs permeable to upper GIT and oral mucosal epithelial cell lining are considered good candidates for FDT[25].
Drugs which can be integrated in the fast dissolving tablets:
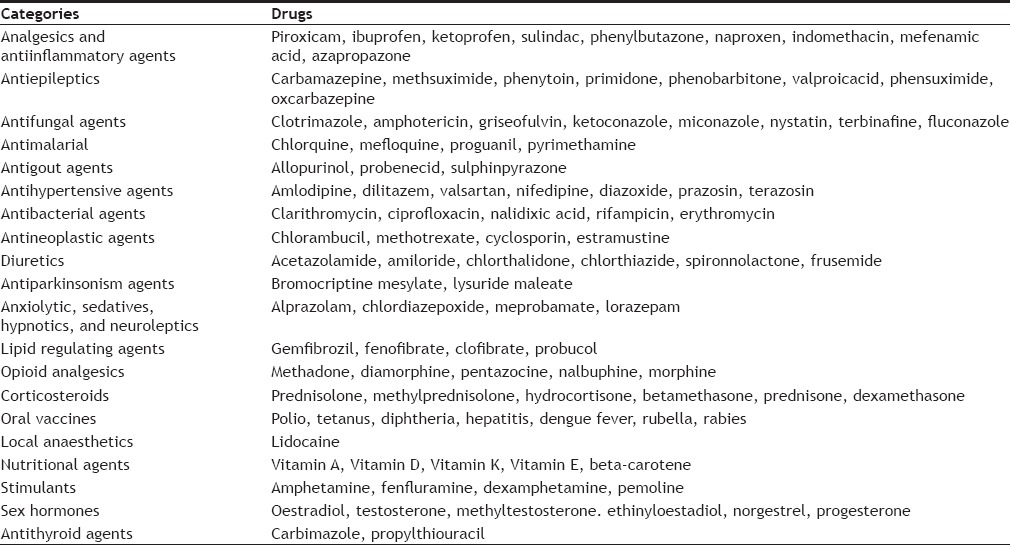
A variety of drugs are being incorporated in FDTs. Examples of drug candidates in various classes are mentioned in Table 1[26,27].
TABLE 1.
DRUGS THAT CAN BE INTEGRATED IN FAST DISSOLVING TABLETS
Excipients required in formulating FDTs:
Excipients used in FDTs contain one superdisintegrant, a diluent/bulking agent, a lubricant and optionally swelling agent, a permeabilizing agent (depending upon drug nature), sweeteners and flavorings agents. Names of excipients classes and their percentages are given in Table 2[28].
TABLE 2.
NAMES AND WEIGHT PERCENTAGE OF VARIOUS MAJOR EXCIPIENTS

TECHNIQUES FOR PREPARING ORODISPERSIBLE TABLETS
Various techniques are currently used in preparing fast disintegrating/dissolving tablets; some of them are discussed briefly in the following section.
Direct compression:
The most easiest and cost effective way to prepare tablets. Conventional compression machines with common ingredients are used, by limited number of processing steps. Microcrystalline cellulose (MCC) and low substituted hydroxypropyl cellulose (HPC) are used to manufacture rapidly disintegrating tablets. Rapid disintegration can also be achieved by adding effervescent material in a tablet to generate carbon dioxide, which also helps in taste masking of a drug. Major drawback of effervescent form, is hygroscopicity i.e., the ability to absorb atmospheric moisture. Sometime super disintegrants are added in optimal concentration, to achieve good oral dispersibility with pleasant feeling. Common examples of superdisinterants include sodium starch glycolate, crospovidone, alginic acid, calcium silicate and crosscarmellose. They provide rapid disintegration by swelling due to water absorption[29]. Characteristics of direct compression are cost effective, much similar to the conventional dosage form with an exception of containing high amount of disintegrants in some cases which can result in low tablet hardness[29].
Freeze drying or lyophilization:
It is a pharmaceutical process that allows the drying of heat sensitive drugs and biological under low temperature by the application of vacuum to remove water by sublimation. Drugs are dissolved or dispersed in aqueous solution of a carrier, transferred to preformed blister packs and subjected to nitrogen flush to freeze out, then placed in refrigerator to complete the process[30]. Characteristics of lyophilization techniques are, they possess high porosity and specific surface area, and gets dissolve rapidly in mouth presenting high drug bioavailability[30]. Major drawback of this system is high cost, time consuming procedure and fragility, making conventional packing inappropriate for packing this dosage form and stability issues under stress condition[23].
Molding method:
Tablets are designed using hydrophilic ingredients, with the aim to get maximum drug dissolution. Powder mass is wetted with hydroalcoholic solvent and compressed into dosage form. The solvent system is then allowed to evaporate. Taste of drug particles is developed by spray congealing the molten mixture of hydrogenated cottonseed oil, sodium carbonate, lecithin, polyethylene glycol with an active ingredient into lactose based tablet triturate[31]. Characteristics of moulding method are, very porous as solvents are removed by drying leaving porous mass which promotes rapid dissolution[31].
Sublimation:
Rapid disintegration and dissolution is acquired by formulating into porous mass by incorporating inert solid ingredients that volatilize rapidly like urea, camphor ammonium carbonate, ammonium bicarbonate and hexamethylene-tetramine. They were mixed with other ingredients and compressed. The volatile material is evolved by reduced pressure and applying slight temperature leaving the mass in porous form[32]. Characteristics of sublimation method are, they are porous in nature, solvents like cyclohexane and benzene can be used[32].
Spray-drying:
By this method ingredients are integrated by hydrolyzed and nonhydrolyzed gelatins as supporting agents, mannitol as bulking agent, sodium starch glycolate or crosscarmellose sodium as disintegrating and an acidic material (e.g. citric acid) and or alkali material (e.g. sodium bicarbonate) to enhance disintegration and dissolution[33]. Characteristics of spray-drying method is this method gives rapid dissolution (within 20 seconds) when dosage form gets in contact with aqueous medium[33].
Mass-extrusion:
In this the mixed ingredients are softened by water soluble ingredient i.e. polyethylene glycol, using methanol as solvent, passing through an extruder to form thin cylinders. Which further get sliced with heated blade to form small tablets[34]. Characteristics of this method is these products can be used to mask bitter tasting drugs making small granules thus enhancing oral bioavailability[34].
Cotton candy process:
By this method matrix of polysaccharides are prepared by simultaneous action of rapid melting and spinning. This candy floss matrix is then recrystalized milled and mixed with active drug along with excipients and compressed to form a fast dissolving tablet[30]. Characteristics of this method is high quantity of doses can be accommodated in this dosage form with high mechanical strength[30].
Nanonization:
It involves particle size reduction to nano size by using the wet grinding procedure. The formed nano crystals are then stabilized to prevent agglomeration by physical attachment on the surface of inert material[24]. Characteristics of this technique is suitable for water insoluble drugs with low bioavailability, a cost effective process and can withstand stress and hold wide range of doses (≥200 mg)[24].
Compaction:
By Melt granulation, it is formulated by addition of hydrophilic waxy binder (super polystate) PEG-6-stearate. This binder possesses dual action; increasing physical strength it also enhances the disintegration. Drugs such as griseofulvin can be easily administered in such dosage form[35]. Characteristics of compaction method is that it rapidly melts in mouth leaving no residue[35].
Phase-transition method:
By Phase-transition method, these dosage forms are established my mixing and compressing the mixture containing two sugar alcohols one of high melting point and 2nd of low melting point and successive heating them between their melting points making the tablet harden due to amplification of bondages induced by phase transition of lower melting point sugar alcohols. A fast dissolving tablet produced by Kuno et al., 2005 contained erythritol (MP: 122°) and xylitol (MP: 93-95°). They were heated at about 93° for 15 min resulted in increased median pore size along with increase in tablet hardness[36]. Characteristics of method is this method can withstand with the rigors of manufacturing and shipping conditions. As enough hardness is gained during heating[36]but not suitable for heat unstable drugs.
Fast dissolving films:
It contains a nonaqueous solution having water soluble film forming polymers (pullulan, carboxymethyl cellulose, hydroxypropyl methylcellulose, hydroxyethyl cellulose, hydroxypropy cellulose polyvinyl pyrrolidone, polyvinyl alcohol or sodium alginate.), a drug and other taste masking agent which are used to develop a film as solvent evaporates. In case of bitter tasting drugs resin adsorbate or coated micro particles of a drug can be used into a film[37]. Characteristics: These are thin films of 2×2 inches dimensions; dissolve fast within 5 seconds, leaving a good after taste[37].
ADVANTAGES OF FORMULATING ORODISPERSIBLE TABLETS
The advantages of orodispersible tablets are enumerated as, it can be administered easily to patients having difficulty in swallowing like elderly, stroke victims, and pediatrics. This increases the bedridden patient's compliance, people travelling having less access to water. Drugs with good mouth feeling may help in strengthens the psychological belief on medication. Ease of administration to both young and elderly patients[15]. More rapid and high absorption of drugs from pregastric parts of GIT improving the bioavailability and efficacy[8]. Cost effective as minimum number of ingredients are required. Improved safety by prevention from the chocking or obstruction as in case of conventional dosage form during swallowing[38]. Provide medication in dissolved or dispersed form through solid dosage form[39].
APPLICATIONS
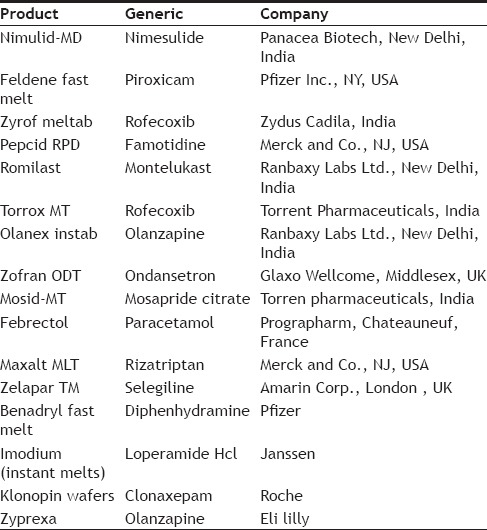
Numbers of drugs are being marketed applying different methods of formulating ODTs. List of some formulated and marketed drugs are mentioned in Table 3[28].
TABLE 3.
MARKETED PRODUCTS OF FAST DISSOLVING TABLETS
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Footnotes
Hannan, et al.: Orodispersible Drug Delivery System
REFERENCES
- 1.Mahato RI, Narang AS. Pharmaceutical Dosage Forms and Drug Delivery. 2nd ed. New York: CRC Press; 2011. Drug delivery systems; pp. 217–34. [Google Scholar]
- 2.Allen LV., Jr Dosage form design and development. Clin Ther. 2008;30:2102–11. doi: 10.1016/j.clinthera.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Parul S, Anoop K, Pankaj S, Sharad V. Fast disintegrating oral films: A recent trend of drug delivery. Int J Drug Dev Res. 2012;4:80–94. [Google Scholar]
- 4.Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery – A review. Pharm Sci Technolo Today. 2000;3:138–45. doi: 10.1016/s1461-5347(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 5.Reddy PD, Swarnalatha D. Recent advances in novel drug delivery systems. Int J Pharm Technol Res. 2010;3:2025–7. [Google Scholar]
- 6.Rastogi S, Vaya N, Mishra B. Osmotic pump: A novel concept in rate controlled oral drug delivery. East Pharm. 1995;38:79–89. [Google Scholar]
- 7.Bhushan SY, Sambhaji SP, Anant RP, Mahadik KR. New drug delivery system for elderly. Indian Drugs. 2003;37:312–18. [Google Scholar]
- 8.Bradoo R, Shahani S, Poojary S, Deewan B, Sudarshan S. Fast dissolving drug delivery systems. JAMA India. 2001;4:27–31. [Google Scholar]
- 9.Wadhwani A, Prabhu NB, Nandkarni MA, Amin PD. Consumer friendly mucolytic formulations. Indian J Pharm Sci. 2004;7:506–7. [Google Scholar]
- 10.Pahwa R, Piplani M, Sharma PC, Kaushik D, Nanda S. Orally disintegrating tablets-Friendly to pediatrics and geriatrics. Arch Appl Sci Res. 2010;2:35–48. [Google Scholar]
- 11.Hirani JJ, Rathod DA, Vadalia KR. Orally disintegrating tablets: A review. Trop J Pharm Res. 2009;8:161–72. [Google Scholar]
- 12.Ishikawa T, Mukai B, Shiraishi S, Utoguchi N, Fujii M, Matsumoto M, et al. Preparation of rapidly disintegrating tablet using new types of microcrystalline cellulose (PH-M series) and low substituted-hydroxypropylcellulose or spherical sugar granules by direct compression method. Chem Pharm Bull (Tokyo) 2001;49:134–9. doi: 10.1248/cpb.49.134. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh T, Ghosh A, Prasad D. A review on new generation orodispersible tablets and its future prospective. Int J Pharm Pharm Sci. 2011;3:1–7. [Google Scholar]
- 14.Bi Y, Yonezawa Y, Sunada H. Rapidly disintegrating tablets prepared by the wet compression method: Mechanism and optimization. J Pharm Sci. 1999;88:1004–10. doi: 10.1021/js990061z. [DOI] [PubMed] [Google Scholar]
- 15.Kuchekar BS, Badhan AC, Mahajan HS. Mouth dissolving tablets: A novel drug delivery system. Pharm Times. 2003;35:7–14. [Google Scholar]
- 16.Redkar M, Gore S, Devrajan P. D-zolv taste masked mouth dissolving tablet. Indian J Pharm Sci. 2002;3:291–92. [Google Scholar]
- 17.Yeola BS, Pisal SS, Paradkar AR, Mahadik KR. New drug delivery systems for elderly. Indian Drugs. 2000;7:312–18. [Google Scholar]
- 18.Masareddy RS, Kadia RV, Manvi FV. Development of mouth dissolving tablets of clozapine using two different techniques. Indian J Pharm Sci. 2008;70:526–8. doi: 10.4103/0250-474X.44611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekar V, Chellan VR. Immediate release tablets of telmisartan using superdisintegrant-formulation, evaluation and stability studies. Chem Pharm Bull (Tokyo) 2008;56:575–7. doi: 10.1248/cpb.56.575. [DOI] [PubMed] [Google Scholar]
- 20.Liang AC, Chen LL. Fast-dissolving intraoral drug delivery systems. Expert Opin Therapeut Patents. 2001;6:981–6. [Google Scholar]
- 21.Schiermeier S, Schmidt PC. Fast dispersible ibuprofen tablets. Eur J Pharm Sci. 2002;15:295–305. doi: 10.1016/s0928-0987(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 22.Abdelbary G, Prinderre P, Eouani C, Joachim J, Reynier JP, Piccerelle P. The preparation of orally disintegrating tablets using a hydrophilic waxy binder. Int J Pharm. 2004;278:423–33. doi: 10.1016/j.ijpharm.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Bhowmik D, Chiranjib B, Pankaj Fast dissolving tablet: An overview. J Chem Pharm Res. 2009;1:163–77. [Google Scholar]
- 24.Shukla D, Chakraborty S, Singh S, Mishra B. Mouth dissolving tablets I: An overview of formulation technology. Sci Pharm. 2009;77:309–26. [Google Scholar]
- 25.Siddiqui MN, Garg G, Sharma PK. Fast dissolving tablets: Preparation, characterization and evaluation: An overview. Int J Pharm Sci Rev Res. 2010;2:87–96. [Google Scholar]
- 26.Pfister WR, Ghosh TK. Orally disintegrating tablets: Products, technologies, and development issues. Pharm Technol. 2005;10:136–50. [Google Scholar]
- 27.Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier J, Piccerelle P. Determination of the in vitro disintegration profile of rapidly disintegrating tablets and correlation with oral disintegration. Int J Pharm. 2005;292:29–41. doi: 10.1016/j.ijpharm.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Deepak S, Dinesh K, Mankaran S, Gurmeet S, Rathore MS. Fast disintegrating tablets: A new era in novel drug delivery system and new market opportunities. J Drug Deliv Ther. 2012;2:74–86. [Google Scholar]
- 29.Ashish P, Harsoliya MS, Pathan JK, Shruti S. A review-formulation of mouth dissolving tablet. Int J Pharm Clin Sci. 2011;1:1–8. [Google Scholar]
- 30.Gupta AK, Mittal A, Jha K. Fast dissolving tablet-A review. Pharm Innov. 2012;1:1–7. [Google Scholar]
- 31.Nagar P, Singh K, Chauhan I, Verma M, Yasir M, Khan A, et al. Orally disintegrating tablets: Formulation, preparation techniques and evaluation. J App Pharm Sci. 2011;4:35–45. [Google Scholar]
- 32.Parkash V, Maan S, Deepika, Yadav SK, Hemlata, Jogpal V. Fast disintegrating tablets: Opportunity in drug delivery system. J Adv Pharm Technol Res. 2011;2:223–35. doi: 10.4103/2231-4040.90877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sri KV, Raj GB, Ravishanker D, Kumar CA. Preparation and evaluation of montelukast oral dispersible tablets by direct compression method. Int Res J Pharm. 2012;7:315–18. [Google Scholar]
- 34.Velmurugan S, Vinushitha S. Oral disintegrating tablets: An overview. Int J Chem Pharm Sci. 2010;1:1–12. [Google Scholar]
- 35.Yang D, Kulkarni R, Behme RJ, Kotiyan PN. Effect of the melt granulation technique on the dissolution characteristics of griseofulvin. Int J Pharm. 2007;329:72–80. doi: 10.1016/j.ijpharm.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Kuno Y, Kojima M, Ando S, Nakagami H. Evaluation of rapidly disintegrating tablets manufactured by phase transition of sugar alcohols. J Control Release. 2005;105:16–22. doi: 10.1016/j.jconrel.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Bess WS, Kulkarni N, Ambike SH, Ramsay MP. Fast dissolving orally consumable solid film containing a taste masking agent and pharmaceutically active agent at weight ratio of 1:3 to 3:1. United States Patent; US 7067116. 2006 [Google Scholar]
- 38.Indurwade N, Rajyaguru T, Nakhat P. Novel approach: Fast dissolving tablets. Indian Drugs. 2002;8:405–9. [Google Scholar]
- 39.Shyamala B, Narmada G. Rapid dissolving tablets: A novel dosage form. Indian Pharm. 2002;8:9–12. [Google Scholar]


