Abstract
Background:
Dermatomyositis (DM) and polymyositis (PM) are common inflammatory myopathies whose immunopathogenic mechanisms remain poorly understood. The NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome is a type of cytoplasmic multiprotein inflammasome and is responsible for the activation of inflammatory reactivations. Responding to a wide range of exogenous and endogenous microbial or sterile stimuli, NLRP3 inflammasomes can cleave pro-caspase-1 into active caspase-1, which processes the pro-inflammatory cytokines pro-interleukin (IL)-1β and pro-IL-18 into active and secreted IL-1β and IL-18. The NLRP3 inflammasome is implicated in infectious and sterile inflammatory diseases. However, it remains unclear whether it is involved in the pathogenesis of DM/PM, which we aim to address in our research.
Methods:
In this study, 22 DM/PM patients and 24 controls were recruited. The protein and RNA expression of IL-1β, IL-18, NLRP3, and caspase-1 in serum and muscle samples were tested and compared between the two groups.
Results:
The serum IL-1β and IL-18 levels were significantly higher in DM/PM patients than those in the controls by enzyme linked immunosorbent assay (ELISA, DM vs. control, 25.02 ± 8.29 ng/ml vs. 16.49 ± 3.30 ng/ml, P < 0.001; PM vs. control, 26.49 ± 7.79 ng/ml vs. 16.49 ± 3.30 ng/ml, P < 0.001). Moreover, the real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) showed that DM/PM patients exhibited higher RNA expression of IL-1β, IL-18, and NLRP3 in the muscle (for IL-1β, DM vs. control, P = 0.0012, PM vs. control, P = 0.0021; for IL-18, DM vs. control, P = 0.0045, PM vs. control, P = 0.0031; for NLRP3, DM vs. control, P = 0.0017, PM vs. control, P = 0.0006). Moreover, the protein expression of NLRP3 and caspase-1 in muscle samples of DM/PM patients were also significantly elevated compared to that in the muscles of the controls.
Conclusions:
Our findings demonstrate that the NLRP3 inflammasome is implicated in the pathogenesis of DM/PM. High NLRP3 expression led to elevated levels of IL-1β and IL-18 and could be one of the factors promoting disease progress.
Keywords: Autoimmunity, Dermatomyositis, NOD-like Receptor Family, Pyrin Domain Containing 3 Inflammasome, Polymyositis
INTRODUCTION
Dermatomyositis (DM) and polymyositis (PM) are common inflammatory myopathies, which are characterized by subacute or chronic progressive muscle weakness, myalgia, and multi-organ involvement.[1] It is commonly thought that DM is a complement-mediated microangiopathy and PM is T-cell mediated myopathy.[2] In DM, the complement C5b9 membrane attack complex is activated and deposited on endothelial cells, leading to necrosis, ischemia, and muscle fiber destruction resembling microinfarcts. The cluster of differentiation (CD)8− major histocompatibility complex (MHC) Class I complex is characteristic of PM, and the perforin released by CD8 cells causes muscle fiber necrosis.[3,4,5] Both PM and DM are potentially treatable myopathies, and glucocorticoids form the basis of treatment. However, the treatment efficiency is heterogeneous between different patients. The immunopathogenic mechanisms behind these diseases remain poorly understood and require further study.
The inflammasome is a large molecular platform that triggers the activation of inflammatory caspases and the processing of pro-interleukin-1β (pro-IL-1β) and pro-IL-18. The inflammasome, which was first described in 2002,[6] was thought to act as a signaling platform that can respond to infections, as well as endogenous cellular stress products. It was also thought that it played a vital role in innate immunity. Among the kinds of inflammasomes, the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome is the most thoroughly studied inflammasome, which consists of the NLR family member NLRP3, the adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and the effector protein pro-caspase-1.[7] After responding to the stimuli of microbial activators [8] or endogenous danger signals, such as Adenosine Triphosphate (ATP),[9] uric acid crystals,[10] and β-amyloid,[11] the NLRP3 inflammasome assembled through the ASC domain autoclaves the pro-caspase-1 into active caspase-1, which in turn cleaves the pro-forms of thecytokines IL-1β and IL-18 to their active and secreted forms.[12] The proinflammatory cytokines, IL-1β and IL-18, will regulate the immune response by increasing the expression of adhesion molecules on endothelial cells, enabling the transmigration of leukocytes, and by stimulating antibody production.
Activation of the inflammasome during infection can be protective, but unregulated NLRP3 inflammasome activation in response to nonpathogenic endogenous or exogenous stimuli can lead to unintended pathology. The NLRP3 inflammasome has been linked to diseases such as Alzheimer's disease, atherosclerosis, metabolic syndrome, diabetes, multiple sclerosis, and age-related macular degeneration.[13] However, whether or not the NLRP3 inflammasome is involved in DM/PM remains unknown. Previous studies have shown that both IL-1β and IL-18 seem to exert a crucial role in the initiation and progression of DM and PM,[14,15] which indicates the implication of NLRP3 in the pathogenesis of PM and DM. Here, we have compared the mRNA and protein expression of the components in the NLRP3 signaling pathway to address this question.
METHODS
Subjects
A total of 46 patients undergoing muscle biopsy for muscle histopathology at Chinese People's Liberation Army (PLA) General Hospital between May 9, 2014 and December 5, 2014 were retrospectively studied in our research. Among the patients included, 22 DM patients and PM patients were diagnosed according to the Bohan and Peter criteria. General information about DM/PM patients was shown in Table 1. The other 24 patients were selected as age- and gender-matched controls, who underwent muscle biopsy because of muscle weakness, and the diagnosis of these patients did not show obvious clinical and histopathological signs of any muscle disease. Subjects showing normal muscle tissue were excluded from the control group if they had characteristic clinical features of myopathy and/or autoimmune disease. Ethylenediaminetetraacetic acid-anticoagulated venous blood samples were collected by centrifugation at 400 × g for 10 min. Plasma was stored at −80°C. Biopsies were only performed with patient approval, and all patients signed an informed consent form authorizing the use of muscle tissue and blood, and the study protocol was approved by the ethics committee of Chinese PLA General Hospital.
Table 1.
Demographic and clinical features of dermatomyositis and polymyositis patients
| Groups | Number | Gender (male/female), n | Age (years) | Symptom duration (months) | MW (+/−), n | CK (2–200 U/L) | LDH (100–240 U/L) | EMG (+/−), n |
|---|---|---|---|---|---|---|---|---|
| DM | 12 | 6/6 | 38.92 ± 18.33 | 7.87 ± 6.48 | 12/0 | 2231.58 ± 1991.15 | 591.50 ± 392.46 | 9/3 |
| PM | 10 | 4/6 | 37.50 ± 17.35 | 4.55 ± 3.91 | 10/0 | 2030.30 ± 1349.57 | 796.50 ± 572.89 | 9/1 |
DM: Dermatomyositis; PM: Polymyositis; MW: Muscle weakness; CK: Creatine kinase; LDH: Lactate dehydrogenase; EMG: Electromyography; +: Positive; −: Negative.
Enzyme linked immunosorbent assay
The concentration of IL-18 and IL-1β in human serum was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (eBiosciences, San Diego, CA, USA).
Real-time quantitative reverse transcription-polymerase chain reaction
Using the method described in our previous study,[16] gene expression was analyzed by two-step quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Briefly, total RNA was extracted from ground muscle tissue using TRIzol reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions. Total RNA (2 µg) was reverse transcribed to cDNA using a Reverse Transcription System (Promega, Madison, WI, USA) for use in qRT-PCR. Quantitative real-time PCR was performed with the Bio-Rad IQ5 Optical System (Bio-Rad, Hercules, CA, USA). The qRT-PCR was carried out in a 96-well plate, with each well containing 10 μl of 2 × SYBR Green Master Mix, 0.5 μl of primers (10 pmol/L), and 9.5 μl of the cDNA in a total volume of 20 μl. The thermal cycling conditions were as follows: an initial denaturation step at 95°C for 3 min; forty cycles at 95°C for 10 s, annealing at 60°C for 15 s, and extension at 72°C for 10 s; and 71 cycles at 60°C for 30 s. At the end of the PCR cycles, the specificity of the amplification products was checked by dissociation curve analysis. The relative expression of a gene was determined using the 2-DDCt method, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal control. The sequences of the primers are listed in Table 2.
Table 2.
qRT-PCR primers for NLRP3, IL-1β and IL-18 used in this study
| Primer name | Sense primer | Antisense primer |
|---|---|---|
| hNLRP3 | 5’-GAATGCCTTGGGAGACTCAG-3’ | 5’-ACGTAAGGCCAGAATTCACC-3’ |
| hIL-18 | 5’-TGCAGTCTACACAGCTTCGG-3’ | 5’-ACTGGTTCAGCAGCCATCTT-3’ |
| hIL-1β | 5’-GAGCTCGCCAGTGAAATGAT-3’ | 5’-GGAGATTCGTAGCTGGATGC-3’ |
| hGAPDH | 5’-CTCTGCTCCTCCTGTTCGAC-3’ | 5’-TTAAAAGCAGCCCTGGTGAC-3’ |
qRT-PCR: Quantitative reverse transcription-polymerase chain reaction; hNLRP3: Human NOD-like receptor family, pyrin domain containing 3; hIL-18: Human interleukin 18; hIL-1b: Human interleukin 1b; hGAPDH: Human glyceraldehyde-3-phosphate dehydrogenase.
Western blotting
Western blotting was performed to evaluate the levels of NLRP3, subunit p20 of caspase-1, and GAPDH. Briefly, muscle tissue was homogenized in Western blotting analysis buffer. The protein concentration was determined using a BCA kit. Fifty milligrams of protein was electrophoretically separated on 10% or 6% NaDodSO4-polyacrylamide gel and was transferred to a polyvinylidene difluoride membrane, which was then blocked by incubation for 1 h at room temperature in 5% fat-free dry milk in Tris-buffered saline and Tween 20 (TBST). The blots were then incubated overnight at 4°C with rabbit antibodies (Abs) against NLRP3 (1:1000), caspase-1-p20 (1:1000), or GAPDH (1:1000) (all from Cell Signaling Technology, Danvers, MA, USA) diluted in TBST containing 5% bovine serum albumin (BSA), washed for 25 min with TBST, and incubated for 1 h at room temperature with alkaline phosphatase-conjugated anti-rabbit IgG Abs (KPL, Gaithersburg, MD, USA) (1:20,000 in TBST containing 5% BSA). Then, bound Abs were visualized using ECL kits (Amersham Biosciences, Pittsburgh, PA, USA). NLRP3, caspase-1 p20, and GAPDH were detected at molecular sizes of about 110,000 Da, 20,000 Da, and 37,000 Da, respectively. The Western blotting was repeated 3 times and the signal intensity was quantitatively analyzed with Quantity-One analysis software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Quantitative results are expressed as the mean ± standard deviation (SD). The independent t-test was used to compare the means of two groups for continuous variables. One-way analysis of variance (ANOVA) was applied for comparing continuous variables between three groups. Differences were considered statistically significant at P < 0.05. SPSS software (version 17.0, IBM SPSS, Chicago, IL, USA) was used for all statistical procedures. GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) software was used for creating the figures.
RESULTS
Concentration of serum interlukin-1β and interlukin-18 in patients versus controls
On testing the concentration of cytokine IL-1β and IL-18 in patients and controls by ELISA, we found higher concentrations in patients than in controls. As shown in Figure 1, there was a significant difference between these two groups for IL-1β (DM vs. control, 25.02 ± 8.29 ng/ml vs. 16.49 ± 3.30 ng/ml, P < 0.001; PM vs. control, 26.49 ± 7.79 ng/ml vs. 16.49 ± 3.30 ng/ml, P < 0.001) and IL-18 (DM vs. control, 214.74 ± 198.11 ng/ml vs. 50.90 ± 37.62 ng/ml, P < 0.001; PM vs. control, 184.8 ± 161.7 ng/ml vs. 50.90 ± 37.62 ng/ml, P = 0.0075) concentrations. The IL-1β and IL-18 are both proinflammatory cytokines, which participate and exacerbate the inflammation reaction in patients.
Figure 1.
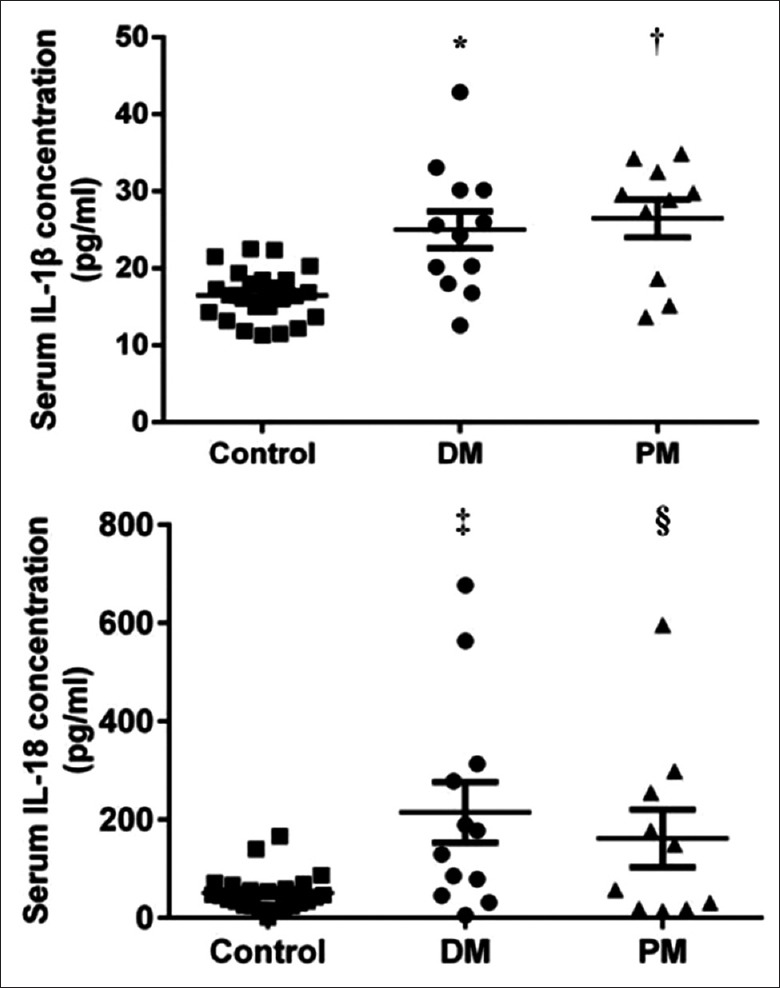
Measurement of serum IL-1β and IL-18 concentrations in the patient and control groups. The concentration of serum IL-1β and IL-18 were higher in patients than that in controls. (IL-1β comparison, DM vs Control *P < 0.001, PM vs. Control †P < 0.001; IL-18 comparison, DM vs. Control ‡P < 0.001, PM vs. Control §P < 0.01) DM: Dermatomyositis; PM: Polymyositis; IL-1β: interleukin-1β; IL-18: interleukin-18.
Increased mRNA expression of interleukin-1β, interleukin-18, and NOD-like receptor family, pyrin domain containing 3 in muscle tissue of dermatomyositis/polymyositis patients
To investigate the reason for higher IL-1β and IL-18 levels in DM/PM patients, we performed qRT-PCR to determine the mRNA expression of IL-1β, IL-18, and the upstream NLRP-3 inflammasome and calculated the quantitative results using the 2-DDCt method. As shown in Figure 2, there is higher relative IL-1β mRNA expression in muscle samples of the patients group than that of the control group (DM vs. control, P = 0.0012; PM vs. control, P = 0.0021). We found a similar result for the relative IL-18 mRNA expression in the case of patients and controls (DM vs. control, P = 0.0045; PM vs. control, P = 0.0031). As for the upstream NLRP-3 inflammasome, the relative mRNA expression of the patients group is much higher than that of the control group (DM vs. control, P = 0.0017; PM vs. control, P = 0.0006). The qRT-PCR result indicates that the NLRP-3 inflammasome expression might be related to high production of IL-1β and IL-18, which will lead to downstream inflammation in DM/PM patients and exacerbate disease progression.
Figure 2.
mRNA expression of IL-1β, IL-18 and the NLRP3 in muscle tissues in relation to GAPDH expression. The data showed that the mRNA expression levels of these three indicators were higher in patients than that in the control groups. The figure depicts the 2−ΔCt of the gene of interest as the mean, and the SEM is calculated as 2-(Ct gene of interest- Ct reference gene) in two groups. The Ct values were obtained from RT-PCR analysis of muscle specimens (mRNA expression of IL-1β comparison, DM vs. control *P < 0.01, PM vs. control †P < 0.01; mRNA expression of IL-18 comparison, DM vs. control ‡P < 0.01, PM vs. control §P < 0.01; mRNA expression of NLRP3 comparison, DM vs. control ||P < 0.01, PM vs. control ¶P < 0.001). DM: Dermatomyositis; PM: Polymyositis; IL-1β: Interleukin-1β; IL-18: Interleukin-18; NLRP3: NOD-like receptor family, pyrin domain containing 3 inflammasome; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; SEM: Standard error of the mean; RT-PCR: Reverse transcription-polymerase chain reaction.
High protein expression of NOD-like receptor family, pyrin domain containing 3 and caspase-1 in the patients group
Next, we aimed to test the protein expression of two components of the signal pathway in DM/PM patients: NLRP3 and caspase-1. Caspase-1 exists as the inactive form, pro-caspase-1, which is activated by the NLRP3 inflammasome, composed of P10 and P20 subunits. We chose subunit P20 to test the expression of caspase-1. As shown in Figure 3, the result of Western blotting, performed using protein extracted from muscle samples of both groups, showed that there was significantly higher protein expression of both NLRP3 and caspase-1 in patient group than in the control group. However, no difference of protein expression between DM and PM patients was found. The result indicates that in DM/PM, the highly expressed NLRP3 cleaves pro-caspase-1 to active caspase-1, which activates pro-IL1β and pro-IL18 into IL-1β and IL-18. The active cytokines might cause cascade inflammation and muscle damage in DM/PM.
Figure 3.
Western blotting analysis of the NLRP3 and caspase-1 p20 expression in muscle tissue (mean ± SD, n = 3–6). The relative grayscale of NLRP3 and p20 was significantly higher in the DM/PM group than that in the control group and no significant difference between the DM and PM groups (Western blotting analysis of NLRP3, DM vs. control *P < 0.01, PM vs. control †P < 0.01; Western blotting analysis of p20, DM vs. control ‡P < 0.01, PM vs. control §P < 0.01). DM: Dermatomyositis; PM: Polymyositis; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; NLRP3: NOD-like receptor family, pyrin domain containing 3 inflammasome; SD: Standard deviation.
DISCUSSION
According to previous studies, DM/PM is generally considered autoimmune muscle disorders. However, the underlying immunopathogenic mechanisms of DM/PM remain poorly understood. According to previous research, the immune response of DM is thought to target the endothelium of capillaries and small blood vessels, leading to the activation of the complement and deposition of C5b9 membrane attack complexes, resulting in the depletion of capillaries and muscle ischemia.[17] Meanwhile, the pathogenesis of PM is mainly mediated by toxic CD8+ T-cells. The CD8/MHC-I complex is characteristic muscle pathology for PM. CD8+ cells surround and invade nonnecrotic muscle fibers, which express MHC Class I molecules and are thought to cause perforin-mediated cytotoxic injury.[18] No matter what kind of immune cells infiltrate the muscle tissue, it is a process that is heavily dependent upon the presence of multiple cytokines.[19] The cellular sources of cytokines are immune cells, endothelial cells, and muscle cells, and their interrelations have the potential to amplify inflammatory responses. Recent studies have demonstrated that the overexpression of tumor necrosis factor (TNF), interferon (IFN), and IL are key mediators in the pathogenesis of DM/PM.[20] TNF-α is believed to be an important regulator of the inflammation associated with DM/PM. A previous study indicated that TNF-α mRNA was upregulated in DM (X12) and PM (X26).[21] In Greenberg's study, gene expression microarray has shown that IFN-α/β-inducible gene and protein expression might be an important part of the pathogenesis of DM.[22] While in the IL family, IL-1β and IL-18 regulate the immune response in DM/PM. Baird also found that there was a significant upregulation of IL-1β compared to IL-1α.[23] In addition, two studies conducted by Tucci et al.[15,24] suggested that the deregulated IL-18/IL-18R pathway might be pathogenetic in inflammatory myopathies and the measurement of IL-18 might be predictive of the disease activity. In our study, the significant elevations of serum IL-1β/IL-18 and mRNA expression of IL-1β/IL-18 in muscle samples are observed in the DM/PM patient group, which indicate the implication of IL-1β/IL-18 in the pathogenesis of DM/PM. The next aim is to find the source of IL-1β/IL-18.
IL-1β and IL-18 exist in the cytoplasm as the proinflammatory cytokines, pro-IL1β and pro-IL18, which will be secreted and functional only after being cleaved by caspase-1. In addition, the NLRP3 inflammasome is a key factor in this process. This inflammasome is an intracellular macromolecular complex that serves as a platform for the activation of proinflammatory caspase-1, which in turn cleaves IL-1β and IL-18 from their preforms. Diverse molecular entities, including bacteria, viruses, purified microbial products, components of dying cells, small molecule immune activators, and crystalline or aggregated materials can activate NLRP3.[25] When activated, NLPR3 senses the ligand via its C-terminal leucine-rich repeat domain and undergoes an ATP-dependent self-oligomerization mediated by an intermediate nucleotide binding and oligomerization domain (NACHT). Then, a homotypic interaction between the N-terminal pyrin domain of NLRP3 and ASC, and subsequently between the caspase activation and recruitment domain of the ASC and pro-caspase-1, will recruit pro-caspase-1 to the high molecular weight complex, leading to its auto-cleavage and activation.[12] Recent studies have demonstrated that NLRP3 inflammasome is involved in the pathogenesis of multiple autoimmune diseases. Our study indicated that in DM/PM patients, the high expression of the NLRP3 inflammasome in muscle tissue led to high expression of activated caspase-1, which in turn caused the upregulation of IL-1β and IL-18 and enhanced cellular immunity. In contrast, we did not find significant differences in NLRP3 and caspase-1 expression for the DM/PM patients and the controls (data not shown), which might indicate that the NLRP3 inflammasome is implicated in the local muscle inflammation in DM/PM, and the released IL-1β/IL-18 could exacerbate disease progression.
The cause of NLRP3 inflammasome activation in DM/PM is poorly understood until now. Cassel and Sutterwala [26] indicated that cell disruption leads to endogenous NLRP3 inflammasome activation. Following cellular disruption, the inflammasome can spontaneously form and acquire the ability to process pro-IL1β into its mature form. In addition, muscle fiber necrosis might release high mobility group box-1, ATP, and hyaluronic acid,[27,28] which are damage-associated molecular patterns and might be activators of the NLRP3 inflammasome. Furthermore, in one of the three NLRP3 activation models generally supported in the current literature,[29] the release of cathepsin B is somehow sensed by NLRP3 and inflammasome activation is triggered. While the upregulation of cathepsin B is related to PM disease activity and the inhibition of cathepsin B showed protective effects in a guinea pig model,[30] the relation of NLRP3 and cathepsin B in DM/PM needs to be studied.
To be noted, there was great heterogeneity of the patients recruited in our study that some received treatment while some did not. This would cause great variation of the result. Moreover, we need to do deep research on animal model to explain the role of NLRP3 inflammasome in the pathogenesis more clearly.
In conclusion, the NLRP3 inflammasome plays a key role in the development of DM/PM. Our research provides a novel insight into the molecular pathway of the pathogenesis of DM/PM and suggests that the NLRP3 pathway might be a therapeutic target for DM/PM.
Financial support and sponsorship
This work was supported by a grant from the National Natural Science Foundation of China (No. 81271399).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372:1734–47. doi: 10.1056/NEJMra1402225. doi: 10.1056/NEJMra1402225. [DOI] [PubMed] [Google Scholar]
- 2.De Paepe B, Creus KK, De Bleecker JL. Role of cytokines and chemokines in idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2009;21:610–6. doi: 10.1097/BOR.0b013e3283317b31. doi: 10.1097/BOR.0b013e3283317b31. [DOI] [PubMed] [Google Scholar]
- 3.Ernste FC, Reed AM. Idiopathic inflammatory myopathies: Current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83–105. doi: 10.1016/j.mayocp.2012.10.017. doi: 10.1016/j.mayocp.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Luo YB, Mastaglia FL. Dermatomyositis, polymyositis and immune-mediated necrotising myopathies. Biochim Biophys Acta. 2015;1852:622–32. doi: 10.1016/j.bbadis.2014.05.034. doi: 10.1016/j.bbadis.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Pestronk A. Acquired immune and inflammatory myopathies: Pathologic classification. Curr Opin Rheumatol. 2011;23:595–604. doi: 10.1097/BOR.0b013e32834bab42. doi: 10.1097/BOR.0b013e32834bab42. [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 7.Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:35–52. doi: 10.1111/imr.12286. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–19. doi: 10.1016/j.cell.2012.07.007. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–228. doi: 10.1038/nature04515. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 10.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–237. doi: 10.1038/nature04516. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 11.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J Inflamm Res. 2015;8:15–27. doi: 10.2147/JIR.S51250. doi: 10.2147/JIR.S51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg I, Kratz AK, Alexanderson H, Patarroyo M. Decreased expression of interleukin-1alpha, interleukin-1beta, and cell adhesion molecules in muscle tissue following corticosteroid treatment in patients with polymyositis and dermatomyositis. Arthritis Rheum. 2000;43:336–48. doi: 10.1002/1529-0131(200002)43:2<336::AID-ANR13>3.0.CO;2-V. doi: 10.1002/1529-0131(200002)43:2<336: AID-ANR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Tucci M, Quatraro C, Dammacco F, Silvestris F. Interleukin-18 overexpression as a hallmark of the activity of autoimmune inflammatory myopathies. Clin Exp Immunol. 2006;146:21–31. doi: 10.1111/j.1365-2249.2006.03180.x. doi: 10.1111/j.1365-2249.2006.03180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, et al. Tcell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. J Immunol. 2013;190:2068–79. doi: 10.4049/jimmunol.1202661. doi: 10.4049/jimmunol.1202661. [DOI] [PubMed] [Google Scholar]
- 17.Dalakas MC. Pathogenesis and therapies of immune-mediated myopathies. Autoimmun Rev. 2012;11:203–6. doi: 10.1016/j.autrev.2011.05.013. doi: 10.1016/j.autrev.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Dalakas MC. Mechanisms of disease: Signaling pathways and immunobiology of inflammatory myopathies. Nat Clin Pract Rheumatol. 2006;2:219–27. doi: 10.1038/ncprheum0140. doi: 10.1038/ncprheum0140. [DOI] [PubMed] [Google Scholar]
- 19.Loell I, Lundberg IE. Can muscle regeneration fail in chronic inflammation: A weakness in inflammatory myopathies?J Intern Med. 2011;269:243–57. doi: 10.1111/j.1365-2796.2010.02334.x. doi:10.1111/j.1365-2796.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 20.Moran EM, Mastaglia FL. Cytokines in immune-mediated inflammatory myopathies: Cellular sources, multiple actions and therapeutic implications. Clin Exp Immunol. 2014;178:405–15. doi: 10.1111/cei.12445. doi: 10.1111/cei.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt J, Barthel K, Wrede A, Salajegheh M, Bähr M, Dalakas MC. Interrelation of inflammation and APP in sIBM: IL-1 beta induces accumulation of beta-amyloid in skeletal muscle. Brain. 2008;131(Pt 5):1228–40. doi: 10.1093/brain/awn053. doi: 10.1093/brain/awn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg SA, Pinkus JL, Pinkus GS, Burleson T, Sanoudou D, Tawil R, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005;57:664–78. doi: 10.1002/ana.20464. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- 23.Baird GS, Montine TJ. Multiplex immunoassay analysis of cytokines in idiopathic inflammatory myopathy. Arch Pathol Lab Med. 2008;132:232–8. doi: 10.5858/2008-132-232-MIAOCI. doi: 10.1043/1543-2165(2008)132[232: MIAOCI] 2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Tucci M, Quatraro C, Dammacco F, Silvestris F. Increased IL-18 production by dendritic cells in active inflammatory myopathies. Ann N Y Acad Sci. 2007;1107:184–92. doi: 10.1196/annals.1381.020. doi: 10.1196/annals.1381.020. [DOI] [PubMed] [Google Scholar]
- 25.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 26.Cassel SL, Sutterwala FS. Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol. 2010;40:607–11. doi: 10.1002/eji.200940207. doi: 10.1002/eji.200940207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zong M, Bruton JD, Grundtman C, Yang H, Li JH, Alexanderson H, et al. TLR4 as receptor for HMGB1 induced muscle dysfunction in myositis. Ann Rheum Dis. 2013;72:1390–9. doi: 10.1136/annrheumdis-2012-202207. doi: 10.1136/annrheumdis-2012-202207. [DOI] [PubMed] [Google Scholar]
- 28.Cseri K, Vincze J, Cseri J, Fodor J, Csernátony Z, Csernoch L, et al. HMGB1 expression and muscle regeneration in idiopathic inflammatory myopathies and degenerative joint diseases. J Muscle Res Cell Motil. 2015;36:255–62. doi: 10.1007/s10974-015-9411-7. doi: 10.1007/s10974-015-9411-7. [DOI] [PubMed] [Google Scholar]
- 29.Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30:628–31. doi: 10.1007/s10875-010-9440-3. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 30.Feng Y, Ni L, Wang Q. Administration of cathepsin B inhibitor CA-074Me reduces inflammation and apoptosis in polymyositis. J Dermatol Sci. 2013;72:158–67. doi: 10.1016/j.jdermsci.2013.06.014. doi: 10.1016/j.jdermsci.2013.06.014. [DOI] [PubMed] [Google Scholar]




