Abstract
Background:
Single-fiber electromyography (SFEMG) has been suggested as a quantitative method for supporting chronic partial denervation in amyotrophic lateral sclerosis (ALS) by the revised EI Escorial criteria. Although concentric needle (CN) electrodes have been used to assess jitter in myasthenia gravis patients and healthy controls, there are few reports using CN electrodes to assess motor unit instability and denervation in neurogenic diseases. The aim of this study was to determine whether quantitative changes in jitter and spike number using CN electrodes could be used for ALS studies.
Methods:
Twenty-seven healthy controls and 23 ALS patients were studied using both CN and single-fiber needle (SFN) electrodes on the extensor digitorum communis muscle with an SFEMG program. The SFN-jitter and SFN-fiber density data were measured using SFN electrodes. The CN-jitter and spike number were measured using CN electrodes.
Results:
The mean CN-jitter was significantly increased in ALS patients (47.3 ± 17.0 μs) than in healthy controls (27.4 ± 3.3 μs) (P < 0.001). Besides, the mean spike number was significantly increased in ALS patients (2.5 ± 0.5) than in healthy controls (1.7 ± 0.3) (P < 0.001). The sensitivity and specificity in the diagnosis of ALS were 82.6% and 92.6% for CN-jitter (cut-off value: 32 μs), and 91.3% and 96.3% for the spike number (cut-off value: 2.0), respectively. There was no significant difference between the SFN-jitter and CN-jitter in ALS patients; meanwhile, there was no significant difference between the SFN-jitter and CN-jitter in healthy controls.
Conclusion:
CN-jitter and spike number could be used to quantitatively evaluate changes due to denervation-reinnervation in ALS.
Keywords: Amyotrophic Lateral Sclerosis, Concentric Needle Electrode, Jitter, Single-fiber Electromyography, Spike Number
INTRODUCTION
Single-fiber electromyography (SFEMG) was introduced by Stålberg and Ekstedt in the 1960s to study neuromuscular junction transmission failure.[1] The single-fiber needle (SFN) electrodes were designed to study single muscle fiber over small hemispheric recording area with a radius of about 300 μm. However, SFN electrodes are expensive, and there has been increasing concern about reusing material for invasive medical procedures; thus, an acceptable, inexpensive alternative to the SFN has been sought. Jitter recorded with concentric needle (CN) electrodes has been studied in healthy controls and in myasthenia gravis (MG) patients.[2,3,4,5,6,7,8] It has been shown that CN and SFN electrodes yield comparable jitter results in most studies.
SFEMG has been suggested as a quantitative method for supporting chronic partial denervation in amyotrophic lateral sclerosis (ALS) by the revised EI Escorial criteria.[9] Due to progressive denervation and reinnervation, immature nerve terminals, and impaired transmission in the endplates, SFEMG in ALS patients showed increased jitter and fiber density (FD), which reflected the neuromuscular junction transmission failure and reinnervation condition due to the progression of ALS. SFEMG could also provide some information for evaluating the prognosis of ALS and differentiating ALS from cervical spondylosis.[10,11,12,13]
Conventional CN electromyography is the standard technique for evaluating denervation and reinnervation in neurogenic diseases. Amplitude, duration, area, and phases of motor unit potential (MUP) are parameters studied conventionally. Instability of the CN-MUP is also routinely qualitatively observed, which reflects the dysfunction of neuromuscular junction transmission. The quantitation of ‘jiggle’ has been attempted by measuring consecutive amplitude differences and cross-correlational coefficient of consecutive discharges.[14,15] However, ‘jiggle’ is not commonly used because the special software required is not routinely installed in electromyogram equipment.
Although CN electrode has been used to assess jitter in MG patients and healthy controls, no report had used CN electrode to assess jitter in neurogenic diseases to investigate motor unit instability. Signals recorded with CN electrodes, even if visibly indistinguishable from single-fiber action potentials, may actually be composed of synchronized and near-simultaneous action potentials from more than one muscle fiber; thus, CN electrodes cannot be used to measure FD as it is defined in SFEMG because of the larger uptake radius.[2,16] We are interested in whether we can use the SFEMG program and CN electrode rather than SFN electrode to study neuromuscular junction transmission failure and reinnervation in ALS.
METHODS
Subjects
Two groups of cases were collected from January 2013 to December 2014. Group 1 comprised 27 healthy controls. Group 2 comprised 23 ALS patients, including 13 definite and ten probable ALS patients according to the Awaji criteria.[17] All patients in Group 2 were followed up for at least 6 months. There was no difference in the mean age (50.3, 52.6 years, respectively) of the two groups at the time of the study. Written informed consent was obtained from each subject. The study was approved by Peking Union Medical College Hospital Human Ethics Committee.
Single-fiber electromyography studies
The study was performed with the SFEMG program of the Counterpoint EMG machine (Dantec Electronics, Denmark). Both CN and SFN electrodes were used for the study. The SFN electrodes used were standard SFN electrodes with a 25 μm recording diameter. The CN electrodes used were facial needle electrodes with recording surface dimensions of 0.019 mm2. Recordings with both electrodes were performed on the extensor digitorum communis (EDC) muscle under voluntary contraction on the same day.[12,13] In ALS patients, the Medical Research Council scale was grade 5 in the EDC muscles studied. The SFN-jitter and single-fiber needle-fiber density (SFN-FD) were defined using SFNs and the SFEMG program. Action potentials with amplitudes >200 µV and rise times <300 µs were selected for calculating the FD and jitter. The CN-jitter and spike number were defined using CNs and the SFEMG program. The methods and criteria for acquiring and analyzing the parameters were the same for SFN and CN, except that the low-frequency filter was 500 Hz when using SFN and 1000 Hz when using CN. Twenty potential pairs were usually collected. The parameter data were collected for each subject as shown in the SFEMG program. When studied with CN electrodes, the CN-jitter was recorded as the jitter data listed in the SFEMG program, and the spike number was recorded as the FD listed in the SFEMG program, although the anatomical substrates of the CN-jitter and spike number are different from those of the SFN-jitter and SFN-FD.
Statistical analysis
Differences in parameters between different groups were analyzed by independent sample Student's t-test. Differences in parameters between two needle types in the same groups were analyzed by paired sample Student's t-test. A value of P < 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curve analysis was performed to explore the accuracy of the CN-jitter and spike number in differentiating ALS patients from the healthy controls.
RESULTS
The parameters recorded using CN electrodes and SFN electrodes in the two groups are shown in Table 1. There was no significant difference between the mean CN-jitter and SFN-jitter in ALS patients (t = 1.854, P = 0.072) and healthy controls (t = 0.986, P = 0.333). The waveforms recorded from an ALS patient and a healthy control using SFNs and CNs in the SFEMG program are shown in Figure 1.
Table 1.
Parameters recorded using SFN electrode and CN electrode in the EDC muscle of ALS patients and healthy controls
| Parameters | Healthy controls (n = 27) | ALS (n = 23) |
|---|---|---|
| SFN-jitter (μs) | 30.0 ± 9.0 | 57.8 ± 18.5* |
| CN-jitter (μs) | 27.4 ± 3.3 | 47.3 ± 17.0* |
| Fiber density (n) | 1.2 ± 0.3 | 2.6 ± 0.5* |
| Spike number (n) | 1.7 ± 0.3 | 2.5 ± 0.5* |
Data are shown as mean ± SD. *A significant difference in the parameter between ALS patients and healthy controls (P<0.001). SFN: Single-fiber needle; CN: Concentric needle; EDC: Extensor digitorum communis; ALS: Amyotrophic lateral sclerosis; SD: Standard deviation.
Figure 1.
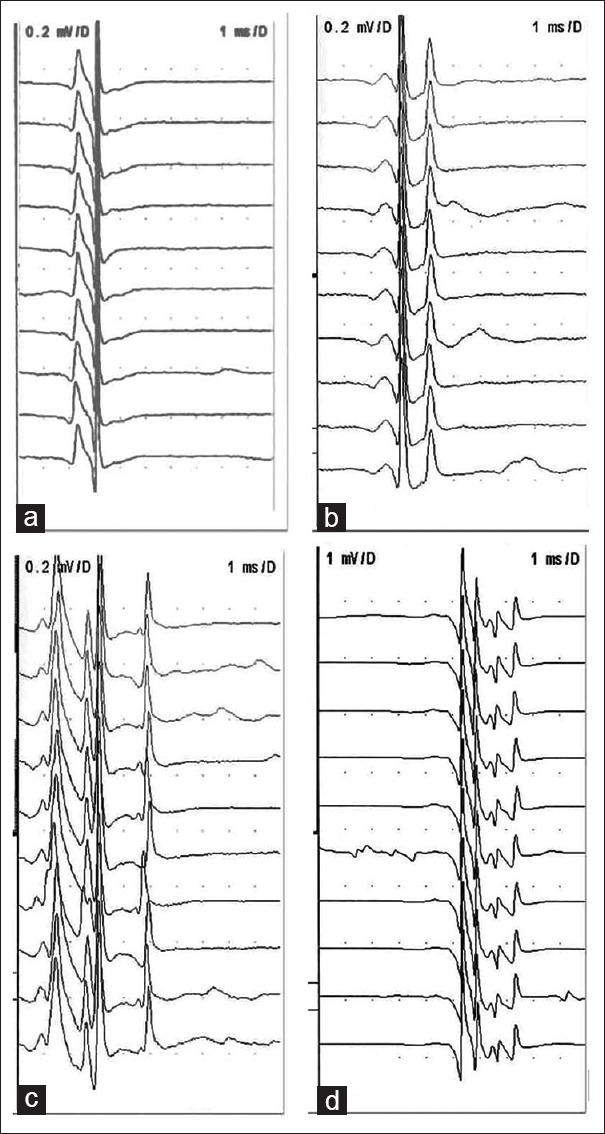
Waveforms obtained using SFN electrode and CN electrode in the SFEMG program in ALS and healthy controls. Consecutive discharges of the same motor unit were recorded from the extensor digitorum communis muscle during voluntary contraction. (a) Recorded by SFN electrode in a normal subject, (b) recorded by CN electrode in a normal subject, (c) recorded by SFN electrode in an ALS patient, and (d) recorded by CN electrode in an ALS patient. The low-cut filter setting was 500 Hz for (a) and (c), 1 kHz for (b) and (d). Signals recorded with CN were visibly indistinguishable from single-fiber waveforms, although the former might actually comprise synchronized and near-simultaneous action potentials from more than one muscle fiber. SFN: Single-fiber needle; CN: Concentric needle; SFEMG: Single-fiber electromyography; ALS: Amyotrophic lateral sclerosis.
The mean CN-jitter was significantly increased in ALS patients than in healthy controls (t = 5.973, P < 0.001). The mean spike number was significantly increased in ALS patients (2.5 ± 0.5) than in healthy controls (1.7 ± 0.3) (t = 9.206, P < 0.001). ROC curve analysis showed that the area under the curve was 0.871 (95% confidence interval [CI]: 0.753, 0.989) for the mean CN-jitter and 0.966 (95% CI: 0.906, 1.000) for the mean spike number [Figure 2]. The sensitivity and specificity in the diagnosis of ALS were 82.6% and 92.6% for the mean CN-jitter (cut-off value: 32 μs) and 91.3% and 96.3% for the mean spike number (cut-off value: 2.0), respectively.
Figure 2.
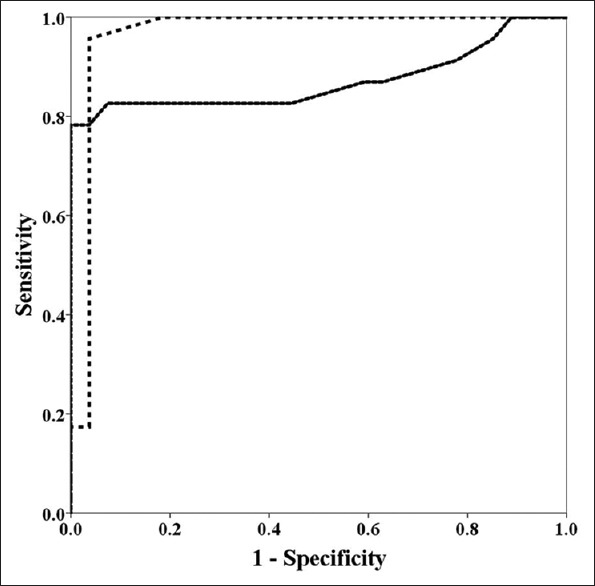
ROC curve analysis of spike number and jitter using concentric needles to differentiate ALS patients from healthy controls. The dotted line indicated the ROC curve of the mean spike number using concentric needles. The black line indicated the ROC curve of the mean jitter using concentric needles. Both ROC curves showed the high area under curve values (0.966 for the mean spike number and 0.871 for the mean jitter). ROC: Receiver operating characteristic. ALS: Amyotrophic lateral sclerosis.
DISCUSSION
SFEMG has been studied mainly in patients with neuromuscular transmission disorders. Although SFEMG has been suggested as one of the quantitative methods for supporting chronic partial denervation in ALS by the revised EI Escorial criteria,[9] few reports have studied SFEMG in neurogenic diseases.[1,10,11,12,13] We are interested in whether the CN-jitter and spike number can substitute the SFN-jitter and SFN-FD to assess neuromuscular junction transmission and chronic reinnervation in neurogenic diseases.
In this study, we found that both CN and SFN electrodes showed significantly increased jitter in ALS patients compared with healthy controls. Despite differences in the anatomical substrates and physiological meanings between SFN-jitter and CN-jitter, jitter recorded using CN electrodes has been accepted as a parameter for assessing neuromuscular transmission in MG in most published reports.[18,19,20,21] The waveform obtained using a CN electrode in the SFEMG program represents the MUP composed of different numbers of spikes. Both the SFN- and CN-jitters reflect a similar physiological phenomenon (variability in the arrival time of muscle fiber action potentials to the recording electrode) and clinical significance. The jitter of those spikes (CN-jitter) reflects the variability of the interval to the recording site between individual components of the MUPs during firing, whereas the SFN-jitter reflects the variability of the interval to the recording site between individual muscle fibers. Unlike in MG, increased jitter in ALS is the result of an immature endplate due to the progressive degeneration and reinnervation of lower motor neurons.[10,11] We suggest that CN-jitter might be used to quantitatively assess MUP instability in neurogenic diseases. In ALS, the variability between MUPs or MUP components results from asynchronous conduction at the motor nerve terminal and neuromuscular junction.
We also found that both the spike number and SFN-FD were increased in ALS patients compared with the healthy controls. The spike number, recorded with CNs in the SFEMG program, is a new term that we first proposed. We adopted the same criteria for calculating spike number as FD in the SFEMG program. Although the signals recorded with the CN electrode are visibly indistinguishable from single-fiber action potentials in the SFEMG program, the spike number and FD had entirely different anatomical substrates. The main difference between SFN electrodes and CN electrodes is the areas of their recording surfaces. With a smaller recording area (0.0005 mm2), SFNs can identify the action potential from an individual muscle fiber (i.e., each spike represents one muscle fiber), which allows for the measurement of FD and jitter between two different muscle fibers. For CN electrodes, with a larger recording area (0.019 mm2), each spike of the signal actually comprises synchronized and near-simultaneous action potentials from a group of muscle fiber s.[2,16] Each motor unit may have one or more groups of summated muscle fibers. The spike number is similar to the number of phases or turns recorded with CN electrodes in conventional EMG; the difference in waveforms between them is due to different filter settings. Each spike mainly reflects the summated action potentials of several muscle fibers nearest to the needle. In healthy controls, the spike number is always no more than two. In ALS patients, due to denervation and reinnervation, more muscle fibers are innervated by survival motor neurons; therefore, the spike number increases. Two pathological and physiological changes may affect the spike number during the denervation-reinnervation process in neurogenic diseases. First, the actual FD of an individual motor unit increases due to collateral reinnervation, and more muscle fibers in the same area can thus be recorded by CN electrodes. Second, asynchronous conduction increases due to an immature motor nerve terminal and neuromuscular junction following denervation-reinnervation; thus, the duration of excitatory signal propagation from muscle fibers to the recording needle significantly varied, allowing more waveform spikes to be obtained. In addition, each spike comprised more muscle fibers.
Although the spike number obtained with CN electrodes is from groups of muscle fibers and not the actual number of muscle fibers in the recording area, the changes in spike number might have the ability to indirectly reflect changes in FD and asynchronous conduction in the motor unit. This condition may be similar to calculating the number of motor units using motor unit number estimation, which is also a relative number and not the actual number of motor units. In fact, many factors might affect the spike number results in neurogenic diseases;[2] however, all of the theoretical problems are hypotheses or result from computer simulations. Neuroelectrophysiological tests are tools that are used to further explore the neuromuscular system. It is not necessary to argue how many exact muscles fibers are involved in composing waveform spikes; instead, we are interested in whether the method can aid in clinical work or in research.
Our data showed that the mean jitter and spike number recorded with CN electrodes in ALS patients were significantly higher than those in healthy controls. Clinicians often prefer to select an individual point defined by an ROC curve to diagnose certain diseases. Our data showed that the mean CN-jitter and spike number had high sensitivity and specificity for differentiating ALS patients from healthy controls. However, the sensitivity and specificity of a diagnostic test largely depend on the clinical context in which the test will be used. Although it is seldom necessary to differentiate ALS patients from healthy controls in practice, the ROC curve data still indicated that the CN-jitter and spike number could provide some quantitative information as those recorded with SFNs in ALS patients.
In conclusion, theoretically, waveform spikes obtained using CN electrodes with the SFEMG program have different anatomical substrates and physiological meaning as those obtained using SFNs; in practice, the parameters recorded with CN and SFN electrodes are highly comparable in ALS patients. CN electrodes may be an alternative to SFNs in ALS studies to quantitatively evaluate the changes in jitter and spike number. The CN-jitter and spike number have high sensitivity and specificity for differentiating ALS from healthy controls and might be helpful to quantitatively investigate the changes due to denervation-reinnervation in ALS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Sanders DB, Stålberg EV. AAEM minimonograph #25: Single-fiber electromyography. Muscle Nerve. 1996;19:1069–1069. doi: 10.1002/(SICI)1097-4598(199609)19:9<1069::AID-MUS1>3.0.CO;2-Y. doi: 10.1002/(SICI)1097-4598(199609)19:9%3C1069: AID-MUS1%3E3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 2.Stålberg EV, Sanders DB. Jitter recordings with concentric needle electrodes. Muscle Nerve. 2009;40:331–9. doi: 10.1002/mus.21424. doi: 10.1002/mus.21424. [DOI] [PubMed] [Google Scholar]
- 3.Kouyoumdjian JA, Stålberg EV. Reference jitter values for concentric needle electrodes in voluntarily activated extensor digitorum communis and orbicularis oculi muscles. Muscle Nerve. 2008;37:694–9. doi: 10.1002/mus.21043. doi: 10.1002/mus.21043. [DOI] [PubMed] [Google Scholar]
- 4.Kouyoumdjian JA, Stålberg EV. Concentric needle single fiber electromyography: Comparative jitter on voluntary-activated and stimulated extensor digitorum communis. Clin Neurophysiol. 2008;119:1614–8. doi: 10.1016/j.clinph.2008.03.008. doi: 10.1016/j.clinph.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Kouyoumdjian JA, Stålberg EV. Concentric needle jitter on stimulated orbicularis oculi in 50 healthy subjects. Clin Neurophysiol. 2011;122:617–22. doi: 10.1016/j.clinph.2010.07.012. doi: 10.1016/j.clinph.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Orhan EK, Deymeer F, Oflazer P, Parman Y, Baslo MB. Jitter analysis with concentric needle electrode in the masseter muscle for the diagnosis of generalised myasthenia gravis. Clin Neurophysiol. 2013;124:2277–82. doi: 10.1016/j.clinph.2013.04.344. doi: 10.1016/j.clinph.2013.04.344. [DOI] [PubMed] [Google Scholar]
- 7.Sarrigiannis PG, Kennett RP, Read S, Farrugia ME. Single-fiber EMG with a concentric needle electrode: Validation in myasthenia gravis. Muscle Nerve. 2006;33:61–5. doi: 10.1002/mus.20435. doi: 10.1002/mus.20435. [DOI] [PubMed] [Google Scholar]
- 8.Kokubun N, Sonoo M, Imai T, Arimura Y, Kuwabara S, Komori T, et al. Reference values for voluntary and stimulated single-fibre EMG using concentric needle electrodes: A multicentre prospective study. Clin Neurophysiol. 2012;123:613–20. doi: 10.1016/j.clinph.2011.07.044. doi: 10.1016/j.clinph.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 10.Balice-Gordon RJ, Smith DB, Goldman J, Cork LC, Shirley A, Cope TC, et al. Functional motor unit failure precedes neuromuscular degeneration in canine motor neuron disease. Ann Neurol. 2000;47:596–605. doi: 10.1002/1531-8249(200005) 47:5%3C596: AID-ANA7%3E3.0.CO;2-I. [PubMed] [Google Scholar]
- 11.Stålberg E, Schwartz MS, Trontelj JV. Single fibre electromyography in various processes affecting the anterior horn cell. J Neurol Sci. 1975;24:403–15. doi: 10.1016/0022-510x(75)90166-5. doi: 10.1016/0022-510X(75)90166-5. [DOI] [PubMed] [Google Scholar]
- 12.Cui LY, Liu MS, Tang XF. Single fiber electromyography in 78 patients with amyotrophic lateral sclerosis. Chin Med J. 2004;117:1830–3. [PubMed] [Google Scholar]
- 13.Liu M, Cui L, Guan Y, Li B, Du H. Single-fiber electromyography in amyotrophic lateral sclerosis and cervical spondylosis. Muscle Nerve. 2013;48:137–9. doi: 10.1002/mus.23767. doi: 10.1002/mus.23767. [DOI] [PubMed] [Google Scholar]
- 14.Stålberg EV, Sonoo M. Assessment of variability in the shape of the motor unit action potential, the “jiggle,” at consecutive discharges. Muscle Nerve. 1994;17:1135–44. doi: 10.1002/mus.880171003. doi: 10.1002/mus.880171003. [DOI] [PubMed] [Google Scholar]
- 15.Campos C, Malanda A, Gila L, Segura V, Lasanta I, Artieda J. Quantification of jiggle in real electromyographic signals. Muscle Nerve. 2000;23:1022–34. doi: 10.1002/1097-4598(200007)23:7<1022::aid-mus4>3.0.co;2-3. doi: 10.1002/1097-4598(200007)23:7%3C1022: AID-MUS4%3E3.3.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Stålberg E, Kouyoumdjian J, Sanders D. Reference values in concentric needle electrode studies. Clin Neurophysiol. 2013;124:1255–6. doi: 10.1016/j.clinph.2012.09.028. doi: 10.1016/j.clinph.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 17.de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119:497–503. doi: 10.1016/j.clinph.2007.09.143. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- 18.Benatar M, Hammad M, Doss-Riney H. Concentric-needle single-fiber electromyography for the diagnosis of myasthenia gravis. Muscle Nerve. 2006;34:163–8. doi: 10.1002/mus.20568. doi: 10.1002/mus.20568. [DOI] [PubMed] [Google Scholar]
- 19.Farrugia ME, Weir AI, Cleary M, Cooper S, Metcalfe R, Mallik A. Concentric and single fiber needle electrodes yield comparable jitter results in myasthenia gravis. Muscle Nerve. 2009;39:579–85. doi: 10.1002/mus.21151. doi: 10.1002/mus.21151. [DOI] [PubMed] [Google Scholar]
- 20.Ertas M, Baslo MB, Yildiz N, Yazici J, Oge AE. Concentric needle electrode for neuromuscular jitter analysis. Muscle Nerve. 2000;23:715–715. doi: 10.1002/(sici)1097-4598(200005)23:5<715::aid-mus8>3.0.co;2-v. doi: 10.1002/(SICI)1097-4598(200005)23:5%3C715: AID-MUS8%3E3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Kouyoumdjian JA, Stålberg EV. Concentric needle jitter on voluntary activated frontalis in 20 healthy subjects. Muscle Nerve. 2013;47:440–2. doi: 10.1002/mus.23710. doi: 10.1002/mus.23710. [DOI] [PubMed] [Google Scholar]


