Abstract
Objective
Septa within the hematoma cavity are common, especially in the mixed density chronic subdural hematomas (CSHs). Although CT remains the diagnosis of choice, MRI is superior to detect the membranes in CSHs. We could obtain MRIs in 64 patients with CSH. We examined the value of MRI to understand the history of CSH.
Methods
We retrospectively examined the medical records and MRIs of 64 consecutive patients. MRI was selected to find any organic causes of neurologic symptoms. We classified the CSHs into septated or non-septated group, since classification of the septa was frequently obscure.
Results
Septa were identified by MRI in 43 patients (67%). They were more common in the over 70-years-old group. Unknown causes were more common in the septated group, which implies they might suffer from multiple traumas. The signal intensity of the CSH was variable. The methods of treatment were different between two groups. Surgery was more common in the septated group (p=0.021). Surgery was performed in 57 patients (89%). Burr-hole drainage was successful in 55 patients, even in the septated group.
Conclusion
Septa within the hematoma cavity may be related to the multiple episodes of head trauma. Repeated trauma may cause acute bleedings over the CSHs, which is one of the pathogenic mechanisms of hematoma enlargement. MRI could show the history of CSH.
Keywords: Chronic subdural hematoma, Magnetic resonance imaging, Craniocerebral trauma, Diagnosis
Introduction
Septum formation or multiple membranes within the hematoma cavity is relatively common, especially in the mixed density chronic subdural hematomas (CSHs).1,13,15,18) Septation in preoperative computed tomographic (CT) scans of CSHs were reported as high as 34.8% in a consecutive series of 320 cases.9) Although CT remains the diagnosis of choice, magnetic resonance imaging (MRI) is superior to CT when detecting membranes in CSHs. MRI can also provide important information concerning the nature of the hematoma.3,8,10,16,17,18) We could obtain a series of MRI in 64 patients with CSH. We examined the value of MRI to understand the pathogenic mechanism and history of CSH.
Materials and Methods
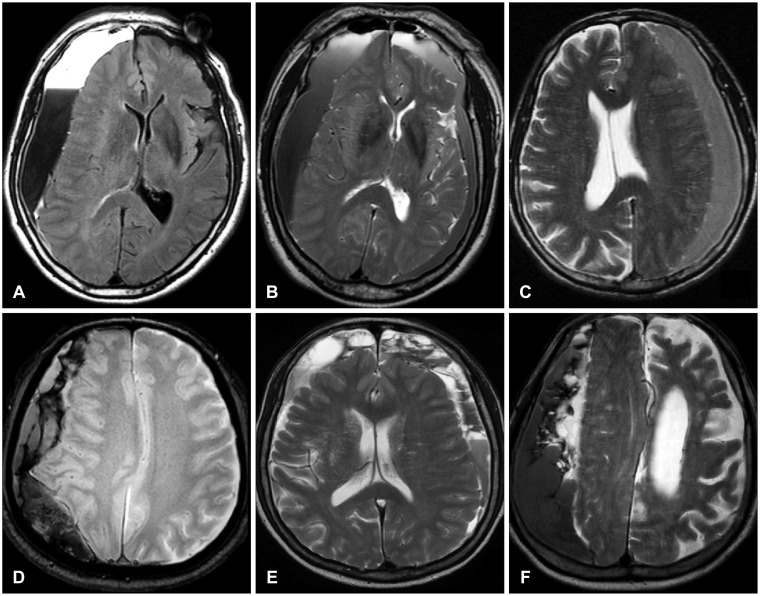
We retrospectively examined the medical records and MRI of 64 consecutive patients with CSHs, which was diagnosed by MRI with or without CT scans from January 2006 to March 2013. When a CSH was suspected, we usually performed CT scan, however, in these patients, MRI was usually selected to rule out any organic causes of hemiparesis or unexplained headache before CT scans. We classified the CSHs simply into two groups; septated or non-septated group on the basis of MRIs (Figure 1). The septum is defined as a membrane clearly separating hematomas with inhomogeneous contents between the inner and the outer membranes of the CSH.
FIGURE 1.
Examples of the non-septated and the septated groups. The layered (A) or graded (B) signal intensity is also classified into the non-septated group like the homogeneous (C) signal intensity. Since the patterns of the septa are usually mixed (D-F), it is hard to classify them into any specific subtypes.
Statistical analysis was performed using the chi-square test or Fisher's exact test. For the statistical significance, we divided the etiology into either known or unknown groups. Differences were considered significant if the probability value was less than 0.05.
Results
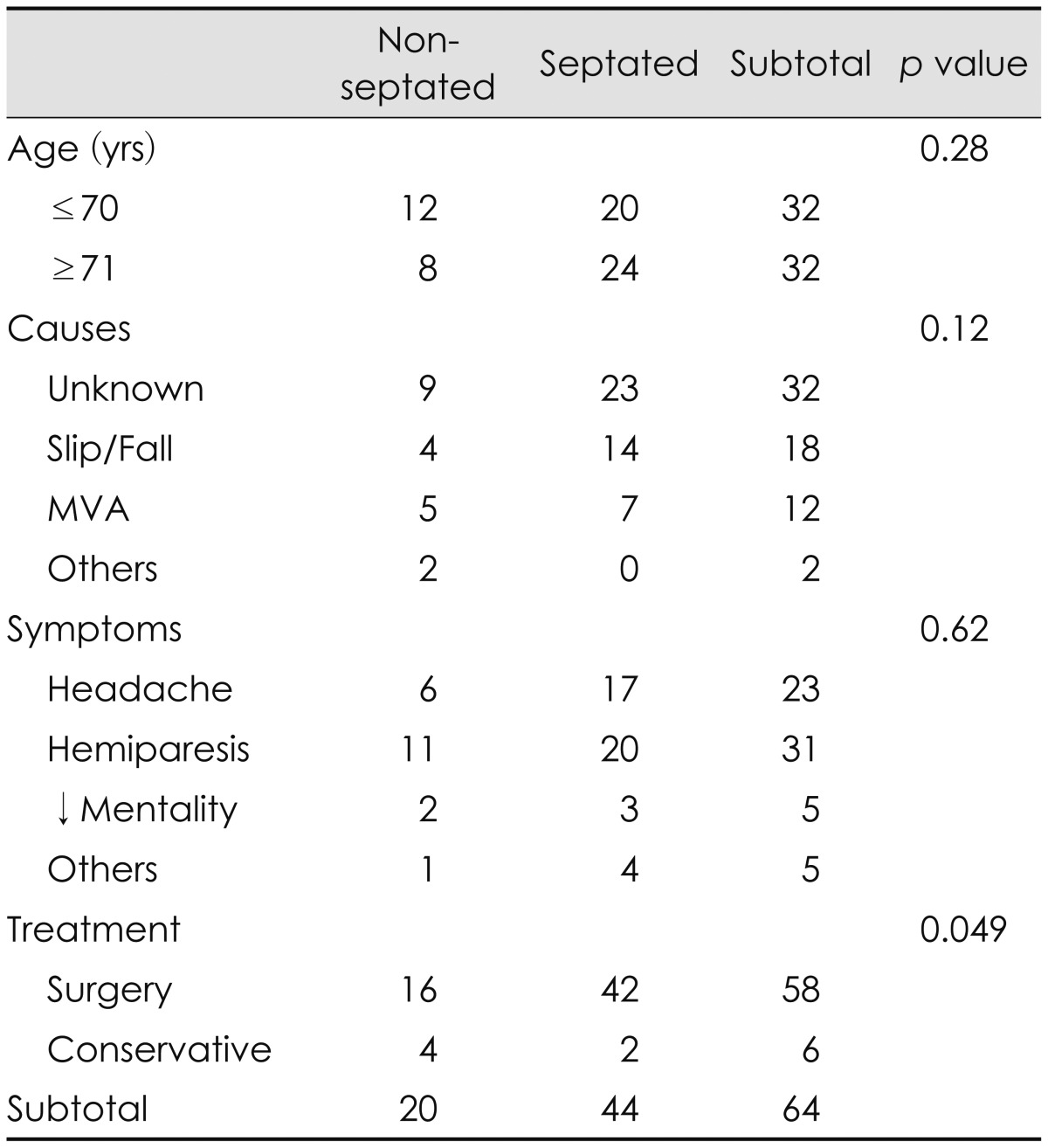
Septa or membranes were identified within the hematoma cavity by MRI in 44 patients (69%). The septa were more common in the over 70-years-old group (Table 1). However, this difference was not statistically significant (p=0.28 by chi-square test). The signal intensity of the CSH with septa was variable. The most common signal intensity was mixed one on T1-weighted, T2-weighted, or fluid attenuated inversion recovery (FLAIR) images (Figure 2). The male/female ratio was 49/15. Although we examined the history of head trauma minutely, the etiology was identifiable in only 32 patients (50%). The frequency of unknown causes was more common in the septated group, however, this difference was not statistically significant (p=0.12 by chi-square test). Slipping or falling was the most common cause of head trauma in the septated group, while motor vehicle accident was more common in the non-septated group. Common symptoms were contralateral hemiparesis, variable degrees of headache, confusion, and seizure in both groups. Clinically, there were also no statistically significant differences between the septated and non-septated groups, except methods of treatment. Surgery was more common in the septated group, while conservative management was more common in the non-septated group (p=0.049 by Fisher's test). Conservative treatment was done in 4 cases of the non-septated group, while it was successful in 2 cases of the septated group. Surgery was performed in 58 patients (91%). Burr hole with retained drainage was successful in 57 patients, even in the septated group. In one patient with septated CSH, revision surgery was needed after a craniotomy with limited membranectomy. Two patients died of malignancy, lung cancer and stomach cancer each, after recovery from the CSH. One patient with alcoholism expired by an acute subdural hematoma after a slip in drunken state.
TABLE 1.
Clinical features of 64 patients with CSHs diagnosed by MRI
CSHs: chronic subdural hematomas, MVA: motor vehicle accident
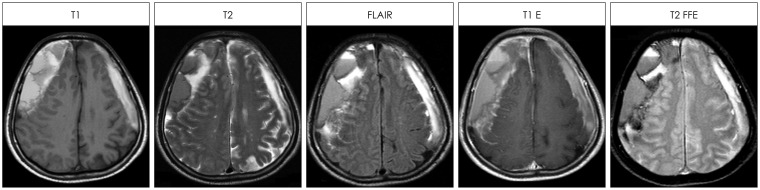
FIGURE 2.
The signal intensity of the chronic subdural hematoma with septa was mixed one on T1-weighted, T2-weighted, fluid attenuated inversion recovery (FLAIR), T1-enhanced (T1 E) or T2 fast field echo (T2 FFE) images.
Discussion
Septa or membranes were identified within the hematoma cavity by MRI in about 2/3 of the patients. Membranes were frequently observed within the hematoma cavity, especially in mixed density hematomas by the CT scans.3,16,17,18) Repeated bleeding into the hematoma cavity may induce new inflammatory processes, with accompanying neomembranes or formation of septa, which is responsible for compartmentalization of the hematoma cavity.13) The septa were more common in the over 70-years-old group, although this difference lacked statistical significance in this study. Mixed density CSHs by the CT scan were more common in the oldest age.16) Since atrophy or enough reserving capacity is more common in the aged brain, the chance of rebleeding into the existing hematoma cavity is more common in the oldest age.
Although some patients with CSH asymptomatic, they are prone to fall or slip down since they are usually aged and enjoy drinking.12,16) If they slip, even though the injury itself is trivial, it may tear the cortical bridge veins or fragile vessels in the neomembrane. The bridge veins in the potential subdural space are thin-walled, and might be under significant tension by the hematoma.12) Like the repeated microhemorrhages from the outer membrane, repeated trauma may cause acute bleeding over the CSH as a mechanism of hematoma enlargement.16) Acute-on-CSH often show layers of extraaxial fluid of varied density separated by internal membranes, septa.12) Sometimes repeated trivial trauma may cause a subdural hygroma, which became a CSH.16) Septa may differentiate such a chronic-on-chronic SDH according to the different ages of the CSHs.
Although there was no statistical significance, unknown etiology was more common in the septated group. Septa within the hematoma cavity may be related to the multiple episodes of head trauma. Multiple episodes of trauma are hard to remember compared to a single traumatic event. In the septated group, it might be hard to remember all the trivial traumas. Mixed density of CSH by CT scans results from multiple episodes of trauma, especially in the oldest age.16) The etiology was frequently obscure in the mixed density CSHs by CT scans, too.16)
According to the internal structure of the hematoma, subtypes were proposed.3,18) Theoretically, the acute-on-chronic SDHs tend to produce so-called multi-lobule type membrane, while chronic-on-chronic SDHs would produce mul-ti-layer type membrane.16) However, it is hard to classify the septa in the hematoma cavity into any specific patterns or subtypes. The septa frequently appeared mixed patterns. The CSHs with septa were appeared as mixed signal intensity in various images of MRI. In general, chronic subdural hematomas were hyperintense on both T1- and T2-weight-ed MRI.6) The signal intensity of the CSH was variable. The most common signal intensity was mixed one on T1-weighted, T2-weighted, or FLAIR images. The septa within the hematoma cavity implied episodes of rebleeding. Thus, MRI could show the history of CSHs including multiple episodes of trivial traumas.
Surgery was performed in 57 patients, more commonly in the septated group. Some insisted that the burr-hole trephination was insufficient to remove the septated CSHs.5,18) Craniotomy or neuroendoscopic techniques have been used for multiloculated septated or organized hematomas.2,7) However, the septa were usually fragile and rarely enclose completely, separated from any cavities. There was free communication, which allows draining out the hematomas.16) Even in completely separated cavities, drainage of the hematoma from the relatively large cavity may decompress to recover the clinical symptoms or may tear the fragile septa. The neomembrane of the hematoma contains several layers of capillaries with large lumens and many thin new vessels deriving from the middle meningeal artery. These blood vessels, in the presence of coagulopathy, are more vulnerable and can easily rebleed. Thus, craniotomy with partial membranectomy or any mechanical manipulation of the hematoma membrane in patients with coagulopathy carry a distinctly higher risk of rebleeding.11) Endoscopic removal of the hematoma with membrane is rarely recommended.4) Burr hole with retained drainage was successful in 55 patients, even in the septated group. Even in acute-on-chronic subdural hematomas, removal of the liquefied hematoma only by a burr hole was possible to relieve the displacement.12) Although there were membranes within the hematoma, burr hole was usually enough to drain the hematomas.16) Craniotomy may be necessary for those instances in which the subdural collection reaccumulates, the brain fails to expand, or there is solid hematoma.14)
Conclusion
The septa within the hematoma cavity implied episodes of rebleeding. Thus, repeated trauma may cause acute bleedings over the CSHs, which is one of the pathogenic mechanisms of hematoma enlargement. MRI could show the history of CSH including multiple episodes of trivial traumas.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012;154:1541–1548. doi: 10.1007/s00701-012-1399-9. [DOI] [PubMed] [Google Scholar]
- 2.Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Anderson K, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 2012;35:155–169. doi: 10.1007/s10143-011-0349-y. discussion 169. [DOI] [PubMed] [Google Scholar]
- 3.Fujisawa H, Nomura S, Kajiwara K, Kato S, Fujii M, Suzuki M. Various magnetic resonance imaging patterns of chronic subdural hematomas: indicators of the pathogenesis? Neurol Med Chir (Tokyo) 2006;46:333–338. doi: 10.2176/nmc.46.333. discussion 338-339. [DOI] [PubMed] [Google Scholar]
- 4.Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107:223–229. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Hellwig D, Kuhn TJ, Bauer BL, List-Hellwig E. Endoscopic treatment of septated chronic subdural hematoma. Surg Neurol. 1996;45:272–277. doi: 10.1016/0090-3019(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 6.Hosoda K, Tamaki N, Masumura M, Matsumoto S, Maeda F. Magnetic resonance images of chronic subdural hematomas. J Neurosurg. 1987;67:677–683. doi: 10.3171/jns.1987.67.5.0677. [DOI] [PubMed] [Google Scholar]
- 7.Imaizumi S, Onuma T, Kameyama M, Naganuma H. Organized ch-ronic subdural hematoma requiring craniotomy--five case reports. Neurol Med Chir (Tokyo) 2001;41:19–24. doi: 10.2176/nmc.41.19. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kang DS, Kim JH, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 2011;50:103–108. doi: 10.3340/jkns.2011.50.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krieg SM, Aldinger F, Stoffel M, Meyer B, Kreutzer J. Minimally invasive decompression of chronic subdural haematomas using hollow screws: efficacy and safety in a consecutive series of 320 cases. Acta Neurochir (Wien) 2012;154:699–705. doi: 10.1007/s00701-012-1294-4. discussion 705. [DOI] [PubMed] [Google Scholar]
- 10.Le TH, Gean AD. Neuroimaging of traumatic brain injury. Mt Sinai J Med. 2009;76:145–162. doi: 10.1002/msj.20102. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61:523–527. doi: 10.1016/j.surneu.2003.10.026. discussion 527-528. [DOI] [PubMed] [Google Scholar]
- 12.Lee KS, Shim JJ, Yoon SM, Doh JW, Yun IG, Bae HG. Acute-on-chronic subdural hematoma: not uncommon events. J Korean Neurosurg Soc. 2011;50:512–516. doi: 10.3340/jkns.2011.50.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo WL, Lee TC, Fang PS, Huang YH. Chronic subdural hematoma in patients under age 65 years: a comparative study of age cohort. Formosan J Surg. 2013;46:10–14. [Google Scholar]
- 14.Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637–645. doi: 10.3171/jns.1981.54.5.0637. [DOI] [PubMed] [Google Scholar]
- 15.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256–262. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 16.Park HR, Lee KS, Shim JJ, Yoon SM, Bae HG, Doh JW. Multiple densities of the chronic subdural hematoma in CT Scans. J Korean Neurosurg Soc. 2013;54:38–41. doi: 10.3340/jkns.2013.54.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senturk S, Guzel A, Bilici A, Takmaz I, Guzel E, Aluclu MU, et al. CT and MR imaging of chronic subdural hematomas: a comparative study. Swiss Med Wkly. 2010;140:335–340. doi: 10.4414/smw.2010.12867. [DOI] [PubMed] [Google Scholar]
- 18.Tanikawa M, Mase M, Yamada K, Yamashita N, Matsumoto T, Banno T, et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir (Wien) 2001;143:613–618. doi: 10.1007/s007010170067. discussion 618-619. [DOI] [PubMed] [Google Scholar]