Abstract
Background:
Talaromyces (Penicillium) marneffei (TM) is an emerging dimorphic human pathogenic fungus that is endemic to Southeast Asia. TM mostly occurs as an opportunistic infection in patients with human immunodeficiency virus (HIV). The objective of this study was to compare the clinical and laboratory parameters of patients with TM infections who were HIV-positive and HIV-negative and to assess therapies and outcomes.
Methods:
This was a retrospective analysis of 26 patients diagnosed with disseminated TM infection from September 2005 to April 2014 at Fujian Provincial Hospital, China.
Results:
Patients with TM infection tend to present with fever, weight loss, and anemia. The time from symptom onset to confirmed diagnosis was greater for HIV-negative patients (n = 7; median: 60 days, range: 14–365 days) than for HIV-positive patients (n = 19; median: 30 days, range: 3–90 days, Mann–Whitney U = 31.50, P = 0.041). HIV-negative patients were more likely to have dyspnea (57.1% vs. 5.3%, χ2 = 8.86, P = 0.010), low neutrophil count (Mann–Whitney U = 27.00, P = 0.029), high CD4 count (Mann–Whitney U = 0.00, P = 0.009), and high lymphocyte count (Mann–Whitney U = 21.00, P = 0.009). There were no significant differences in other demographic, clinical, or biochemical characteristics. Among all the patients, 12 HIV-positive patient and 1 HIV-negative patient received amphotericin and fluconazole treatment, 9 of whom improved, 1 died, 2 had kidney damage, 1 had hypokalemia due to exceeded doses.
Conclusions:
HIV-negative patients with TM infections tend to have a longer diagnostic interval, a higher percentage of dyspnea, higher levels of CD4 and lymphocytes, and lower neutrophil counts than TM infection in HIV-positive patients. Treatment programs with amphotericin and fluconazole are mostly effective.
Keywords: Clinical Characteristics, Human Immunodeficiency Virus, Talaromyces marneffei
INTRODUCTION
Talaromyces marneffei (TM) infection is a deep fungal infection caused by the dimorphic fungus TM (formerly known as Penicillium marneffei), a species endemic to Southeast Asia, including some districts in China (e.g., Guangdong, Guangxi, Yunnan, Hong Kong), and nearby countries including Thailand, Laos, and Malaysia.[1,2,3,4] There are also reports of TM infection outside of Southeast Asia in patients who traveled to this region.[5,6] There is some indication that the symptoms of TM infection are different in human immunodeficiency virus (HIV)-positive and HIV-negative patients,[7,8] presumably because of the differences in the causes and characteristics of immune suppression. However, little is known about TM infection in HIV-negative patients, and there have been no previous reports of TM infection in Fujian, China. TM infection typically disseminates hematogenously, and invades and damages numerous organs.[9] Fever is the most common clinical symptom and occurs in 80–90% of cases. Previous research indicated a higher incidence of fever in HIV-positive than HIV-negative patients with TM infections.[6]
In this study, we retrospectively analyzed 26 cases of TM infection in Fujian, China and compared the characteristics of HIV-positive and HIV-negative patients with TM infection who presented in Fujian Provincial Hospital from September 2005 to April 2014. Thus, this study addressed certain clinical and scientific questions. From the clinical perspective, TM infection in China is occasionally found in several southern cities, but seldom elsewhere. We therefore sought to identify the clinical manifestations and outcomes after therapy for TM infection in HIV-positive and HIV-negative patients from Southern China. We focused on the clinical manifestations and efficacy of therapies for TM infection in an effort to provide evidence that can be used for clinical diagnosis and treatment. From the scientific perspective, we sought to identify the clinical manifestations of TM infection in HIV-negative and HIV-positive patients in order to shed light on the pathogenesis of this disease in these two groups of patients.
METHODS
Patient enrollment
This study retrospectively analyzed the records of 26 patients who were admitted to Fujian Provincial Hospital (a tertiary level hospital with 2500 beds) with diagnoses of TM infection from September 2005 to April 2014. All HIV-positive and HIV-negative patients were from the same population. The last follow-up time was June 2014. This study was approved by the Institutional Review Board of the Fujian Provincial Hospital in Fuzhou City, Fujian Province, China (No. K2014-023-01). The need for informed consent was waived because this study was retrospective.
Data on demographic factors (gender, age, weight, smoking, drinking, employment rate, and coexisting disease), personal history (visit prostitutes, drug addiction, and opportunistic infections), clinical symptoms and signs (cough, expectoration, dyspnea, chest pain, diarrhea, vomiting, fever, body temperature, respiratory rate, moist rales, and dry rales) and laboratory test results (white blood cell [WBC] counts, neutrophil, lymphocyte, hematocrit, and platelets [PLT] [XE-500 Blood Analyzer, Sysmex, Japan] and albumin [ALB], alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase [ALP], total bilirubin [Tbil], blood creatinine, blood sodium, prothrombin time and activated partial thromboplastin time [the Cobas 8000 Automatic Biochemical Analyzer, Roche, Switzerland], pH, PaO2, PaO2/FiO2 [ABL8000, Radiometer, Danes]) T-cell immune function was assessed by measuring CD3, CD4, NK, and CD19, and humoral immune function was assessed by measuring IgG, IgM, IgA, C3, C4, and C-reactive protein (CRP) (FACSCalibur Flow Cytometry, BD Biosciences, Becton, Dickinson and Company, USA). Chest radiology (X-ray or computed tomography [CT], Siemens, Germany) was also examined. All treatments and outcomes were recorded.
Diagnosis of acquired immune deficiency syndrome
Acquired immune deficiency syndrome (AIDS) was diagnosed by the 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults from the Centers for Disease Control and Prevention (CDC) of the United States (1992) and the 2005 Guidelines for HIV/AIDS Diagnosis and Treatment in China, which was jointly issued by the Chinese Medical Association and Ministry of Health (2005).[10] Positive HIV antibodies were verified by immunoblotting in the HIV/AIDS laboratory at the Fujian Provincial Center for Disease Control and Prevention.
Diagnosis of Talaromyces marneffei infection
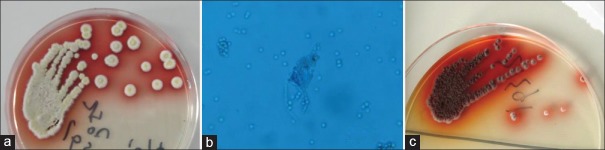
TM infection was diagnosed based on the presence of this species in body fluids (blood [n = 23, 92%], bone marrow [n = 5, 20%], bronchoalveolar lavage fluid [n = 1, 4%], bronchial secretions [n = 1, 4%], ascites [n = 1, 4%]), and in tissue biopsy [n = 1, 4%]).[11,12] Blood and bone marrow were directly inoculated into neutralizing antibiotic flasks of BacT/Alert three-dimensional blood culture system (BioMérieux, Inc., Durham, NC, USA). If the cultures were positive, specimens were inoculated onto Sabouraud Dextrose Agar plate (Beiruite Bio-technology Co., Ltd., Zhengzhou, Henan, China) as well as other body fluids, at 25°C for 48 h and then identified by the mycology laboratory of the Fujian Provincial Hospital, which is a Biosafety level-2 certified laboratory. Identification was based on the characteristic appearance of the mycelial phase, with a soluble red pigment in the background that gradually diffused into the agar [Figure 1a]. The squash specimen is flower-like, with characteristic “broom sticks” under microscopy [Figure 1b]. The yeast phase grows at 37°C in culture, appears white or brown with a gyrus-shaped or radial-cleft-like surface, and has no pigments. The yeast spores are sausage-shaped and partitioned by septa [Figure 1c].
Figure 1.
Representative figures of Talaromyces marneffei colonies. (a) Fungi were cultured in Sabouraud's medium at 25°C, at which mycelial phase and typical red products develop. (b) Rose-shaped fungi appear after smearing (×200). (c) Fungi were cultured in Sabouraud's medium at 37°C, at which the yeast phase and typical red products develop.
An oral temperature above 37.2°C was defined as fever. We defined four levels of fever: mild (37.3 to 38.0°C), moderate (38.1–39.0°C), severe (39.1–40.9°C), and very severe (≥41.0°C).
Treatment regimens
Treatment regimens were according to the Guidelines for Diagnosis and Treatment of HIV/AIDS in China (2005).[10] Among HIV-positive patients, four patients were lost to follow-up. Therapeutic regimens for the remaining 15 cases are described below. Twelve cases were treated with amphotericin B liposomal complex (1 mg/kg qd) or amphotericin B (0.5–0.6 mg/kg qd). Eight of these 12 patients received additional treatments as follows: amphotericin B liposomal complex or amphotericin B with fluconazole (0.4 g qd) for 13–32 days, plus oral fluconazole (0.4 g qd) for 3–4 months. Among the 15 cases who received antifungal therapy, 12 cases also received antiretroviral therapy using sanilvudin (d4T, 30 mg bid) + lamivudine (3TC, 0.3 g qd) + nevirapine (NVP, 200 mg bid) or zidovudine (AZT, 300 mg bid) +3TC (0.3 g qd) + NVP (200 mg qd). The other three patients were treated with fluconazole (0.4 g qd) alone due to intolerance to the other treatment regimens.
Among HIV-negative patients, two patients were treated with liposomal amphotericin B (1 mg/kg daily) or amphotericin B (0.5–0.6 mg/kg qd) combining with itraconazole (200 mg/d), one was treated with itraconazole, one was treated with fluconazole (0.4 g qd) alone due to economic difficulty, and the other three patients did not receive any treatment without timely diagnosis.
Statistical analysis
Continuous variables with normal distributions were presented as mean ± standard deviations (SDs) and continuous variables with nonnormal distributions were shown as medians and interquartile ranges. The differences between groups were assessed by an independent t-test (for age and weight) and the Mann–Whitney U-test (for disease duration and other biochemical indexes). Counts and percentages were calculated for categorical variables and compared by Fisher's exact test. All statistical assessments were two-sided and evaluated at the 0.05 level of significance. All analyses were performed using SPSS 22.0 statistics software (IBM SPSS Inc., Chicago, Illinois, USA).
RESULTS
Baseline characteristics
Table 1 shows the baseline characteristics of HIV-positive and HIV-negative patients who were diagnosed with TM infection and treated at Fujian Provincial Hospital from September 2005 to April 2014. These two groups had similar age, sex distribution, body weight, employment rate, and rates of smoking and drinking. However, the HIV-negative group had a significantly longer diagnostic interval (time from onset of symptoms to confirmed diagnosis of TM infection; Mann–Whiney U = 31.50, P = 0.041) and a greater rate of opportunistic infection (Mann–Whitney U = 6.18, P = 0.041). The data also show that the HIV-negative group had a higher prevalence of malignancy (14.3% vs. 0%), systemic lupus erythematosus (SLE) (28.6% vs. 0%), primary immunodeficiency (14.3% vs. 0%), and leukemia (14.3% vs. 0%), and the HIV-positive group had a higher prevalence of liver dysfunction (58.1% vs. 28.6%). However, these other differences were not statistically significant. There was one case from Yunnan, one from Jiangsu, one from Jianxi, and one who traveled to Vietnam; all other patients were from Fujian province and never visited Thailand or other Southeast Regions (e.g., Guangdong, Guangxi, or Hong Kong) of China (data not shown).
Table 1.
Characteristics of 26 patients diagnosed with TM from September 2005 to April 2014 in Fujian, China
| Characteristics | HIV-negative (n = 7) | HIV-positive (n = 19) | Statistical values | P |
|---|---|---|---|---|
| Age (years) | 44 ± 25 | 42 ± 12 | 0.17* | 0.867 |
| Weight (kg)¶ | 54 ± 25 | 57 ± 6 | 5.27† | 0.751 |
| Duration from symptom onset to diagnosis (days) | 60 (20, 180) | 30 (10, 45) | 31.50† | 0.041 |
| Male | 6 (86) | 15 (79) | 0.15‡ | 0.999 |
| Employment | 1 (15) | 1 (6) | 2.53‡ | 0.474 |
| Smoking | 2 (29) | 7 (37) | 0.16‡ | 0.999 |
| Drinking | 1 (14) | 3 (16) | 0.01‡ | 0.999 |
| Underlying diseases§,|| | 5 (71) | 11 (58) | 0.40‡ | 0.668 |
| Malignancy | 1 (14) | 0 | 2.82‡ | 0.269 |
| Systemic lupus erythematosus | 2 (29) | 0 | 5.88‡ | 0.065 |
| Primary immunodeficiency | 1 (14) | 0 | 2.82‡ | 0.269 |
| Leukemia | 1 (14) | 0 | 2.82‡ | 0.269 |
| Opportunistic infection¶ | 1 (17) | 14 (74) | 6.18‡ | 0.023 |
| Fungus†† | 1 | 9 | – | |
| Herpes†† | 0 | 3 | – | |
| Syphilis†† | 0 | 3 | – | |
| Hepatitis B virus†† | 0 | 1 | – | |
| Heart disease | 0 | 1 (5) | 0.38‡ | 0.999 |
| Liver dysfunction** | 2 (29) | 10 (59) | 1.82‡ | 0.371 |
| Hepatitis B | 0 | 1 (5) | 0.38‡ | 0.999 |
| Multiple organ dysfunction syndrome | 0 | 1 (5) | 0.38‡ | 0.999 |
Age and weight are presented as mean ± SD, disease duration as median (P25, P75), and other parameters as count (%). P values were estimated by an *Independent t-test, †Mann–Whitney U-test, or ‡Fisher’s exact test;§Underlying disease indicates malignancy, systemic lupus erythematosus, primary immunodeficiency and any disease of the heart, liver or kidney;|| One patient may have more than one underlying disease; ¶Missing data were found in six patients for weight and in one patient for opportunistic infection; **Liver dysfunction was defined as aminotransferase >40 U/L and aspartate aminotransferase >40 U/L; ††One patient might be infected by two or more pathogens. Dash indicates no comparisons were done. SD: Standard deviation; TM: Talaromyces marneffei; HIV: Human immunodeficiency virus.
Initial symptoms, diagnosis, and admitting departments
Most patients were admitted with fever as the main complaint (n = 23/26, 88.5%). Among the HIV-positive patients, seven cases were initially diagnosed with pulmonary infections at the Respiratory Departments, seven were diagnosed with gastrointestinal tract infections at the Gastroenterology Department, two were diagnosed with leukemia at the Hematology Department, one was diagnosed with rheumatic fever at the Rheumatology Department, one was diagnosed with an intracranial infection at the Neurology Department, and one was diagnosed with infective endocarditis at the Cardiology Department. In the HIV-negative group, three patients were admitted to the Department of Respiratory and Critical Care Medicine, two were diagnosed with SLE and pulmonary infection at the Rheumatology Department, one was admitted to the Pediatrics Department with a pulmonary infection, and one was diagnosed with postchemotherapy infection at the Hematology Department. One patient with SLE who received prednisone for 3 months had oral leukoplakia on admission. The throat secretion was collected with a swab and oral Candida albicans infection was diagnosed.
Clinical symptoms and computed tomography findings
Table 2 shows the clinical symptoms and lung CT findings of the HIV-negative and HIV-positive groups. The two groups were similar in most clinical symptoms, including fever, cough, weight loss, lymphadenectasis, hepatosplenomegaly, rash, wheezing in the lungs, and pleural effusion. However, dyspnea was significantly more common in patients who were HIV-negative (57% vs. 5%, χ2 = 8.86, P = 0.010).
Table 2.
Clinical signs and symptoms of 26 patients diagnosed with Penicilliosis marneffei in Fujian, China
| Clinical sign/symptom | HIV-negative (n = 7) | HIV-positive (n = 19) | Statistical values | P |
|---|---|---|---|---|
| Fever* | ||||
| None | 2 (29) | 1 (5) | 1.73 | 0.785 |
| Mild | 1 (14) | 4 (21) | ||
| Moderate | 2 (29) | 8 (42) | ||
| Severe | 1 (14) | 6 (32) | ||
| Very severe | 1 (14) | 0 | ||
| Cough | 3 (43) | 5 (26) | 0.66 | 0.635 |
| Dyspnea | 4 (57) | 1 (5) | 8.86 | 0.010 |
| Abdominal pain | 0 | 5 (26) | 2.28 | 0.278 |
| Weight loss | 5 (71) | 7 (37) | 2.46 | 0.190 |
| Lymphadenopathy | 1 (14) | 4 (21) | 0.15 | 0.999 |
| Splenomegaly | 3 (43) | 9 (47) | 0.04 | 0.999 |
| Hepatomegaly | 1 (14) | 1 (5) | 0.59 | 0.474 |
| Necrotic skin rash | 0 | 5 (26) | 2.28 | 0.278 |
| Rales, rhonchi, or wheezes in lung | 2 (29) | 3 (16) | 0.54 | 0.588 |
| Pleural effusion | 0 | 3 (16) | 1.25 | 0.540 |
| Computed tomography results | ||||
| Nodus | 2 (29) | 3 (16) | 0.51 | 0.588 |
| Increase in density of plaques | 2 (29) | 2 (11) | 1.28 | 0.287 |
| Cavitary change | 1 (14) | 1 (5) | 0.59 | 0.474 |
| Interstitial lung disease | 2 (29) | 0 | 5.88 | 0.065 |
Data are shown as count (%) and tested by Fisher’s exact test. *Fever was classified as mild (37.3–38.0°C), moderate (38.1–39.0°C), severe (39.1–40.9°C), or very severe (≥41.0°C). HIV: Human immunodeficiency virus.
Figure 2 shows representative chest X-rays of an HIV-positive patient and an HIV-negative patient. Figure 2a shows a ground-glass opacity in the upper lobe of the left lung of an HIV-negative patient. Figure 2b shows nearly normal lungs of an HIV-positive patient. Figure 3 shows representative CT imaging of lesions in the lungs of four TM patients. These images show multinodular lesions in both lungs of an HIV-negative patient [Figure 3a], patchy shadows in both lungs of an HIV-positive patient [Figure 3b], ground-glass-like lesions in both lungs of an HIV-positive patient [Figure 3c], and diffused interstitial and multiple cavity-like changes in both lungs of an HIV-negative patient [Figure 3d]. In general, the intrapulmonary lesions were more severe in HIV-negative patients.
Figure 2.
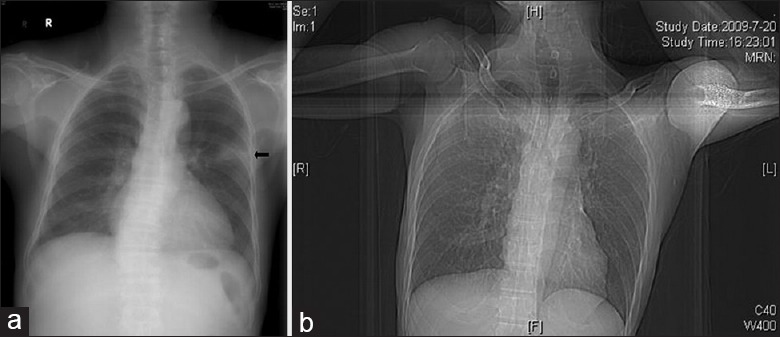
Representative chest X-rays of human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with Talaromyces marneffei infection. (a) Male human immunodeficiency virus (negative), 71-year-old with chronic lymphocytic leukemia, the X-ray shows patchy shadows in the upper lobe of the left lung (arrow). (b) Male, human immunodeficiency virus (positive), 54-year-old with repeated fever for 2 weeks, the X-ray shows nearly normal in both lungs.
Figure 3.
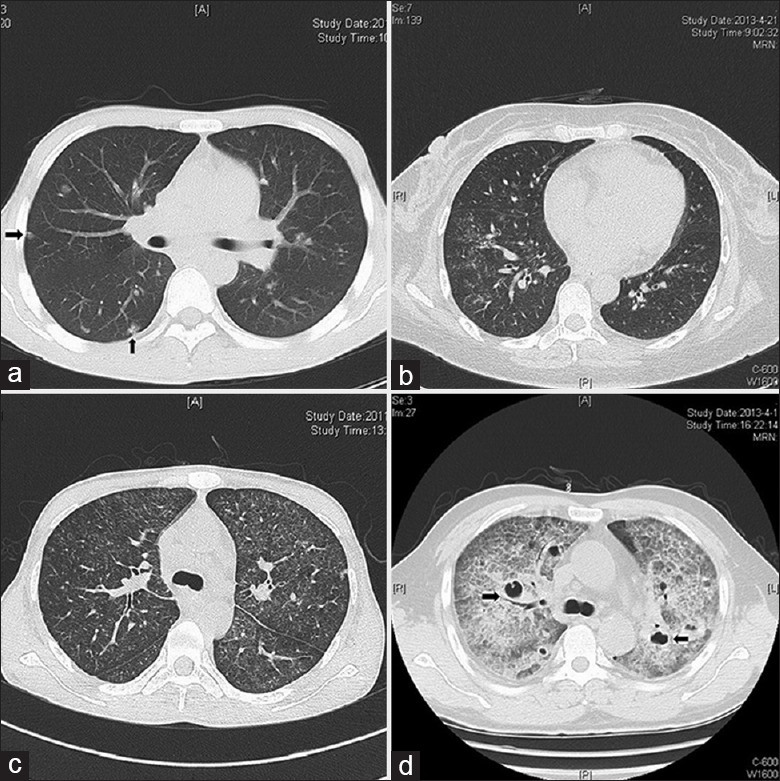
Representative computed tomography images of human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with Talaromyces marneffei infections. (a) Male, 31 years, human immunodeficiency virus (positive), computed tomography shows multinodular lesions (arrows) in both lungs. (b) Female, 47 years, human immunodeficiency virus (positive), computed tomography shows scattered patchy shadows in both lungs. (c) Male, 31 years, human immunodeficiency virus (positive), computed tomography shows diffuse ground-glass-like lesions in both lungs. (d) Male, 56 years, human immunodeficiency virus (negative), computed tomography shows diffuse interstitial lesions with cavity-like changes (arrows) in both lungs.
Biochemical indexes
Table 3 compares the biochemical indexes of HIV-positive and HIV-negative patients. The two groups were similar with regard to WBCs, hemoglobin, PLT, ALB, ALT, AST, ALP, Tbil, and C3. However, HIV-positive patients had a significantly higher level of neutrophils (Mann–Whiney U = 27.00, P = 0.029) and lower levels of CD4-positive cells (Mann–Whiney U = 0.00, P = 0.009) and lymphocytes (Mann–Whiney U = 21.00, P = 0.009).
Table 3.
Biochemical indexes of 26 patients diagnosed with Penicilliosis marneffei in Fujian, China
| Biochemical index | HIV-negative (n = 7) | HIV-positive (n = 19) | U† | P |
|---|---|---|---|---|
| WBC (109/L), median (IQR)* | 9 (3–12) | 4 (3–6) | 43.00 | 0.178 |
| <4×109/L , n (%) | 2 (29) | 9 (50) | 0.252 | |
| (4–10)×109/L, n (%) | 3 (42) | 8 (44) | ||
| >10×109/L, n (%) | 2 (29) | 1 (6) | ||
| Hemoglobin (g/L), median (IQR)§ | 110.0 (95.0–150.0) | 106.0 (89.0–118.0) | 54.00 | 0.497 |
| Normal, n (%) | 3 (43) | 5 (26) | 0.671 | |
| Mild anemia, n (%) | 3 (43) | 9 (48) | ||
| Severe anemia, n (%) | 1 (14) | 5 (26) | ||
| Platelets (109/L), median (IQR) | 147.0 (95.0–201.0) | 113.0 (80.0–151.0) | 48.00 | 0.306 |
| <100×109/L, n (%) | 2 (29) | 6 (32) | 0.243 | |
| 100–300×109/L, n (%) | 4 (57) | 13 (68) | ||
| >300×109/L, n (%) | 1 (14) | 0 | ||
| Albumin (g/L), median (IQR)|| | 30 (27–35) | 29 (21–31) | 43.50 | 0.244 |
| <30 g/L, n (%) | 4 (57) | 8 (44) | 0.673 | |
| ≥30 g/L, n (%) | 3 (43) | 10 (56) | ||
| ALT (U/L), median (IQR) | 38 (16–48) | 48 (33–70) | 35.70 | 0.130 |
| AST (U/L), median (IQR) | 36.0 (27.0–133.0) | 70.0 (56.0–141.0) | 37.50 | 0.125 |
| Neutrophils (%), median (IQR) | 71 (45–80) | 82 (73–91) | 27.00 | 0.029 |
| ALP (U/L), median (IQR) | 93.0 (52.0–120.0) | 90.0 (75.0–148.0) | 51.00 | 0.901 |
| Total bilirubin (μmol/L), median (IQR) | 9 (7–12) | 9 (7–11) | 57.00 | 0.874 |
| CD4 (%), median (IQR)|| | 41 (35–46) | 2 (1–3) | 0.00 | 0.009 |
| C3 (g/L), median (IQR)|| | 1 (0–1) | 1 (0–1) | 19.50 | 0.941 |
| Lymphocytes (%), median (IQR) | 21 (15–42) | 12 (7–19) | 21.00 | 0.009 |
| Lymphocyte (109/L), median (IQR) | 2 (1–4) | 0 (0–1) | 20.00 | 0.008 |
*Data were not available for one patient for WBC and albumin, ten patients for CD4, and 13 patients for C3. †Mann–Whitney U-test §Hemoglobin was defined as normal (>120 g/L), mild anemia (90–120 g/L for males and 90–110 g/L for females), or moderate anemia (60–90 g/L); ||Data were not available for one patient for WBC and albumin, ten patients for CD4, and 13 patients for C3. IQR: Interquartile range; ALT: Alanine aminotransferase; AST: Aspartate transaminase; ALP: Alkaline phosphatase; WBC: White blood cells; HIV: Human immunodeficiency virus.
Results from different treatment regimens
Among 12 HIV-positive patients with amphotericin B treatment, one patient died due to treatment failure and two patients had kidney damage. One of the patients who had renal toxicity took itraconazole (400 mg qd) and the other took voriconazole (200 mg q12h) instead of the drugs above and both improved. One patient took amphotericin B for more than 105 days and the total dose exceeded 2.5 g; this medication was stopped due to hypokalemia. The symptoms of the remaining eight patients improved without obvious side effects. For the three patients treated with Fluconazole (0.4 g qd) alone, one patient died and two patients improved without recurrence, and oral fluconazole (0.4 g qd) as sequential therapy was carried out for 3–4 months. Two cases in the HIV-positive group were infected with other pathogens (Escherichia coli, Streptococcus agalactia, and Pseudomonas aeruginosa) and were treated with antibiotics as well.
Among HIV-negative patients, only two patients received timely treatments. One patient improved after combined administration of amphotericin B liposomal (1 mg/kg qd) and itraconazole (200 mg, qd) for 2–3 weeks. Thereafter, oral itraconazole (200 mg, bid) was given for 4 months. This patient was cured and no recurrence was observed. The other patient was given fluconazole (0.4 g qd) for only 2 months due to financial hardship, and the body temperature gradually decreased to normal. No subsequent treatment was given and there was no recurrence. Both patients had histories of glucocorticoid misuse, and gradual tapering was carried out after diagnosis. One patient with primary immunodeficiency (C3 complement deficiency) and one patient with chronic lymphocytic leukemia were treated with itraconazole, but both died due to serious underlying diseases. Two patients with SLE were initially misdiagnosed with aggravation of SLE and were discharged. The other patient with an intratracheal infection following radiotherapy for lung cancer was discharged without timely diagnosis. Most of the HIV-negative patients had a relatively long course of disease.
DISCUSSION
The HIV-positive and HIV-negative groups both had typical symptoms of systemic TM infection, including fever, weight loss, anemia, and hepatosplenomegaly. Lymph node enlargement, diarrhea, and necrotic skin rash were more common in the HIV-positive group, but dyspnea was more common in the HIV-negative group. There were no significant decreases in PLT numbers, absolute neutrophil count, and CRP (data not shown) in either group.
Most of our patients were males (HIV-positive group: 86%; HIV-negative group: 79%), in contrast to a previous study in Thailand, which reported that females accounted for 50% (17/34) of the HIV-negative population with TM infection.[8] The time from symptom onset to diagnosis of TM infection was significantly longer in our HIV-negative patients [Table 1]. The average time from symptom onset to diagnosis in our HIV-negative patients was similar to that reported in Nanning China,[3] but longer than that reported in Thailand.[8] This may be because TM infection progresses more rapidly in HIV-positive patients, and these cases tend to present with specific skin rashes, making diagnosis easier. In our HIV-negative group, 71.4% of patients (5/7) had an underlying disease-related to immune function, higher than reported in Thailand (38.2%)[8] and Guangdong (35.3%).[9]
Hematogenous dissemination can lead to hepatosplenic damage and lymph node enlargement, although in contrast to the results of Kawila et al.,[8] our HIV-positive and HIV-negative groups had no significant differences in lymphadenopathy or hepatosplenomegaly. Our results indicated that HIV-negative patients were more likely to develop dyspnea than HIV-positive patients, but patients in the two groups had similar lung CT results, in contrast to some previous reports.[13,14,15]
TM mostly occurs as an opportunistic infection in patients who are infected with HIV. Previous studies indicated that about 50% of patients with TM infections with HIV-positive have additional opportunistic infections.[16,17] More than 70% of our HIV-positive cases had co-infections, mostly with fungi, herpes, and syphilis, but none with tuberculosis or cytomegalovirus, in contrast to previous reports.[8] HIV-positive patients with low CD4 counts (<100 cells/µl) have greatly increased risk of TM infection.[18]
Bamboo rats (Rhizomys spp.) serve as an animal vector for TM, and the increasing incidence of TM infection in the endemic region parallels the increasing incidence of HIV/AIDS in the same region.[19] Patients may be infected by inhalation of asexual spores or by skin injury, and the weakened immune systems of HIV-infected patients may awaken latent infections.[20]
Rapid diagnosis and treatment of TM infection are critical because untreated patients have greatly increased risk of mortality from sepsis.[21] In cases of restricted infection of the lung, the symptoms may resemble bronchitis, bronchiectasis, acute respiratory distress syndrome, or tuberculosis, possibly complicating diagnosis. Patients with disseminated disease develop fever, anemia, weight loss, and skin lesions that typically occur on the face, extremities, and genitalia.[22] Such patients may also have thrombocytopenia, hepatosplenomegaly, and various symptoms of the pulmonary and digestive systems.
Drug susceptibility testing of TM showed that the minimum inhibitory concentration (MIC) for TM increases from low to high for voriconazole, itraconazole, terbinafine, fluconazole, to amphotericin B.[23] The CDC of the USA recommends 2 weeks of intravenous liposomal amphotericin B (3–5 mg/kg body weight) and then oral itraconazole (400 mg qd) for 10 weeks as a standard treatment for an HIV-infected patient.[24] However, in Fujian, China, itraconazole treatment is not possible in most patients due to its high cost. Thus, in our study, a combined therapy with amphotericin B and fluconazole was used in most patients although standard treatment with amphotericin B and itraconazole was used in several critically ill patients. The results showed therapeutic effectiveness in most patients. Our findings, together with previously reported results, indicate that amphotericin B is preferred for the therapy of TMinfection. Sequential therapy with fluconazole is also acceptable considering its low cost. Although some patients are responsive to fluconazole in the intensified treatment, caution is needed due to the limited effects.
Several limitations of this study should be mentioned. First, this is a retrospective study with a small sample size that studied patients admitted to a single institution. Second, some patients were lost to follow-up because they were from different provinces of China. Finally, the patients ranged widely in age (1–71 years old), so we cannot make any inferences regarding the characteristics or treatments of different age groups.
In conclusion, patients with TM infection tend to present with fever, weight loss, and anemia. Compared with HIV-positive patients, HIV-negative patients have a longer diagnostic interval, a higher percentage of dyspnea, higher levels of CD4-positive cells and lymphocytes, and lower neutrophil counts. Treatment programs with amphotericin and fluconazole are mostly effective.
Financial support and sponsorship
This study was supported by grants from the National Science and Technology Major Special Project (No. 2014ZX10004005), Key Project of Science and Technology of Fujian Province (No. 2012Y0016), the Development and Reform Commission of Fujian Province (No. 2013045), Key Project of Fujian Provincial Hospital (No. 2014080).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Sirisanthana T, Supparatpinyo K. Epidemiology and management of penicilliosis in human immunodeficiency virus-infected patients. Int J Infect Dis. 1998;3:48–53. doi: 10.1016/s1201-9712(98)90095-9. doi: 10.1016/s1201-9712(98)90095-9. [DOI] [PubMed] [Google Scholar]
- 2.Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: A series from 1994 to 2004. Hong Kong Med J. 2008;14:103–9. [PubMed] [Google Scholar]
- 3.Zhang JQ, Yang ML, Zhong XN, He ZY, Liu GN, Deng JM, et al. Acomparative analysis of the clinical and laboratory characteristics in disseminated penicilliosis marneffei in patients with and without human immunodeficiency virus infection. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31:740–6. [PubMed] [Google Scholar]
- 4.Larsson M, Nguyen LH, Wertheim HF, Dao TT, Taylor W, Horby P, et al. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in Northern Vietnam. AIDS Res Ther. 2012;9:24. doi: 10.1186/1742-6405-9-24. doi: 10.1186/1742-6405-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristofaro P, Mileno MD. Penicillium marneffe i infection in HIV-infected travelers. AIDS Alert. 2006;21:140–2. [PubMed] [Google Scholar]
- 6.De Monte A, Risso K, Normand AC, Boyer G, L'Ollivier C, Marty P, et al. Chronic pulmonary penicilliosis due to Penicillium marneffei: Late presentation in a french traveler. J Travel Med. 2014;21:292–4. doi: 10.1111/jtm.12125. doi: 10.1111/jtm.12125. [DOI] [PubMed] [Google Scholar]
- 7.Wong SY, Wong KF. Penicillium marneffei infection in AIDS. Patholog Res Int. 2011;2011:764293. doi: 10.4061/2011/764293. doi: 10.4061/2011/764293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: A retrospective study. BMC Infect Dis. 2013;13:464. doi: 10.1186/1471-2334-13-464. doi: 10.1186/1471-2334-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liyan X, Changming L, Xianyi Z, Luxia W, Suisheng X. Fifteen cases of penicilliosis in Guangdong, China. Mycopathologia. 2004;158:151–5. doi: 10.1023/b:myco.0000041842.90633.86. doi: 10.1023/b:myco.0000041842.90633.86. [DOI] [PubMed] [Google Scholar]
- 10.Chinese Medical Association;Chinese Center for Disease Control and Prevention. Guidelines for diagnosis and treatment of HIV/AIDS in China (2005) Chin Med J. 2006;119:1589–608. [PubMed] [Google Scholar]
- 11.Segretain G. Penicillium marneffei n.sp. agent of a mycosis of the reticuloendothelial system. Mycopathologia. 1959;11:327–53. doi: 10.1007/BF02089507. doi: 10.1007/bf02089507. [DOI] [PubMed] [Google Scholar]
- 12.DiSalvo AF, Fickling AM, Ajello L. Infection caused by Penicillium marneffei: Description of first natural infection in man. Am J Clin Pathol. 1973;60:259–63. doi: 10.1093/ajcp/60.2.259. [DOI] [PubMed] [Google Scholar]
- 13.Breton P, Bani Sadr F, Germaud P, Leautez S, Morin O, Raffi F. Unusual lung mycosis Penicillium marneffe i infection. Rev Pneumol Clin. 1998;54:85–7. [PubMed] [Google Scholar]
- 14.Saadiah S, Jeffrey AH, Mohamed AL. Penicillium marneffei infection in a non AIDS patient: First case report from Malaysia. Med J Malaysia. 1999;54:264–6. [PubMed] [Google Scholar]
- 15.Jung JY, Jo GH, Kim HS, Park MY, Shin JH, Chin BS, et al. Disseminated penicilliosis in a Korean human immunodeficiency virus infected patient from Laos. J Korean Med Sci. 2012;27:697–700. doi: 10.3346/jkms.2012.27.6.697. doi: 10.3346/jkms.2012.27.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong SS, Wong KH, Hui WT, Lee SS, Lo JY, Cao L, et al. Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. J Clin Microbiol. 2001;39:4535–40. doi: 10.1128/JCM.39.12.4535-4540.2001. doi: 10.1128/jcm.39.12.4535-4540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nor-Hayati S, Sahlawati M, Suresh-Kumar C, Lee KC. A retrospective review on successful management of Penicillium marneffei infections in patients with advanced HIV in Hospital Sungai Buloh. Med J Malaysia. 2012;67:66–70. [PubMed] [Google Scholar]
- 18.Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammachai C, Nelson KE, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–4. doi: 10.1093/clinids/14.4.871. doi: 10.1093/clinids/14.4.871. [DOI] [PubMed] [Google Scholar]
- 19.Vanittanakom N, Cooper CR, Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110. doi: 10.1128/CMR.19.1.95-110.2006. doi: 10.1128/cmr.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le T, Wolbers M, Chi NH, Quang VM, Chinh NT, Lan NP, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis. 2011;52:945–52. doi: 10.1093/cid/cir028. doi: 10.1093/cid/cir028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Supparatpinyo K, Nelson KE, Merz WG, Breslin BJ, Cooper CR, Jr, Kamwan C, et al. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated Penicillium marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrob Agents Chemother. 1993;37:2407–11. doi: 10.1128/aac.37.11.2407. doi: 10.1128/aac.37.11.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–3. doi: 10.1016/s0140-6736(94)91287-4. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Liang L, Chen J. In vitro antifungal drug susceptibilities of Penicillium marneffei from China. J Infect Chemother. 2013;19:776–8. doi: 10.1007/s10156-012-0511-7. doi: 10.1007/s10156-012-0511-7. [DOI] [PubMed] [Google Scholar]
- 24.Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE. National Institutes of Health;Centers for Disease Control and Prevention;HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:1308–11. doi: 10.1093/cid/ciu094. doi: 10.1093/cid/ciu094. [DOI] [PMC free article] [PubMed] [Google Scholar]