Abstract
Plant-derived natural products are known to have cancer chemo-preventive and chemo-therapeutic properties. Plant extracts or their active constituents are used as folk medicine in traditional therapies by 80% of the world population. The aim of the present study was to determine the anti-proliferative potential of Fumaria vaillantii extracts on three different cancer cell lines including malignant melanoma SKMEL-3, human breast adenocarcinoma MCF-7 and human myelogenous leukemia K562 as well as human gingival fibroblast (HGF) as normal cell line. Anti-proliferative activity was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), flowcytometry and annexin methods. Total phenolics and flavonoids were determined by Folin-Ciocalteu and aluminum chloride methods. Chloroform fraction had the lowest IC50 value at 72 h (0.1 μg/ml) in MCF-7 cells. Flowcytometry and annexin-V analysis indicated that the chloroform fraction induced necrosis in MCF-7 cells. In addition, the colorimetric methods showed that the methanolic fraction possessed the highest amount of total phenolics (33.03 ± 0.75 mg/g of dry powder) and flavonoids (10.5 ± 2.0 mg/g of dry powder). The collective data demonstrated that F. vaillantii chloroform fraction may contain effective compounds with chemo-therapeutic potential act through an apoptotic independent pathway.
Keywords: Fumaria vaillantii, Cancer, Apoptosis, MTT assay, Flowcytometry
INTRODUCTION
Traditional medicine and its relevance to public health have received a great deal of attention in developing countries (1,2). According to World Health Organization (WHO), more than 80% of the people around the world use plant extracts or their active constituents as folk medicine in traditional therapies (3). The advantages of using traditional medicine include dissimilarity, flexibility, easy to approach, relatively low cost, low levels of technological input and increasing economic importance (4). There are reports on more than 150 plant herbs still being used all over the world in daily life to treat different diseases (5).
Fumaria, a well-known herb, has for a long time been recognized for its medicinal properties. The genus Fumaria L. comprises 60 species, mostly native to the Mediterranean region, with only eight species reported in Iran (6). Antihypertensive, diuretic, liver protection, purgative, treatment of gastro-intestinal disorders and abdominal cramps are some of the conventional properties of Fumariaceae family (7,8,9,10,11,12). Fumaria vaillantii, a member of the Fumariaceae family with a wide distribution in Iran, has traditionally been used for treating hepatobiliary, dermatological and gastrointestinal disorders (13).
The growing cost for conventional treatments of cancer has prompted people to depend more on traditional medicine (14). As a therapeutic alternative and a safe choice, herbal medicine might even increase the success rate of most cancer treatments by having a lower systemic toxicity indicated in chemotherapy (15). Despite the afore-mentioned advantages, little is known about the possible medicinal application of these plants or their cytotoxicity (16,17). The present study was conducted to screen and evaluate the anticancer potential of Fumaria vaillantii extract against malignant melanoma cell line SKMEL-3, human breast cancer cell line MCF-7 and human myelogenous leukemia cell line K562.
MATERIALS AND METHODS
Plant material and extraction procedure
F. vaillantii plant was collected from North of Tehran, Iran in August 2014. A voucher specimen (No. 6563 TEH) was deposited at the Herbarium of Faculty of Pharmacy at Tehran University of Medical Sciences and authenticated by Dr. Amin.
The aerial parts of the plant were separated and dried in the dark for 3 days. Total extract was prepared by thoroughly mixing 30 g of dried powder with ethanol: water (80:20) at room temperature through maceration procedure. Furthermore, 30 g of the powder was extracted by solvents with different polarities including hexane, chloroform, ethyl acetate and methanol (Merck, Germany) by maceration. Total extract obtained from dried powder and the yield of extract was 21%. Chloroform, ethyl acetate and methanol fractions also obtained from the dried powder with the yield of 1.6, 2, 0.4 and 6%, respectively.
Two methods for fractionation of the raw plant material have been reported in the literature; one is starting from dried powder and the other from total extract. In the present study, the former method was used to make sure that all compounds have been completely extracted (18,19,20).
Estimation of total phenolics and flavonoids
Phenolic extract of all samples were prepared according to Wang's method (21) with some modifications. Total phenolics and flavonoids content in the phenolic extract were estimated by Folin-Ciocalteu and Aluminium chloride methods, respectively (22,23). The results are expressed as mg of gallic acid and quercetin equivalent/g of dry leaf extract.
Cell culture
The human cancer cell lines SKMEL-3, MCF-7, K562 and HGF were obtained from the National Cell Bank of Pasture Institute of Iran (NCBI). Cells were cultured and routinely maintained in Dulbecco's Modified Eagle Medium (DMEM) or Roswell Park Memorial Institute medium (RPMI) medium (Gibco-BRL, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin (Gibco-BRL, USA) and were incubated at 37°C in a humidified atmosphere containing 5% CO2 inside a CO2 incubator.
In vitro cytotoxicity assay
Total extract and fractions were tested for their cytotoxicity toward cancer and normal cell lines using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, cells were placed in 96-well plates, and sample solutions were added at concentrations ranging from 0.1–250 μg/ml to each well and incubated for 24, 48 and 72 h. Dimethyl sulfoxide (DMSO) (0.5%, Merck, Germany) treated cells serve as the solvent control. Treated cells were incubated with MTT (0.5 mg/ml in phosphate buffered saline) for 4 h at 37°C. The medium was removed and dye crystal formazan was solubilized in DMSO. The absorbance was measured at 545 nm. The 50% inhibitory concentration (IC50) value, defined as the amount of extract that inhibits 50% of cell growth, was calculated from concentration-response curves following a 24, 48 and 72 h exposure periods. Three independent experiments, performed in triplicate, were used for these calculations.
Flowcytometric analysis
Cell cycle phase distribution was determined by analytical DNA flowcytometry. SKMEL-3, MCF-7 and K562 cells were incubated for 72 h with 1.5, 0.05 and 3.5 μg/ml of chloroform fraction, respectively. Cells were harvested and adjusted to 106 cells/plate in 6-well plates (SPL, Korea) and stained with propidium iodide (PI) (Sigma-Aldrich, USA) reagent at 37°C for 15 min in the dark. PARTEC flowcytometer (Partec GmbH, Munster, Germany) with Flowjo software v.10 was used to analyze DNA content using UV light.
Identification of apoptosis by PI-Annexin-V staining
This assay was performed to detect apoptosis using an annexin-V-FLUOS apoptosis detection kit, following the instructions provided by the manufacturer. In brief, harvested cells were resuspended in 100 μl of the annexin-V-FLUOS labeling solution containing 2 μl annexin-V-FLUOS labeling agent, 2 μl PI solution and 1 ml incubation buffer to achieve a concentration of 106 cells/ml and incubated at 37°C. Finally, each tube was diluted with buffer before the cells were analyzed by a flowcytometer. All extracts were treated at ½ IC50 concentrations for both flowcytometry and annexin-V assays. This concentration was selected to observe the possible mechanism of apoptosis or necrosis induced by the extract (24).
Statistical analysis
Statistical analysis among groups was performed using multiple comparisons by one-way ANOVA followed by Tukey's post hoc test. All data are presented as arithmetic mean test. All data are presented as arithmetic mean ± S.E.M of at least triplicate determinations. Significance was accepted at P<0.05.
RESULTS
Total phenolics and flavonoids content
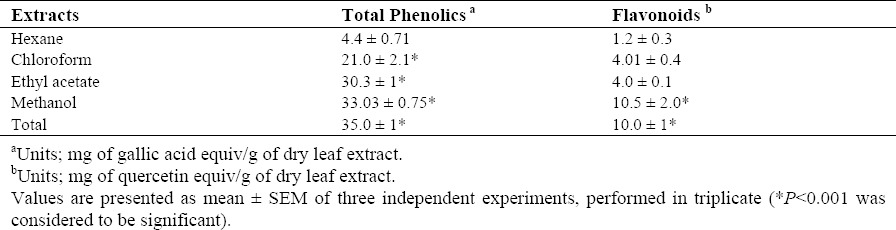
Total phenolics and flavonoids of total extract and different fractions obtained from F. vaillantii were reported in Table 1. Due to the polar nature of methanol, the amount of phenols and flavonoids in the methanolic fraction were significantly higher than hexane and chloroform fractions. Ethyl acetate fraction contained a considerable amount of total phenolics, but it did not reach to the quantity of the methanolic farction. As shown in Table 1, all fractions contained a certain amount of total phenolics and flavonoids, but methanolic fraction possessed the maximum quantity. These findings are in agreement with previous studies indicating presence of the phenolic compounds in the most species of this family (9).
Table 1.
Contents of total phenolics and flavonoids in aerial parts of Fumaria vaillantii extracts.
Cytotoxic activity of F. vaillantii extracts
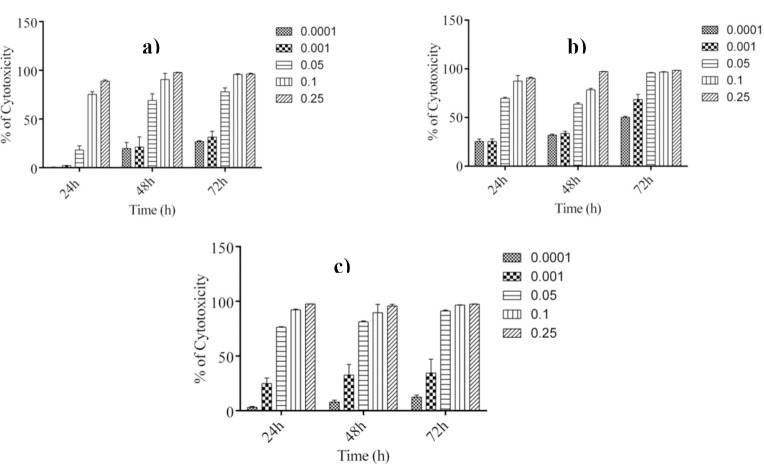
To screen the growth inhibitory activity of total extract and various fractions from aerial parts of F. vaillantii against different cancer cell lines including SKMEL-3, MCF-7 and K562 cells, cultures of the three cell lines were incubated in the absence and presence of 0.1–250 μg/ml of extracts for 24, 48 and 72 h. The extracts caused cell toxicity in a concentration-dependent manner (Fig. 1).
Fig. 1.
Concentration-dependent cytotoxicity of chloroform fraction in a; malignant melanoma (SKMEL-3), b; breast adenocarcinoma (MCF-7) and c; human myelogenous leukemia (K562) cells. Anti-proliferative activity of the fractions was evaluated after 24, 48 and 72 h of treatments. Values are presented as mean ± SEM of three independent experiments, performed in triplicate.
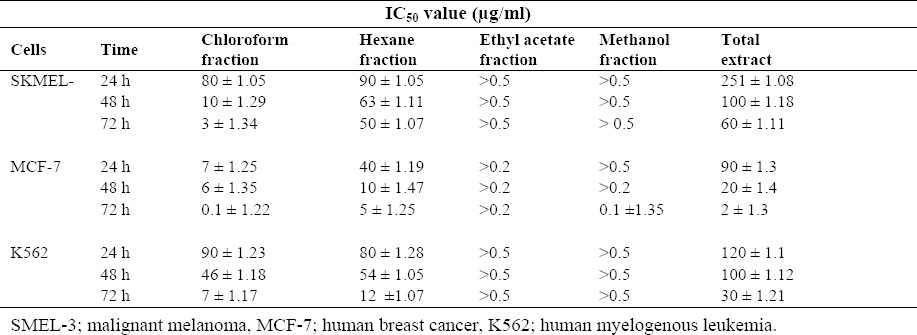
IC50 values for various extracts in three different cell lines were calculated and reported in Table 2. In order to evaluate cell toxicity of the chloroform fraction in normal cell line, the viability assay of this fraction was performed on HGF (human gingival fibroblast) after 72 h incubation. The IC50 value of the chloroform fraction against HGF cells [IC50 = 20 (8 - 30) μg/ml] was higher in comparison to cancer cell lines.
Table 2.
IC50 values (μg/ml) for anti-proliferative activity of different extracts towards SKMEL-3, MCF-7 and K562 cells. Values are presented as Mean ± SEM of three independent experiments performed in triplicate.
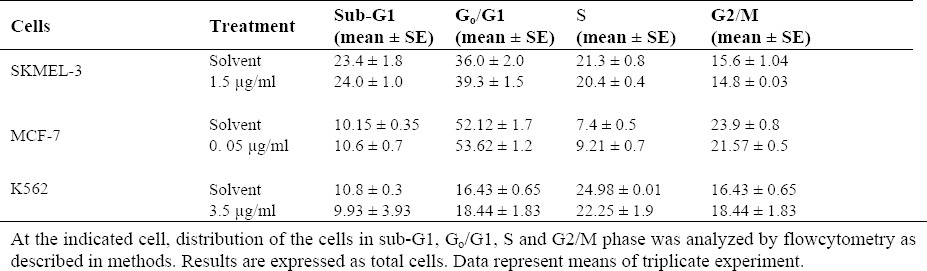
Effect of F. vaillantii chloroform fraction on cell cycle progression
To determine the ability of the most effective fraction (i.e., chloroform) of F. vaillantii to induce SKMEL-3, MCF-7 and K562 cell cycle arrest, flowcytometric analysis of DNA content arrest, flowcytometric analysis of DNA content and cell cycle distribution was performed. Cultures of the three cell lines were incubated with ½ IC50 concentration of the chloroform fraction for 72 h, stained with PI and analyzed by flowcytometry in order to determine the total population distribution in the different phases of G0/G1, S, and G2/M. Control cells were treated only with the highest amount of DMSO used in the experiments proceeded through a normal cell cycle. When SKMEL-3 cells were treated with 1.5 μg/ml of chloroform fraction, an insignificant increase in population of sub-G1 cells from 23.4 ± 1.8 % for control to 24.0 ± 1.0 % after treatment was detected. In addition, treatment of MCF-7 cells with 0.05 μg/ml of chloroform fraction led to an inconsiderable enrichment of sub-G1 cell population from 10.15 ± 0.35 % for control to 10.6 ± 0.7 % after treatment. The same occurred after treatment of K562 cells with ½ IC50 concentration of chloroform fraction. Moreover, cell population in G0/G1 exhibited no significant change for the three treated cancer cell lines (Table 3).
Table 3.
Effect of chloroform fraction on cell cycle progression with respect to solvent-control on SKMEL-3, MCF-7 and K562 cell lines.
Induction of apoptosis or necrosis by chloroform fraction of F. vaillantii
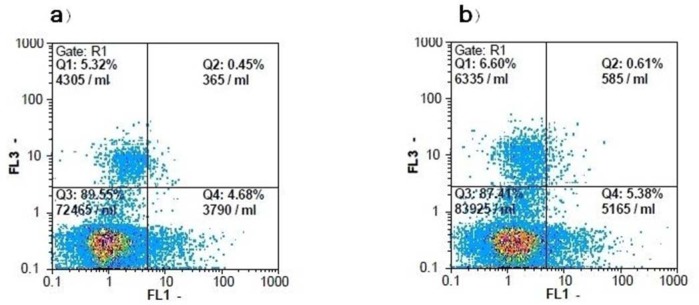
To detect a correlation between the cytotoxic effect of F. vaillantii chloroform fraction on MCF-7 cells and apoptotic cell death, phosphatidylserine exposure as an early marker of apoptosis was evaluated using annexin-V/PI double staining. To do so, MCF-7 cells were treated with chloroform fraction at ½ IC50 for 72 h. As shown in Table 4, in the DMSO-treated cells, 89.4 ± 1.7% of cells were viable, while 5.5 ± 0.6% were in the early and 0.3 ± 0.1% were in the late stages of apoptosis. After a 72 h treatment with 0.05 μg/ml of F. vaillantii chloroform fraction, the number of annexin V+/PI- and annexin V+/PI+ cells did not increase significantly, while the population of necrotic cells increased by 1.74%. These results are suggestive of a necrotic response induced by the chloroform fraction (Fig. 2).
Table 4.
Percentage of MCF-7 cells in each state after treatment with solvent and chloroform fractions at 0.05 μg/ml for 72 h of incubation.

Fig. 2.
Flowcytometric analysis of PI-annexin-V to quantify chloroform fraction-induced apoptosis in MCF-7 cells. a; Dot plot of MCF-7 cells with DMSO treatment, b; Dot plot of MCF-7 cells were treated with chloroform fraction at 0.05 μg/ml for 72 h. The results shown are representative of three independent experiments. Quadrant 3; living cells An−/PI−, Quadrant 4; early apoptotic cells An+/PI−, Quadrant 2; late apoptotic cells An+/PI+, Quadrant 1; necrotic cells An−/PI+.
DISCUSSION
Hundreds of years, plants have been used to treat diseases worldwide. Nowadays, traditional herbal medicine is a favorable alternative to chemotherapy, and basic research has opened the doors for scientists in the field of phytomedicine (25). Most of the current chemopreventive agents are nonspecific and cytotoxic for both cancerous and normal cells leading to various side effects (26). But the use of plant derived agents reduces this risk and it is more specific to the cancer cells.
F. vaillantii, commonly known as “Shahtarah” in Persian herbal medicine, possesses antioxidant, anti-inflammatory and cytotoxic effects (27). It has been reported that these properties are mostly due to the presence of phenolics and alkaloids (28). Furthermore, aqueous, ethanol, chloroform and hexane extracts of F. indica were analyzed and found that diverse secondary metabolites containing flavonoids and alkaloids were present in all the extracts. Interestingly, among them, hexane and chloroform extracts showed the highest cytotoxic property (29).
In this study, the antiproliferative activity of F. vaillantii extracts on MCF-7, SKMEL-3 and K562 cells was examined. To do this, the obtained fractions as well as total extract were tested on three cell lines using MTT assay at 24, 48 and 72 h. Considerable differences in sensitivity of the cell lines and fractions were evident. In addition, there was a significant difference between 24, 48 and 72 h treatments of the three cell lines with various fractions. MCF-7 cells showed a better inhibitory activity toward different fractions and chloroform fraction indicated more remarkable anti-proliferative efficiency at 72 h. Importantly, the noncancerous cell line, HGF was not susceptible to this extract showing selectivity between cancer and non-cancer cells. Based on the data reported by Gao and colleagues, Kim and colleagues and Shi and coworkers (30,31,32,33) ethanolic extract of some genera of this family including Corydalis yanhusuo and Corydalis heterocarp exhibited anti-metastatic and anti-proliferative activity against different cancerous cell lines. Our observed results are similar to the previous results confirming anti-cancer activity of the extracts obtained from F. vaillantii. However, the chloroform fraction indicated a very striking IC50 value among other fractions.
Alteration of DNA content induced by disturbance of cell cycle progression plays a pivotal role in the proliferation of cancer cells (34). Therefore, to explore if F. vaillantii chloroform fraction caused antiproliferative activity through change in cell cycle distribution in MCF-7, SKMEL-3 and K562 cells, a standard PI-staining protocol was applied to the treated cells. Our results revealed that cytotoxic effects are independent of the cell cycle blockage. Furthermore, it has been previously demonstrated that induction of apoptosis is one of the mechanisms for the anticancer activities of extracts in different cell lines (24). To validate the possible apoptosis occurred in MCF-7 cells were treated with chloroform fraction at 72 h, annexin V/PI profiles of flowcytometry were generated. Our results showed that chloroform fraction caused cell death not via apoptosis, but by other mechanisms containing necrosis.
Since plant phenolics constitute one of the major groups of compounds and have a protective role in carcinogenesis, it was reasonable to determine their total amounts in various leaf extracts to discover if there is any correlation between anticancer properties and the amount of phenolic compounds (18). According to the amount of total phenolic compounds in different fractions, no obvious correlation was found between phenolics and anti-proliferative activity in our study.
On the other hand, previous studies showed that Fumaria species contain some types of alkaloids with various structures possessing antiproliferative activity (35,36). These reports are in line with our obtained results suggesting a possible role of alkaloids present in chloroformic fraction, which are responsible for its considerable cytotoxic effect.
CONCLUSION
Taken together, our data suggest that F. vaillantii chloroform fraction can induce cell death possibly by necrosis. This is very promising, since it indicates that the chloroform fraction of F. vaillantii may contain molecules that can be potentially used in cells death other than flavonoids or phenolic compounds. Also, it exhibited increased toxicity towards cancer cell lines, including breast cancer cells.
ACKNOWLEDGEMENTS
This research was supported by Pasteur Institute of Iran. The authors greatly appreciate Dr. Gholamreza Amin (School of Pharmacy, Tehran University of Medical Sciences) for his kind cooperation in collecting and identifying the plant specimen.
REFERENCES
- 1.Tachjian A, Maria V, Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol. 2010;55:515–525. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran P, Govindarajan P. Cancer an ayurvedic perspective. Pharmacol Res. 2005;51:19–30. doi: 10.1016/j.phrs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Shaik D, Malika FA, Rafi SM, Naqui B. Studies of antibacterial activity of ethanolic extract from Nericum indicum and Hibiscus rosasinensis. J Islamic Acad Sci. 1994;7:167–168. [Google Scholar]
- 4.Nembo NE, Dimo T, Sylvain O, Bopda M, Hescheler J, Nguemo F. The proliferative and chronotropic effects of Brillantaisia nitens Lindau (Acanthaceae) extracts on pluripotent stem cells and their cardiomyocytes derivatives. J Ethnopharmacol. 2014;156:73–81. doi: 10.1016/j.jep.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Irmaileh BE, Afifi FU. Herbal medicine in Jordan with special emphasis on commonly used herbs. J Ethnopharmacol. 2003;3:193–197. doi: 10.1016/s0378-8741(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 6.Heydari Nasrabadi M, Aboutalebi H, Naseri M. Effect of Fumaria parviflora alcoholic extract on male rat's reproductive system. J Med Plants Res. 2012;6:2004–2010. [Google Scholar]
- 7.Ozalson M. A Comparison of Fumaria parviflora Lam and Momordica balsamina Linn. Hepatoprotection. Pak J Bio Sci. 2011;14:1034–1035. doi: 10.3923/pjbs.2011.1034.1035. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi M, Singh BK, Raisuddin S, Kakkar P. Abrogation of nimesulide induced oxidative stress and mitochondria mediated apoptosis by Fumaria parviflora Lam. Extract. J Ethnopharmacol. 2011;136:94–102. doi: 10.1016/j.jep.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Orhan IE, Sener B, Musharraf SG. Antioxidant and hepatoprotective activity apprasial of four selected Fumaria species and their total phenol and flavonoid quantities. Exp Toxicol Pathol. 2012;64:205–209. doi: 10.1016/j.etp.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Suau R, Cabezudo B, Rico R, Najera F, Lopez-Romero M. Direct determination of alkaloid contents in Fumaria species by GC – MS. Phytochem Analysis. 2002;13:363–367. doi: 10.1002/pca.669. [DOI] [PubMed] [Google Scholar]
- 11.Tripathi M, Singh BK, Raisuddin S, Kakkar P. Involvement of mitochondria mediated pathway in hepathoprotection conferred by Fumaria parviflora Lam.extract against nimesulid induced apoptosis in vitro . Toxicol In Vitro. 2010;24:495–508. doi: 10.1016/j.tiv.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Gilani AH, Janbaz KH, Akhtar MS. Selective protective effect of an extract from Fumaria parviflora on paracetomal induced hepatotoxicity. Gen Pharmacol. 1996;27:979–983. doi: 10.1016/0306-3623(95)02140-x. [DOI] [PubMed] [Google Scholar]
- 13.Ebrahimzadeh F, Keshavarzi M, Sheidaii M, Ghadam P. Fruit and seed morphology of the Fumaria L.species (Papaveraceae) of Iran. Turk J Bot. 2011;35:167–173. [Google Scholar]
- 14.Sheldon JW, Balick MJ, Laird SA. Advances in economic botany, The New York Botanical Garden. New York: Bronx; 1997. Medicinal plants: can utilization and conservation coexist? [Google Scholar]
- 15.Gao Y, Su Y, Qu L, Xu S, Meng L, Cai SQ, et al. Mitochondrial apoptosis contributes to the anti-cancer effect of Smilax glabra Roxb. Toxicol Lett. 2011;207:112–120. doi: 10.1016/j.toxlet.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Mahasneh AM, El-Oqlah AA. Antimicrobial activity of extracts of herbal plants used in the traditional medicine of Jordan. J Ethnopharmacol. 1999;64:271–276. doi: 10.1016/s0378-8741(98)00132-9. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hussaini R, Mahasneh AM. Microbial growth and quorum sensing antagonist activities of herbal plants extracts. Molecules. 2009;9:3425–3435. doi: 10.3390/molecules14093425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.M, Majd A, Sepahdar Z, Azadmanesh K, Irian S, Ardestaniyan MH, et al. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm Biol. 2012;50:1416–1422. doi: 10.3109/13880209.2012.682118. [DOI] [PubMed] [Google Scholar]
- 19.Salimi M, Ardestaniyan MH, Mostafapour Kandelous H, Saeidnia S, Gohari AR, Amanzadeh A, et al. Anti-proliferative and apoptotic activities of constituents of chloroform extract of Juglans regia leaves. Cell Prolif. 2014;47:172–179. doi: 10.1111/cpr.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mostafapour Kandelous H, Salimi M, Khuri V, Rastkari N, Amanzadeh A, Salimi M. Mitochondrial apoptosis induced by Chamaemelum nobile extract in breast cancer cells. Iran J Pharm Res. 2015 In press. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YJN, Shi GL, Zhao LL, Liu SQ, Yu TQ, Clarke SR, et al. Acaricidal activity of Juglans regia leaf extracts on Tetranychusviennensis and Tetranychuscinnabarinus (Acari: Tetranychidae) J Econ Entomol. 2007;100:1298–1303. doi: 10.1603/0022-0493(2007)100[1298:aaojrl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Porter LJ, Hrstich LN, Chan BG. The conversion of procyanidin and prodelphinidins to cyaniding and delphindin. Phytochemistry. 1986;25:223–230. [Google Scholar]
- 23.Makkar HPS, Blummel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agr. 1993;61:161–165. [Google Scholar]
- 24.Gudarzia H, Salimi M, Irian S, Amanzadeh A, Mostafapour Kandelous H, Azadmanesh K K, Salimi M. Ethanolic extract of Ferula gummosa is cytotoxic against cancer cells by inducing apoptosis and cell cycle arrest. Nat Prod Res. 2015;29:546–550. doi: 10.1080/14786419.2014.951854. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Park SY, Lee HM, Seo DI, Kim YM. Antiproliferative effect of the methanol extract from the roots of Petasites japonicus on Hep3B hepatocellular carcinoma cells in vitro and in vivo. Exp Ther Med. 2015;9:1791–1796. doi: 10.3892/etm.2015.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plackal Adimuriyil George B, Tynga IM, Abrahamse H. In Vitro antiproliferative effect of the acetone extract of Rubus fairholmianus Gard. Root on human colorectal cancer cells. Biomed Res Int 2015. 2015 doi: 10.1155/2015/165037. ID 165037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava S, Choudhary GP. Pharmacognostic and pharmacological study of Fumaria vaillantii Loisel: a review. J Pharmacogn Phytochem. 2014;3:194–197. [Google Scholar]
- 28.Ivanov IG, Vrancheva RZ, Marchev AS, Petkova NT, Aneva IY, Denev PP, et al. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int J Curr Microbiol. Appl Sci. 2014;3:296–306. [Google Scholar]
- 29.Shambaleed H, Muhammad I, Barkatullah, Imtiaz A. Comparison of three extracts of Fumaria indica for the evaluation of cytotoxic and phytotoxic activities. IntJ Biosci. 2012;2:112–119. [Google Scholar]
- 30.Gao JL, Shi JM, He K, Zhang QW, Li SP, Lee SM, et al. Yanhusuo extract inhibits metastasis of breast cancer cells by modulating mitogen-activated protein kinase signaling pathways. Oncol Rep. 2008;20:819–824. [PubMed] [Google Scholar]
- 31.Gao JL, Shi JM, Lee SM, Zhang QW, Wang YT. Angiogenic pathway inhibition of Corydalis yanhusuo and berberine in human umbilical vein endothelial cells. Oncol Res. 2009;17:519–526. doi: 10.3727/096504009789745575. [DOI] [PubMed] [Google Scholar]
- 32.Shi J, Zhang X, Ma Z, Zhang M, Sun F. Characterization of aromatase binding agents from the dichloromethane extract of Corydalis yanhusuo using ultrafiltration and liquid chromatography tandem mass spectrometry. Molecules. 2010;15:3556–3566. doi: 10.3390/molecules15053556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YA, Kon CS, Yea SS, Seo Y. Constituents of Corydalis heterocarpa and their anti-proliferative effects on human cancer cells. Food Chem Toxicol. 2010;48:722–728. doi: 10.1016/j.fct.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Pieme CA, Santosh G, Tekwu E, Askun T, Aydeniz H, Ngogang J, et al. Fruits and barks extracts of Zanthozyllum heitzii a spice from Cameroon induce mitochondrial dependent apoptosis and Go/G1 phase arrest in human leukemia HL-60 cells. Biol Res. 2014;47:1–13. doi: 10.1186/0717-6287-47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi WY, Jin CY, Han MH, Kim GY, Kim ND, Lee WH, et al. Sanguinarine sensitizeshuman gastric adenocarcinoma AGS cells to TRAIL-mediated apoptosis via downregulation of AKT and activation of caspase-3. Anticancer Res. 2009;29:4457–4465. [PubMed] [Google Scholar]
- 36.Burgeiro A, Bento AC, Gajate C, Oliveira PJ, Molliendo F. Rapid human melanoma cell death induced by sanguinarine through oxidative stress. Eur J Pharmacol. 2013;705:109–118. doi: 10.1016/j.ejphar.2013.02.035. [DOI] [PubMed] [Google Scholar]