Abstract
Objective
Re-implantation of autologous skull bone has been known to be difficult because of its propensity for resorption. Moreover, the structural characteristics of the area of the defect cannot tolerate physiologic loading, which is an important factor for graft healing. This paper describes our experiences and results with cranioplasty following decompressive craniectomy using autologous bone flaps.
Methods
In an institutional review, the authors identified 18 patients (11 male and 7 female) in whom autologous cranioplasty was performed after decompressive craniectomy from January 2008 to December 2011. We examined the age, reasons for craniectomy, size of the skull defect, presence of bony resorption, and postoperative complications.
Results
Postoperative bone resorption occurred in eight cases (44.4%). Among them, two experienced symptomatic breakdown of the autologous bone graft that required a second operation to reconstruct the skull contour using porous polyethylene implant (Medpor®). The incidence of bone resorption was more common in the pediatric group and in those with large cranial defects (>120 cm2). No significant correlation was found with sex, reasons for craniectomy, and cryopreservation period.
Conclusion
The use of autologous bone flap for reconstruction of a skull defect after decompressive craniectomy is a quick and cost-effective method. But, the resorption rate was greater in children and in patients with large skull defects. As a result, we suggest compressive force of the tightened scalp, young age, large skull defect, the gap between bone flap and bone edge and heat sterilization of autologous bone as risk factors for bone resorption.
Keywords: Autologous transplant, Cranioplasty, Resorption
Introduction
Decompressive craniectomy is performed in patients with traumatic brain injury that is refractory to medical therapy.1,3) After acute cerebral edema has resolved or when the patient's condition is stable, an area of the skull defect may become concave. A cranioplasty is necessary for aesthetic purposes, protection of the brain, as well as for its role in the prevention of headaches, seizure, or syndrome of the trephined.5) Through cranioplasty, the skull is able to regain its preoperative shape, and normalization of cerebrospinal fluid and cerebral blood flow, and neurological improvement can be expected.5,6,7) But, untoward complications such as infection and fluid collection appear at times.
Cranioplasty has been performed using autologous bone grafts or artificial bone materials such as polymethylmethacrylate (PMMA) and porous polyethylene implants (Medpor®, Stryker, Kalamazoo, MI, USA).9) Cranioplasty using autologous bone grafts is used to reconstruct skull defects following decompressive craniectomy, and has many merits. Cryopreserved autografts are relatively easily preserved because the shape exactly matches the shape of the defect and no additional reformation is necessary. Above all, the advantage of the autologous bone is that there is no rejection.8) On the other hand, the chief problem of such complications is the bone resorption. This re-implantation of the skull bone has been known to be difficult because of its propensity for resorption. In particular, the skull bone has a higher propensity for resorption than other parts of the body. If implanted, skull graft resorption proceeds, and the bone graft may break down requiring further surgery. This study was performed to evaluate autologous bone graft resorption in patients undergoing bone grafting.
Materials and Methods
Study population
Among all patients who underwent decompressive craniectomy to relieve increased intracranial pressure at our institution, we identified 18 patients who underwent cranioplasty using cryopreserved autologous bone, not foreign material, from January 2008 to December 2011. A combination of antibiotics and normal saline was used for irrigation after the bone was removed; the bone was then stored in a sterile plastic bag.8,15) The bone was sealed in at least three bags and placed in a cryogenic freezer at a temperature of -70℃.9,11,13) For those patients scheduled to undergo a cranioplasty in the near future, a gelfoam film was placed over the dura to minimize adhesion prior to skin closure. During cranioplasty, the bone was taken from the freezer and autoclaved. A skin incision was made over the site of the previous incision and the scalp, periosteum, and dura were dissected. The bone flap was placed adjacent to the frontal bone and midline, and fixed with miniplates.
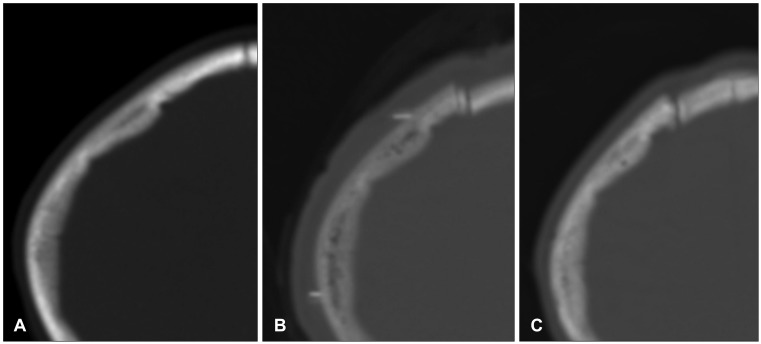
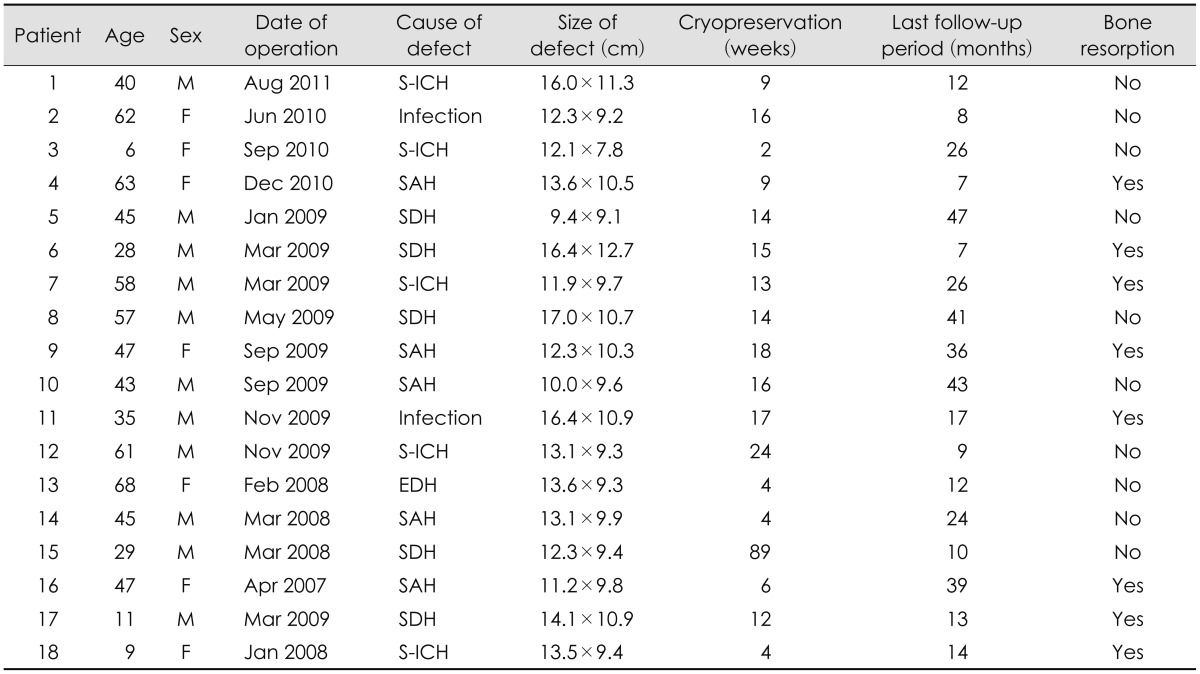
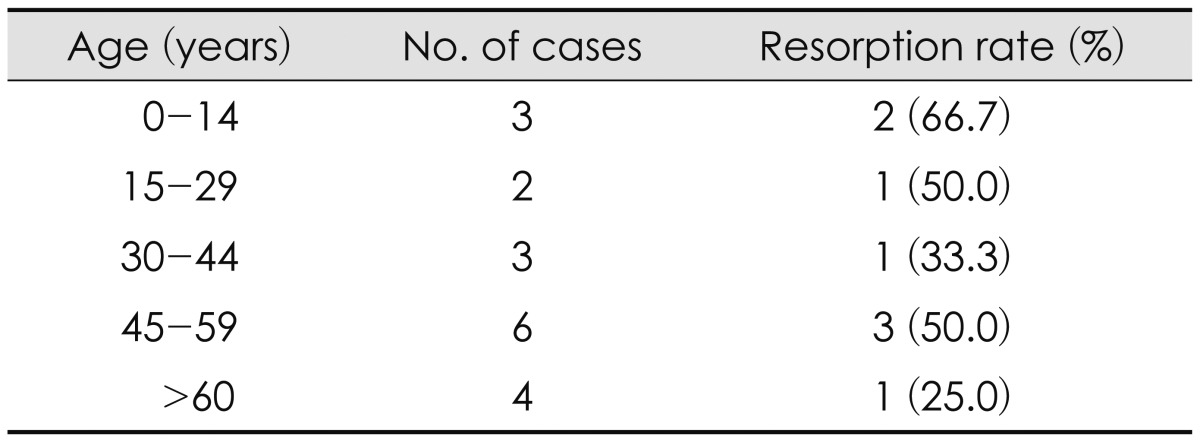
To evaluate the presence of infection, bone resorption, and other complications, a physical examination, routine blood tests, skull radiography, and computed tomography (CT) scan were performed.2) The amount of bone resorption was evaluated by CT of the skull after cranioplasty and at the follow-up examination. Figure 1 shows a case in which the bone reintegrated well. If the gap between the bone graft and the skull increased by >20% during the follow-up period, three cross sections were chosen randomly and the Hounsfield unit (HU) of the graft and the skull were each measured at five different points. In cases where the mean HU measurements of the bone graft decreased by >5% compared with those of the remaining skull, the contour was also compared. Bone breakdown was defined as a decrease in the surface area of the bone that was attributed to resorption and atrophy.
FIGURE 1.
Change in bone density. Serial CT scans taken before operation (A), after replacement of cryopreserved autologous bone (B), and after successful reintegration (C) show changes in bone density.
Statistical analysis
The Pearson correlation test was used to analyze correlations between resorption rates and skull defect size, patient age, and cryopreservation period. All statistical analyses were performed using Statistical Package for the Social Science (SPSS) version 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Age and sex
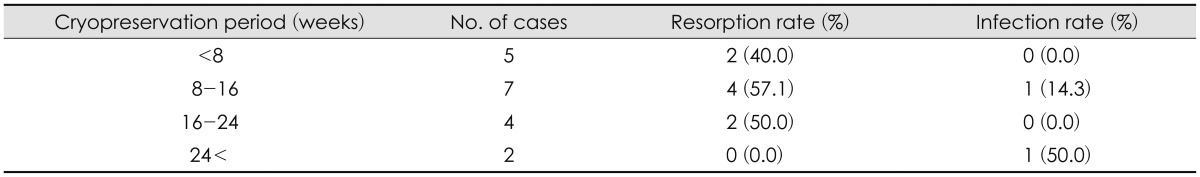
Of the 18 patients who underwent autologous bone cranioplasty, 11 were male and seven were female. Patient age ranged from 6 to 68 years (mean, 44.5 years), and the majority were in their 30 s. The mean cryopreservation period after craniectomy was 118 days (range, 18-625 days), with a follow-up period 22 months (range, 7 to 47 months)(Table 1). No significant correlation was found with sex, reasons for craniectomy, and cryopreservation period.
TABLE 1.
Patients characteristics
S-ICH: spontaneous intracerebral hemorrhage, SAH: subarachnoid hemorrhage, SDH: subdural hemorrhage
Clinical outcomes
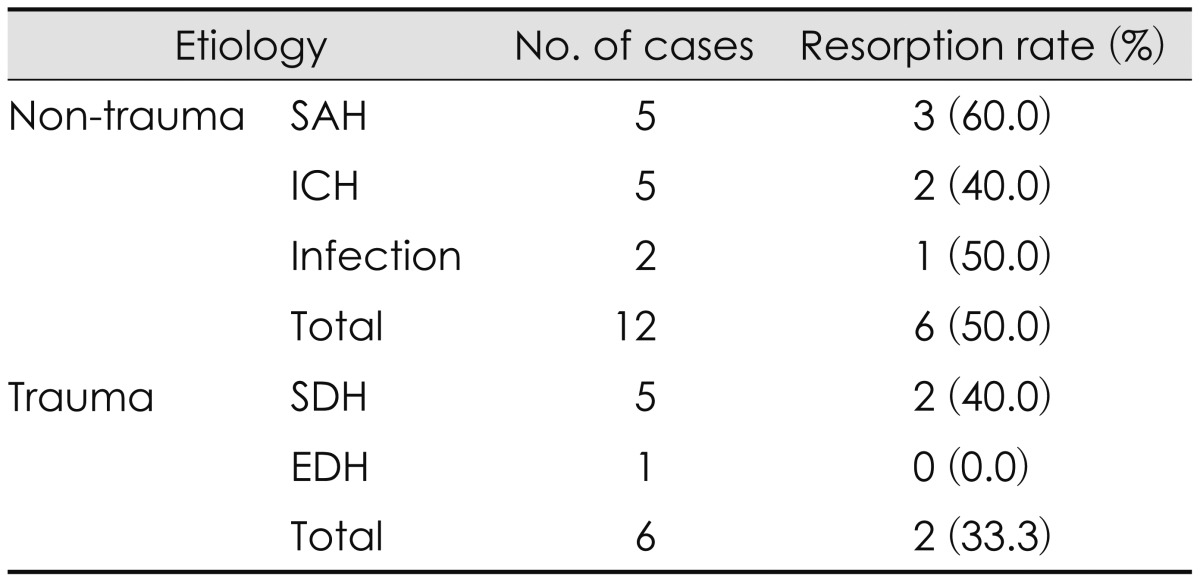
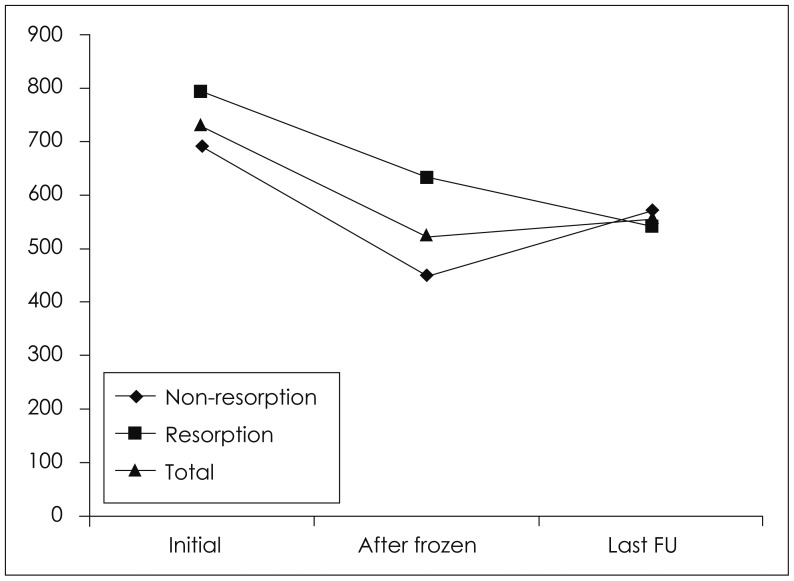
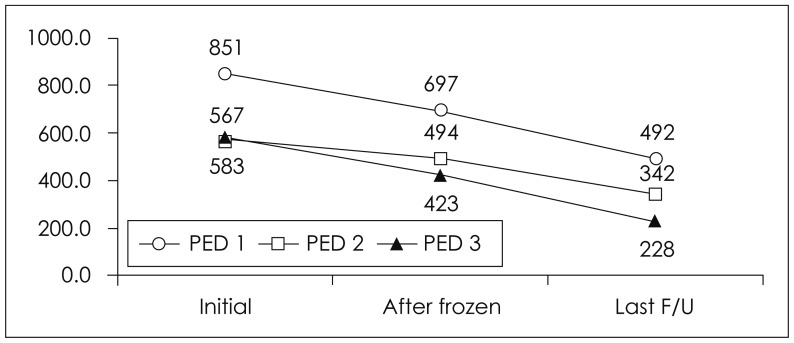
Bone resorption occurred in eight (44.4%) of the 18 cases (Table 2). A summary of resorption rates divided by the distribution of the cranial defect area is showed in Table 3. Six patients underwent craniectomies due to trauma [epidural hematoma (EDH) in one, subdural hematoma in five], and 12 due to non-traumatic hemorrhages (subarachnoid hemorrhage in five, intracerebral hemorrhage in five, and infection in two patients), as shown in Table 4. Symptomatic breakage occurred in two of the eight cases and required reoperation. The HU scores after cranioplasty are showed in Figure 2. Infection occurred in two cases (11.1%) and hematoma in one case (5.6%). In one case in which the cryopreservation period was relatively long, infection was suspected within 2 weeks of the cranioplasty and reoperation was performed (Table 5). Granulation tissue was found as well as an abscess that had formed under the bone. Cultures of the infected site were performed and the bone graft was removed and replaced with Medpor®. Second operations were performed in two cases: one with bone breakdown due to loosening of a plate screw, and the other with bone flap instability. The patient with EDH had to undergo hematoma removal. In our study, overall rate of bone resorption was 44.4% (8 of 18 cases) with autologous bone. In a pediatric patient, the bone resorption rate was especially too high 66.7% (2 of 3 cases), and required reconstructive surgery. Re-operations were performed in two cases due to bone resorption and breakdown. The operation fields in these cases revealed fragmented bones that were very crumbly and fragile. In both cases, the fragmented bones were removed and replaced with Medpor®, and involved pediatric patients (aged 9 and 11 years; as dipicted as PED 1 and 2 in Figure 3).
TABLE 2.
Resorption rates stratified by age
TABLE 3.
Resorption rates stratified by cranial defect area
TABLE 4.
Resorption rates stratified by etiology of decompressive craniectomy
SAH: subarachnoid hemorrhage, ICH: intracerebral hemorrhage, SDH: subdural hemorrhage, EDH: epidural hemorrhage
FIGURE 2.
Follow-up Hounsfield unit scores after cranioplasty. Follow-up Hounsfield unit scores after cranioplasty. Bone resorption occurred in eight (44.4%) out of the 18 cases.
TABLE 5.
Resorption and infection rates stratified by cryopreservation period
FIGURE 3.
Follow-up Hounsfield unit scores after cranioplasty in pediatric patients. PED: pediatric patient.
Discussion
A cranioplasty is considered for patients who have undergone decompressive craniectomy due to increased intracranial pressure once brain edema has resolved. For cranioplasty, the ideal material required to fill the defect must be viable and easy to handle during surgery. The material should also be stable, radiolucent, and hard enough to be protective. In addition, it should also be aesthetically pleasing, cost effective, with a low infection rate. Other than the autologous bone, hydroxyapatite (HA), metal plates, HA cement, PMMA, and polyethylene have been used to fulfill such requirements.9) By the way, several studies on growth factors that induce bone repair and remodeling have been performed. Insulin like growth factor-1 is known to induce osteoblast reproduction and bone matrix formation, and transforming growth factor-b1 has been associated with osteoblast, chondrocyte, and osteoclast stimulation, and plays an important role in remodeling and fracture healing. These different bone growth factors interact to stimulate bone regeneration. Although not authorized in Korea, growth factors such as recombinant human bone morphogenetic protein-2, currently used in the United States, may be used in defect repair to promote osteoinduction and bone remodeling and formation, thereby reducing graft failure. However, such studies have only been performed in animal models.10,12) Ward16) has outlined the tongue and groove method, which involves drilling down the edges of the donor and recipient bone then overlapping the bone to achieve bone-to-bone contact, in an attempt to maximize osteoblastic ingrowth. Citardi and Friedman4) reported that when using a non-vascularized autologous bone, rigid fixation of the graft is important to minimize graft resorption and induce osteoconduction.
Of these many materials, autologous bone has been favored because of its good aesthetic results due to its perfect fit and lack of introduction of foreign body.8) And since children are in the growing phase, autologous bone graft remains the material of choice. But, the propensity of resorption in cranial autografts, unlike other artificial grafts, seems to be related to the compressive force of the tightened scalp that acts on the convex calvaria. And, unlike other areas where bone is grafted, the current resorption rate of autologous bone graft for cranioplasty is somewhat higher than expected, especially in the pediatric population. Son et al.14) have also reported that the use of autologous bone for delayed cranioplasty following decompressive craniectomy should be reconsidered in light of this resorption, in the pediatric population particulary. In cases of large defects or pediatric patients, the rate of bone resorption is higher and can cause bone breakage requiring re-operation. Healing of bone graft is a complex process requiring revascularization, osteoconduction, osteoinduction, and osteogenesis in phase.14) The bone flap acts as a scaffold into which blood vessels must grow. The osteoprogenitor cells from the recipient bone must also move into the flap, allowing bone resorption to occur and stimulating new bone to form, called creeping substitution.10) Because of its structural integrity, very little bone resorption occurs in the calvaria. However, if the microenvironment of the cranium does not promote sufficient osteogenesis, the imbalance between resorption and osteogenesis leads to bone breakage and graft failure. In conclusion, we believe that the most important key for success after cranioplasty with autograft bone was excellent contiguity between the bone graft and the recipient bone's edge. Therefore, the bone flap's boundary should contact the bone defect as closely as possible, especially among the pediatric population. At our institution, we performed cranioplasties using cryopreserved autologous bone after each bone underwent pressurized heat sterilization. We think that the use of this pressurized heat sterilization was a factor that played a role in the high frequency of bone resorption. As a result, we suggest compressive force of the tightened scalp, young age, large skull defect and heat sterilization of autologous bone as risk factors for bone resorption. However, more research on the relationship between the high rate of bone resorption in children and structural features of the graft site will be needed. Additional studies are needed to confirm the significance of such findings, but we believe that resorption rates in certain groups can be reduced by applying augmentation techniques at the initial cranioplasty.
Conclusion
The cranial environment does not receive enough physiologic stress and therefore osteogenesis is poorly promoted. Using autogenous bone for cranioplasty after decompressive craniectomy can lead to reintegration failure resulting from bone resorption. The resorption rate was greater in children and in patients with large skull defects. As a result, we suggest compressive force of the tightened scalp, young age, large skull defect, the gap between bone flap and bone edge and heat sterilization of autologous bone as risk factors for bone resorption.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469–479. doi: 10.3171/jns.2006.104.4.469. [DOI] [PubMed] [Google Scholar]
- 2.Brooks RA. A quantitative theory of the Hounsfield unit and its application to dual energy scanning. J Comput Assist Tomogr. 1977;1:487–493. doi: 10.1097/00004728-197710000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58(3 Suppl):S25–S46. doi: 10.1227/01.NEU.0000210365.36914.E3. discussion Si-Siv. [DOI] [PubMed] [Google Scholar]
- 4.Citardi MJ, Friedman CD. Nonvascularized autogenous bone grafts for craniofacial skeletal augmentation and replacement. Otolaryngol Clin North Am. 1994;27:891–910. [PubMed] [Google Scholar]
- 5.Dujovny M, Agner C, Aviles A. Syndrome of the trephined: theory and facts. Crit Rev Neurosurg. 1999;9:271–278. doi: 10.1007/s003290050143. [DOI] [PubMed] [Google Scholar]
- 6.Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997;47:238–241. doi: 10.1016/s0090-3019(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 7.Durand JL, Renier D, Marchac D. [The history of cranioplasty] Ann Chir Plast Esthet. 1997;42:75–83. [PubMed] [Google Scholar]
- 8.Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003;52:591–596. doi: 10.1227/01.neu.0000047891.86938.46. discussion 595-596. [DOI] [PubMed] [Google Scholar]
- 9.Lee BS, Min KS, Lee MS, Kim YG, Kim DH. Comparison with subcutaneous abdominal preservation and cryoconservation using autologous bone flap after decompressive craniectomy. Korean J Neurotrauma. 2012;8:21–25. [Google Scholar]
- 10.Oppenheimer AJ, Tong L, Buchman SR. Craniofacial Bone Grafting: Wolff's Law Revisited. Craniomaxillofac Trauma Reconstr. 2008;1:49–61. doi: 10.1055/s-0028-1098963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osawa M, Hara H, Ichinose Y, Koyama T, Kobayashi S, Sugita Y. Cranioplasty with a frozen and autoclaved bone flap. Acta Neurochir (Wien) 1990;102:38–41. doi: 10.1007/BF01402184. [DOI] [PubMed] [Google Scholar]
- 12.Park J, Ries J, Gelse K, Kloss F, von der Mark K, Wiltfang J, et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 13.Prolo DJ, Oklund SA. Composite autogeneic human cranioplasty: frozen skull supplemented with fresh iliac corticocancellous bone. Neurosurgery. 1984;15:846–851. [PubMed] [Google Scholar]
- 14.Son S, Park CW, Kim EY, Kim JM, Yoo CJ. Bone resorption of autologous cranioplasty following decompressive craniectomy in children: case report. J Korean Neurotraumatol Soc. 2009;5:118–123. [Google Scholar]
- 15.Vanaclocha V, Sáiz-Sapena N, García-Casasola C, De Alava E. Cranioplasty with autogenous autoclaved calvarial bone flap in the cases of tumoural invasion. Acta Neurochir (Wien) 1997;139:970–976. doi: 10.1007/BF01411307. [DOI] [PubMed] [Google Scholar]
- 16.Ward GWR. The grove encyclopedia of materials and techniques in art. New York: Oxford University Press; 2008. p. 699. [Google Scholar]