Abstract
It has been reported that Rosa damascena hydroalcoholic extract has inhibitory effect at higher concentration but stimulatory action at lower concentrations on ileum. This could be due to the presence of stimulatory components in the extract. R. persica Mich. is another species which belongs to Rose family but so far there is no report about pharmacological action of its extract. Therefore, the objective of this research was to investigate inhibitory effect of hydroalcoholic and hexane extract of this plant on ileum contraction to see which type of extract would be more appropriate as antispasmodic agents. Hydroalcoholic and hexane extracts were prepared by percolation method. A section of rat ileum was suspended in an organ bath containing Tyrode's solution. The tissue was kept under 1 g tension at 37°C and continuously gassed with O2. Effects of R. persica extracts or vehicle were studied on ileum contractions induced by electrical field stimulation (EFS), KCl or acetylcholine (ACh) and compared with that of atropine. Hydroalcoholic extracts of R. persica (10-640 μg/ml) concentration dependently inhibited ileum contraction induced by KCl (IC50 = 244 ± 35 μg/ml), ACh (IC50 = 129 ± 7.4 μg/ml) and EFS (IC50 = 172 ± 18.7 μg/ml). Hexane extract of R. persica (10-320 μg/ml) concentration dependently inhibited ileum contraction induced by KCl (IC50 = 117 ± 12.4 μg/ml), ACh (IC50 = 78 ± 9.1 μg/ml) and EFS (IC50 = 67 ± 10.5 μg/ml). Atropine only inhibited the responses to ACh and EFS. The vehicle had no significant effect on ileum contractions. From this experiment it was concluded that R. persica extract have inhibitory effect on rat isolated ileum. Therefore, separation and identification of active component is recommended.
Keywords: Rosa persica, Hydroalcoholic extract, Hexane, Atropine, Ileum
INTRODUCTION
Diarrhoea is a common disease and a major cause of death in infants and children in the tropics (1). In order to combat the problem of diarrhea globally, the World Health Organization in its Diarrhoeal Disease Control Program, has given a special emphasis on the use of traditional medicine in the control and management of diarrhea (1). Gastrointestinal (GI) system is one of the major target organs for herbal medicines particularly in the treatment of GI motility. Plant extracts have been used as medicine for centuries and they are origin of some clinically useful drugs (2).
Genus Rosa which belongs to family of Rosaceae is reported to have many medicinal properties (3). For example dried flower of Rosa damascena are believed to assist conditions like frigidity, chronic bronchitis, asthma, skin disease, cancer, ulcers, wounds, wrinkles, infections, as well as constipation (4).
Rosa damascena flower are particularly used as folk medicine for GI disorders including antispasmodic action (5,6). Pharmacological studies have revealed that R. damascena extract has both stimulatory and inhibitory effects on isolated rat ileum (7,8) and that explain why this plant are suggested to be used for treatment of both constipation and diarrhoea. However, the proportion of plant constituents is not constant and varies annually from plant to plants. Therefore, if other species of Rosa genus could be found that mainly have inhibitory action on ileum without stimulatory potential it would be a more appropriate antispasmodic remedy.
R. persica Mich. is a native Iranian plant which also belongs to Rosaceae family. This plant grows wildly in all over of Iran and known as local name of Varak (9). However, so far there is no report on pharmacological activities of this species of Rosa. Therefore, the main aim of this research was to investigate the effect of R. persica hydroalcoholic and hexane extracts on isolated ileum, in order to find any excitatory or inhibitory effect it may have on intestinal motility. If one of these extracts show inhibitory effect on ileum contraction without significant stimulatory effect it would have an advantage over R. damascena.
MATERIALS AND METHODS
Plant materials
Flower of R. persica was collected in May 2014 from Deliajn (Iran) and identified by the botanist, Dr. Godarzi, in Research Institute of Jahad and Agricultures in Arak province. A voucher specimen was authenticated and then deposited in the herbarium of the Faculty of Pharmacy and Pharmaceutical Sciences (herbarium number: 3497). The flowers were dried in shade and powdered using electrical miller (Moulinex, France). The total hydroalcoholic extract was obtained by percolation (10) using 70% ethanol with solvent to plant powder ratio of 8:1. The percentage yield of the dried extract was obtained following evaporating the solvents. The hexane extract was obtained by percolation using solvent to plant ration of 10:1 (10).
Drugs and solutions
Tyrode's solution composed of (mM): NaCl, 136.9; KCl, 2.68; CaCl2, 1.8; MgCl2, 1.05; NaHCO3, 11.9; NaH2PO4, 0.42 and glucose, 5.55, was made up in distilled water. The hydroalcoholic extract was made up as 50 mg/ml stock solution in dimethyl sulfoxide (DMSO), dilution being made in 50% DMSO. Hexane extract was made up as stock solution of 20 mg/ml in DMSO and further serial dilution was prepared in 50% DMSO. Atropine sulphate (1 mM) and KCl (2 M) stock solutions were made up in distilled water. Acetylcholine (ACh) hydrochloride (Sigma, Germany) was made up as 100 mM stock solution and acidified by 1% acetic acid, and further serial dilution was made in distilled water. Unless stated, all chemicals and drugs were from Merck Co. (Germany).
Pharmacological studies
Male Wistar rats (Bred in School of Pharmacy animal house, Isfahan-Iran), weighing 180–250 g were killed killed in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals, as recommended by Isfahan University of Medical Science (11). Following opening of abdomen a piece of ileum was cut out and placed in oxygenated Tyrode's solution at room temperature. The ileum was then further cut to 2-3 cm long strips and mounted in an organ bath (Harvard, England) filled with Tyrode's solution at 37°C and continuously gassed with O2. Longitudinal isotonic contraction of the ileum was recorded under 1 g weight tension on a Harvard Universal Oscillograph (England) pen recorder device.
Following mounting the tissue into the organ bath the physiograph was calibrated according to the equipment manual. Then the tissue was subjected to repeated wash and allowed to relax into a stable baseline. When a basal tension was established, the ileum was contracted by either application of electrical field stimulation (EFS) or addition of ACh or KCl.
After an equilibration period, effect of the extracts were determined on above induced contractions using two fold increments in concentration until a full concentration response effect was achieved. The extract effects were evaluated after at least 10 min contact with the tissue. The extract concentrations were selected following a series of initial pilot experiments. All experiments were performed alongside time-matched vehicle treated controls with the tissue from same animal.
Relaxant effect of extracts on EFS
In the case of EFS, the ileum was contracted by applying rectangular pulses of electrical charge using trains of 6 V and 50 Hz from a stimulator for 1 s duration. The EFS was delivered at 15 min intervals to the ileum via a couple of platinum electrodes wires placed on either side of the ileum inside the organ bath. In order to establish a consistent response, initially 3 to 6 successive stimulation pulses were delivered. After completion of contractile response the tissue was washed with fresh Tyrode's solution. Initially a couple of control contractions were performed and then first concentration of extracts or vehicle was added with at least 10 min equilibration time. All the drugs and extracts were added directly into the bath.
Relaxant effect of extracts on acetylcholine
In another group of experiments, contraction was induced by addition of ACh (0.5 μM). ACh was in contact with the tissue for 30 s before it was washed off with fresh Tyrode's solution. The contractile response to ACh was determined at least 10 min after addition of the extracts. As above, the effect of extracts or vehicle, were examined in non-cumulative manner.
Relaxant effect of extracts on KCl
In separate sets of experiments contraction was induced in rat ileum by addition of KCl (80 mM) into the bath. After equilibration period of 15 min, effect of the extracts or vehicle were determined on above induced contractions using two fold increments in concentration until a full concentration response effect was achieved. Extract effects were evaluated after at least 10 min contact with the tissue without washing the tissue (cumulative manner).
Relaxant effect of atropine
In this research atropine was used as a standard drug. Effect of atropine was examined on contraction induced by KCl, ACh and EFS for comparison with the extracts. Effects of the extracts, atropine or vehicle was examined on at least 6 different tissues (n = 6).
Measurements and statistical analysis
The contractile response to extract was measured relative to tissue baseline and expressed as the percentage of the initial contraction for each ileum. All the values are expressed as mean ± standard error of the mean (SEM). Whenever appropriate, the IC50 value (drug concentration causing 50% of maximum response), was calculated.
Statistical significance was assessed using a one-way analysis of variance (ANOVA) for repeated measures and when appropriate were compared with the time-matched vehicle treated control groups using unpaired Student's t-test. Differences was considered statistically significant for P<0.05. SigmaPlot computer (version 11) software was used for statistical analysis and calculation of IC50.
RESULTS
Plant materials analysis
The yields of the hydroalcoholic and hexane extracts were 24.3% and 0.5% (w/w) respectively. Dried hydroalcoholic extract had brownish colour while the hexane extract had green colour.
Relaxant effect of extracts on EFS
Application of EFS induced a fast contraction which then partially relaxed toward the baseline and few seconds later followed by another contraction which lasted few minutes longer and then gradually disappeared. The EFS responses were the same as the biphasic response reported before (7,12).
Direct addition of the hydroalcoholic or hexane extracts of R. persica, with concentrations range of 10 to 640 μg/ml, produced no contractile activity in rat isolated ileum suspended in the organ bath. However, both extracts had pronounced inhibitory effect on EFS responses. The inhibitory effect on hexane extract was observed with bath concentration of 20 μg/ml and total inhibition was achieved with bath concentration of 160 μg/ml (Figs. 1 and 2). The IC50 values for first and second EFS responses were 67 ± 10.5 μg/ml and 50 ± 8.8 mg/ml respectively.
Fig. 1.
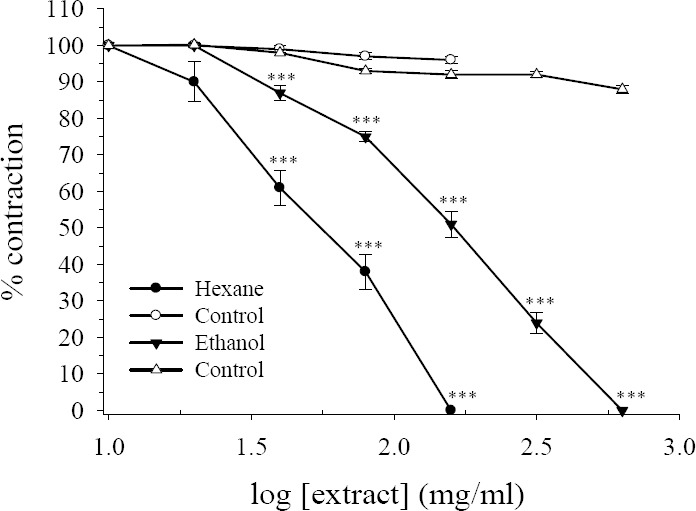
Effect of Rosa persica hydroalcoholic and hexane extracts on tension development to first contractile responses to electrical field stimulation (EFS, 6 V, 50 Hz, 1 s duration) in isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of the extract. Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6). There was no statistically difference in vehicle treated time-matched control tissues contractions over the course of study (ANOVA). Asterisks show statistically significant differences in EFS responses in comparison with its corresponding time-matched vehicle treated control tissues ***P<0.001 (Student's t-test). Maximum concentration of vehicle (DMSO) in the bath was 1.28% and 1.6% for hexane and hydroalcoholic extract respectively.
Fig. 2.
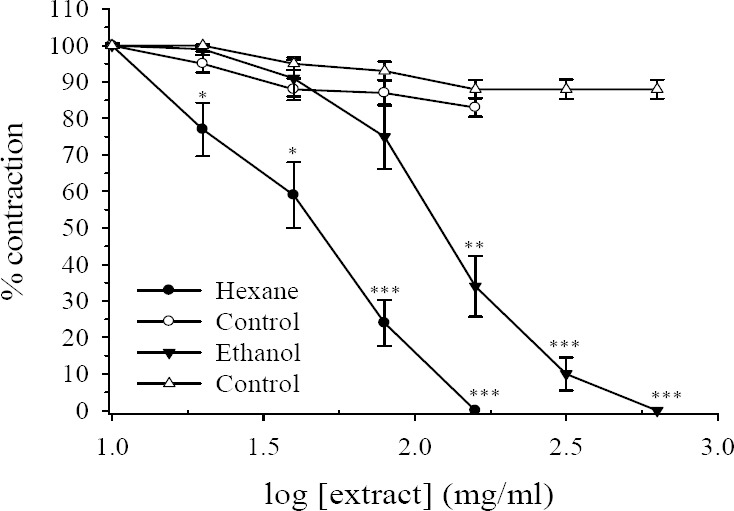
Effect of Rosa persica hydroalcoholic and hexane extracts on tension development to second contractile responses to electrical field stimulation (6 V, 50 Hz, 1 s duration) in isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of the extract. Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6). There was no statistically difference in vehicle treated time-matched control tissues contractions over the course of study (ANOVA). Asterisks show statistically significant differences in electrical field stimulation responses in comparison with its corresponding time-matched vehicle treated control tissues ***P<0.001 (Student's t-test). Maximum concentration of vehicle (DMSO) in the bath was 1.28% and 1.6% for hexane and hydroalcoholic extract respectively.
The hydroalcoholic extract also concentration-dependently inhibited the contractile responses to EFS but at higher concentration. The inhibitory effect of hydroalcoholic extract was started at bath concentration of 40 μg/ml and at concentration of 160 μg/ml still about 51% and 34% of initial responses remained. At bath concentration of 640 μg/ml both EFS responses were completely inhibited (Figs. 1 and 2). The IC50 values for the first and the second EFS responses were 172 ± 18.7 μg/ml and 123 ± 18.9 μg/ml respectively. In the time-matched vehicle treated controls there was no reduction in either EFS response over the course of studies (Figs. 1 and 2).
Relaxant effect of extracts on ACh
Addition of ACh into the bath produced a rapid contraction which reached it peaked within 30 s of the contact time. Following washing the tissue with fresh Tyrode's solution the tissue rapidly relaxed toward the baseline. Addition of 0.5 μg/ml ACh at 10 min intervals produced consistent responses. Both the hexane and hydroalcoholic extracts of R. persica in a concentration-dependent manner attenuated the amplitude of contractile responses induced by ACh. The inhibitory effect of the extracts was started with bath concentration of 40 μg/ml and complete inhibition was observed at bath concentration of 160 μg/ml and 640 μg/ml respectively for the hexane and hydroalcoholic extracts (Fig. 3). For the hydroalcoholic extract with 160 μg/ml bath concentration still 33% of initial contraction remained (Fig. 3). The IC50 values for the hexane and the hydroalcoholic extract were 78 ± 9.1 μg/ml and 129 ± 7.4 μg/ml respectively. There was no reduction in response of tissue treated with equivalent volume of the vehicle (DMSO) over course of the studies (Fig. 3).
Fig. 3.
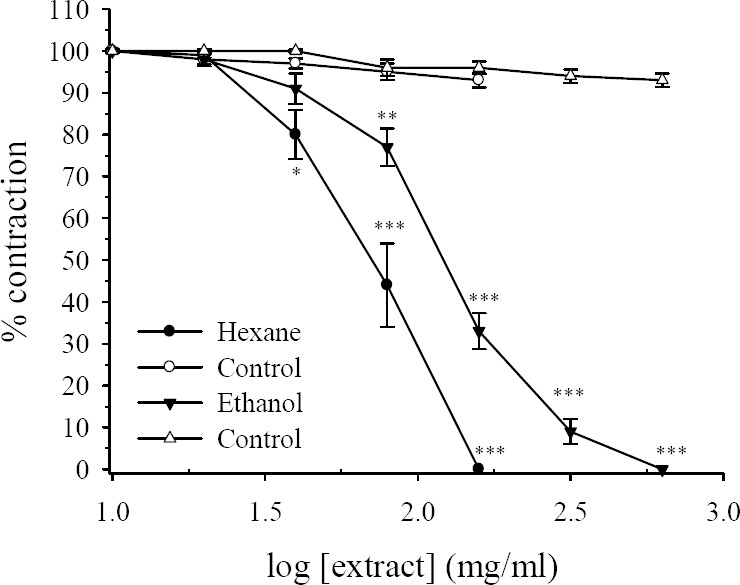
Effects of Rosa persica hydroalcoholic and hexane extracts on tension development to acetylcholine (0.5 μM) in the isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of the extract. Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6). There was no statistically difference in vehicle treated time-matched control tissues contractions over the course of study (ANOVA). Asterisks show statistically significant differences in acetylcholine responses in comparison with its corresponding time-matched vehicle treated control tissues *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Maximum concentration of vehicle (DMSO) in the bath was 1.28% and 1.6% for hexane and hydroalcoholic extract respectively.
Relaxant effect of extracts on KCl
Addition of KCl (80 mM) into the organ bath caused a rapid contraction followed by tonic contraction which maintained during the course of experiments. Cumulative addition of R. persica extracts concentration-dependently attenuated the tonic contraction induced by KCl with IC50 =117 ± 12.4 μg/ml and IC50 = 244 ± 35.3 μg/ml for hexane and hydroalcoholic extract respectively (Fig. 4). For the hexane extract relaxation of the ileum began with 20 μg/ml extract in the bath, while the inhibitory effect of the hydroalcoholic extract was seen with 80 μg/ml bath concentration (Fig. 4). For comparison, at bath concentration of 320 μg/ml the hexane extract abolished the tonic contraction to KCl while, with this concentration of hydroalcoholic extract still 28% of tonic contraction remained. Cumulative addition of equivalent volume of the vehicle (DMSO) reduced the tonic contraction of KCl by 30% and 24% for ethanol and hexane extract of R. persica respectively (Fig. 4) at its highest used concentration (P<0.001, ANOVA).
Fig. 4.
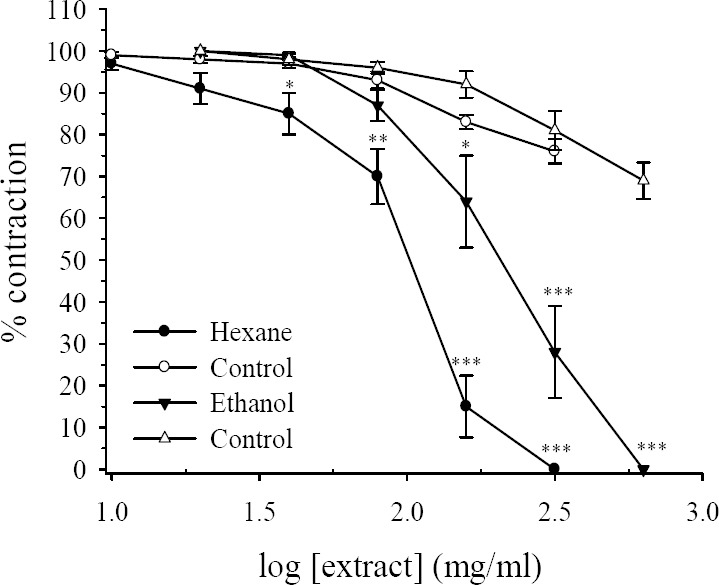
Effects of Rosa persica hydroalcoholic and hexane extracts on tension development to potassium chloride (KCl, 80 mM), in isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of the extract. Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6). The reduction in response to vehicle treated timematched control tissues are statistically significant (P<0.001, ANOVA). Asterisks show statistically significant differences in KCl responses in comparison with its corresponding vehicle treated time matched control tissues *P<0.05, **P<0.01 (Student's t-test). Maximum concentration of vehicle (DMSO) in the bath was 3.2% and 2.65% for hexane and hydroalcoholic extracts respectively.
Relaxant effect of atropine
As expected atropine concentration-dependently (0.1-10 nM) inhibited the contractile response to ACh with IC50 value of 0.67 ± 0.15 nM (Fig. 5). At above concentrations atropine had no effects of contractile response to EFS, however, at higher concentration (50 nM-50 μM), atropine inhibited both responses of EFS (Fig. 5). On the other hand, atropine at above concentration had no major inhibitory effect on tonic contraction induced by KCl (Fig. 5).
Fig. 5.
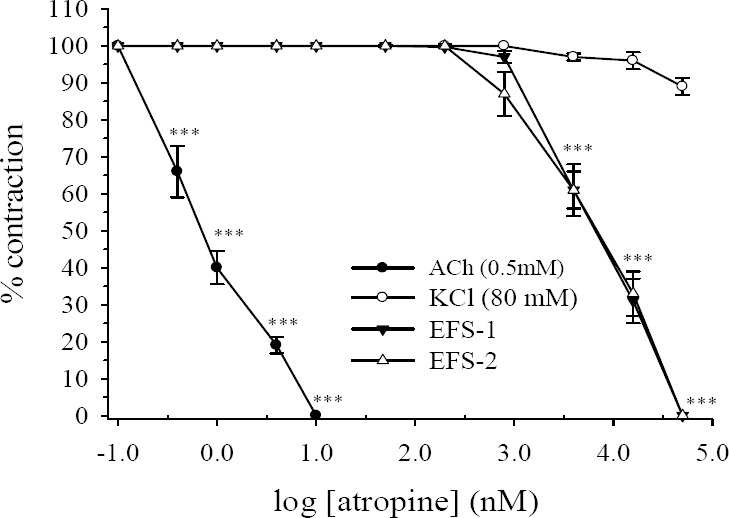
Effect of atropine on tension development to potassium chloride (KCl, 80 mM), acetylcholine (ACh, 0.5 μM) and first (EFS-1) and secondary (EFS-2) contractile responses to electrical field stimulation (EFS, 6V, 50 Hz, 1 s duration) in isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of atropine. The points are mean and the vertical bars show the SEM (n=6). Asterisks show statistical differences in comparison with corresponding time-matched vehicle treated control groups, ***P<0.001 (Student's t-test).
DISCUSSION
Flowers of R. damascena traditionally are used to alleviate GI disorders (5). In previous research we have shown that depending on concentration used, hydroalcoholic extract of R. damascena could have stimulatory and/or inhibitory effects on rat ileum smooth muscle. The excitatory effect of R. damascena extract confirms its emollient action (7). However, the laxative and antispasmodic effects of R. damascena extract are seen at close concentrations which limit its use as laxative or antispasmodic agent (7). R. persica is another species of Rosa family and we have shown that the extracts of this plant only had inhibitory activities on rat isolated ileum. The R. persica extracts inhibited the ileum contractions induced by either neuronal stimulation (EFS), ACh or KCl; indicating a powerful inhibitory action on ileum. Stimulation of the myenteric plexus (nerve net) situated between the longitudinal and the circular muscle layers of the GI tract are mainly responsible for muscle contraction seen with transmural stimulation (EFS). The neurotransmitters ACh, serotonin (5-HT) and a number of peptides including opioid peptide are the important regulators of motility of gastrointestine (13). ACh is a major neurotransmitter that is released by neurons in the ileum. ACh produce contractions by activating M3 muscarinic receptors on ileum smooth muscle cells (14). The M3 receptor activation, results in the activation of phospholipase C and the formation of inositol trisphosphate (IP3) and diacylglycerol (15,16). IP3 causes Ca2+ release from intracellular stores (17,18) and can also mobilize Ca2+ secondarily through Ca2+-sensitive or store-dependent mechanisms (19,20). Muscarinic receptor antagonists are one of the known pharmacological agents that decrease GI motility (21). As expected atropine completely abolished the contractile effect of ACh, but at much higher concentrations transmural stimulation (EFS) was also inhibited. This is in consistence with the literature reports that the inhibitory effect of muscarinic antagonist on gut neuronal stimulation are seen with concentration above those which remove response to exogenous ACh (22). Atropine had no effect on KCl induced contraction. This is because increase in extracellular concentration of KCl, results in depolarization cell membrane and activation of voltage gated Ca2+ channels (23). Voltage gated Ca2+ channels are located on the plasma membrane and are activated by membrane depolarization. Therefore, atropine had no effect on KCl response because in this situation muscarinic antagonist are no involved.
R. persica extracts concentration-dependently inhibited the ileum contraction due to membrane depolarization, muscarinic receptor or neuronal stimulation suggesting a more general mechanism of actions. The contractile machinery of smooth muscle is activated when the myosin light chain undergoes phosphorylation. Activation of the enzyme myosin light chain kinase (MLCK) is responsible for phosphorylation process. Interaction of intracellular Ca2+ ion with calmodulin activates the enzyme MLCK (24,25). Therefore, if constituents of the extract affects the final pathway of cell contraction, that could explain the inhibitory effect of the extracts. Another possibility is the existence of several active compounds with different mechanism of action and this may explain why inhibitory effect of KCl was seen with higher concentration of the extracts.
Comparison of concentrations of the extracts at IC50 levels shows that the hexane extract is 2 to 2.5 times more potent than the hydroalcoholic extract. Statistical analysis also indicate a significant differences between the IC50 values of the extracts (P<0.001). Although the hexane extract is more potent than the hydroalcoholic extract, however, the yield of the hexane extract is far less than the hydroalcoholic extract. It is possible that the different active compounds could be presence in these extract, nevertheless, the difference could simply be due amount of the active compound in each extract as less polar compound tends to be concentrated in the hexane extract.
When the potency of hydroalcoholic extract of R. persica at IC50 level is compared with that of R. damascena extract, great differences could be seen. In fact, the hydroalcoholic extract of R. persica is 8 to 14 times more potent than the R. damascena extract.
CONCLUSION
In conclusion, we have demonstrated that unlike R. damascene, the extract of R. persica only has inhibitory effect on rat isolated ileum smooth muscles contractions. Furthermore, the extract of R. persica is more potent and is useful for GI disordered accompanied with gut spasm or diarrhoea. Further research for isolation and identification of active compounds are recommended.
ACKNOWLEDGEMENTS
The content of this paper is extracted from the Pharm. D thesis NO. 393658 submitted by F. Jalali which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Syder JH, Merson MH. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Organ. 1982;60:605–613. [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian V. A dictionary of Iranian plant names. Tehran: Farhang Moaser; 1996. pp. 443–444. [Google Scholar]
- 3.Mozaffarian V. Identification of Iranian aromatic and medicinal plants. Tehran: Farhang Moaser; 2012. pp. 994–998. [Google Scholar]
- 4.Boskabady M, Shafei M, Saberi Z, Amini S. Pharmacological effects of Rosa damascena. Iranian J Basic Med Sci. 2011;14:295–307. [PMC free article] [PubMed] [Google Scholar]
- 5.Sharafkandy A. Ave-sina Law in Medicine. Tehran: Ministry of Guidance publication; 1990. pp. 129–131. [Google Scholar]
- 6.Mirheydar H. Plant Science. 1st ed. Vol. 3. Tehran: Islamic Culture Press Iran; 1993. pp. 392–396. [Google Scholar]
- 7.Sadraei H, Asghari G, Emami S. Effect of Rosa damascena Mill.flower extract on rat ileum. Res Pharm Sci. 2013;8:227–284. [PMC free article] [PubMed] [Google Scholar]
- 8.Sadraei H, Asghari G, Emami S. Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction. Res Pharm Sci. 2013;8:17–23. [PMC free article] [PubMed] [Google Scholar]
- 9.Khatamsaz A. Flora of Iran Rosaceace. Tehran: research institute of forest and rangelands; 2008. pp. 37–40. [Google Scholar]
- 10.European Pharmacopoeia. 4th ed. Vol. 1. Strasbourg: Council of Europe; 2002. pp. 183–184. [Google Scholar]
- 11.Washington DC: The National Academies Press; 2010. Committee for the update of the guide for the care and use of laboratory animals, National Research Council. Guide for the Care and use of Laboratory animals; pp. 11–37. [Google Scholar]
- 12.Ekblad E, Sundler F. Motor responses in rat ileum evoked by nitric oxide donors vs.field stimulation: Modulation by pituitary adenylate cyclase-activating peptide, forskolin and guanylate cyclase inhibitors. J Pharmacol Exp Ther. 1997;283:23–28. [PubMed] [Google Scholar]
- 13.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;17:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 14.Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 15.Candel LM, Yun SH, Tran LL, Ehlert FJ. Differential coupling of subtypes of the muscarinic receptor to adenylate cyclase and phosphoinositide hydrolysis in the longitudinal muscle of the rat ileum. Mol Pharmacol. 1990;38:689–697. [PubMed] [Google Scholar]
- 16.Prestwich SA, Bolton TB. G-protein involvement in muscarinic receptor-stimulation of inositol phosphates in longitudinal smooth muscle from the small intestine of the guinea-pig. Br J Pharmacol. 1995;114:119–126. doi: 10.1111/j.1476-5381.1995.tb14915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komori S, Bolton TB. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morel J L, Macrez N, Mironneau J. Specific Gq protein involvement in muscarinic M3 receptor-induced phosphatidylinositol hydrolysis and Ca2+ release in mouse duodenal myocytes. Br J Pharmacol. 1997;121:451–458. doi: 10.1038/sj.bjp.0701157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito S, Ohta T, Nakazato Y. Inward current activated by carbachol in rat intestinal smooth muscle cells. J Physiol. 1993;470:395–409. doi: 10.1113/jphysiol.1993.sp019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohta T, Kawai K, Ito S, Nakazato Y. Ca2+ entry activated by emptying of intracellular Ca2+ stores in ileal smooth muscle of the rat. Br J Pharmacol. 1995;114:1165–1170. doi: 10.1111/j.1476-5381.1995.tb13329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasricha PJ. Treatment of disorders of bowel motility and water flux; antiemetics; agents used in biliary and pancreatic disease. In: Hardman JG, Limbird LE, editors. Goodman & Gilmans. The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. pp. 983–1008. [Google Scholar]
- 22.Rang HP, Dale MM, Ritter JM, Flower RJ. Cholinergic transmission In: Rang & Dale's Pharmacology. 6th ed. London: Churchill Livingstone; 2007. pp. 144–167. [Google Scholar]
- 23.Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288:769–783. doi: 10.1152/ajpcell.00529.2004. [DOI] [PubMed] [Google Scholar]
- 24.Triggle DJ. The pharmacology of ion channels: with particular references to voltage gated Ca2+ channels. Eur J Pharmacol. 1999;375:311–325. doi: 10.1016/s0014-2999(99)00329-5. [DOI] [PubMed] [Google Scholar]
- 25.Kuriyama H, Kitamura K, Itoh T, Inour R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]


