Abstract
Persons with Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) often complain of fatigue states (e.g. post-exertional malaise, brain fog) that are qualitatively different than normal, daily fatigue. Given the heterogeneous nature of ME/CFS, it is likely that individuals with this illness experience these fatigue types differently in terms of severity and frequency. It is also possible that meaningful subgroups of patients exist regarding different patterns of the fatigue experience. The purpose of this study was to investigate whether individuals with ME/CFS can be classified in a meaningful way according to the different types of fatigue they experience. One hundred individuals with ME/CFS participated in the study. Individuals that met inclusion criteria were administered the Multiple Fatigue Types Questionnaire (MFTQ), a five-factor instrument that distinguishes between different types of fatigue. A cluster analysis was used to classify patients into various clusters based upon factor subscale scores. Using a three-factor solution, individuals were classified according to illness severity (low, moderate, severe) across the different fatigue factors. However, a 5-cluster solution enabled participants with moderate to severe fatigue levels to fall out into more differentiated clusters and demonstrate distinct fatigue state patterns. These results suggest that fatigue patterns of individuals with ME/CFS are heterogeneous and patients may be classified into meaningful subgroups.
Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating illness that is characterized by severe fatigue, post-exertional malaise, cognitive interruption, sleeping difficulties, and often irregularities in neurological, immunological, endocrine, and autonomic areas.1 Although several biological abnormalities have been observed in ME/CFS2–4, its’ etiology and pathophysiology remains unclear. As such, researchers have relied on self-report scales and symptom inventories to capture information about the illness. Among those with ME/CFS, it is likely that persons have distinct illness subtypes characterized by different causal and/or maintenance mechanisms.1
To explore this illness heterogeneity, researchers have employed factor analysis has been used to find meaningful summary variables for the large number of symptoms experienced with ME/CFS. Using principal components analysis, Nisenbaum, Reyes, Mawle, and Reeves 5 administered a telephone survey to 1,150 respondents that had experienced severe fatigue for longer than one month. Their analysis of a symptom checklist revealed a three-factor structure accounting for the intercorrelations among the various symptoms (14 items) for those respondents experiencing fatigue for more than 6 months’ duration—specifically, a “fatigue/mood/cognition” factor, “flu-like” factor, and “visual impairment” factor emerged. The researchers failed to find an interpretable factor solution for those respondents with severe fatigue for 1–5 months’ duration, thus lending support to the CDC consensus CFS case definition’s6 major criterion of severe, unexplained fatigue for > 6 months. In a later study conducted on a community-based sample from Wichita, KS, Nisenbaum, Reyes, Unger, and Reeves7 used an exploratory and confirmatory factor analysis to investigate the underlying structure of ME/CFS. Although their findings again supported the Fukuda6 case definition, a slightly different factor structure emerged that contained the following three latent illness factors: musculoskeletal, infection and cognition–mood–sleep.
Three other studies have used principal components analysis to explore the underlying structure of ME/CFS symptomology. Friedberg, Dechene, McKenzie, and Fontanetta8 found a factor structure similar to that of Nisenbaum et al.5 for a group of 286 persons with ME/CFS. Specifically, a principal components analysis revealed the following three factors: cognitive problems, flu-like symptoms, and neurologic symptoms. In a community-based study of ME/CFS-like illness, Jason et al.9 extracted four-factors including Lack of Energy, Physical Exertion, Cognitive Functioning, and Fatigue and Rest. In this study, participants with a confirmed diagnosis of ME/CFS reported significantly higher scores on all of the factor scales as compared to other chronically-fatigued, non-ME/CFS groups. Finally, Arroll and Senior10 identified five illness factors and labeled them as follows: FMS-like, depression/anxiety, fatigue/post-exertional malaise, cognitive/neurological and IBS-like symptoms. In general, these studies suggest that there is a distinction between physical fatigue and cognitive interruption, and other factors, such as immune manifestations (“flu-like” and “IBS-like”) and post-exertional malaise.
Recently, Hickie et al.11 conducted an exploratory factor analysis on symptom data drawn from 37,724 subjects that were compiled through the dataset merging of 33 studies from 21 countries. These researchers obtained a five-factor solution including musculoskeletal pain/fatigue, neurocognitive difficulties, inflammation, sleep disturbance/fatigue, and mood disturbance. The authors concluded that mood disturbance is a core component of this illness. Unfortunately, the authors used the factor structure from a heterogeneous fatigue sample (subjects with and without a diagnosis of ME/CFS) and then applied these factors to the ME/CFS group, rather than employing an exploratory factor analysis on only the ME/CFS group. In addition, the lack of procedure reporting (estimation method, fit indices, model specifications) and tests of factorial invariance12 warrant a cautious interpretation of the results.
A different approach has been also used, and that involves cluster analysis, which classifies individuals into subgroups—called clusters—according to their similarity on specific measures of interest.13 The purpose of such an analysis is to identify a meaningful typology or taxonomy that is characterized by a small amount of within-cluster variation and a large amount of between-cluster variation. Using their community-based sample of chronically-fatigued participants from Wichita, Nisenbaum, Reyes, Unger, and Reeves7 identified a three cluster solution that classified individuals according to the number of symptoms (21 possible) they were currently experiencing. They found that the clusters represented illness severity as the majority of respondents who had been diagnosed with ME/CFS (34/43) were assigned to the same cluster and experienced more symptoms than those in the other two clusters. A similar solution was found by Jason and Taylor14 using factor scores of symptom severity that were based on their previous principal components analysis.9 Recently, a study by Arroll and Senior10 again replicated these findings using the Profile of Fatigue Related Symptoms15 and a symptom checklist. In sum, these studies suggest that across different ME/CFS indices subgroups emerge based on the severity of experienced symptoms.
Conversely, Hickie et al.16 identified a two-cluster solution that was differentiated by more than just illness severity. One cluster involved a “somatization-like” group, including those who have a higher prevalence of CFS symptoms and atypical symptoms, greater disability attributed to CFS and psychiatric symptoms, and a greater percentage unemployed. The second cluster included individuals with lower prevalence of CFS and atypical symptoms, less disability attributed to CFS and psychiatric symptoms, and a greater percentage employed. This study has been replicated in a multi-site study17, but there were significant inter-site differences in subclass distributions. As the distribution of symptoms differed between clusters, the researchers concluded the existence of ME/CFS subgroups not attributable to symptom severity.
One recent study by Kerr et al.18 examined the genetic profile of patients with ME/CFS versus healthy controls and found 88 genes (85 upregulated, 3 down-regulated) that were differentially expressed between groups. The researchers were then able to identify 7 subgroups of ME/CFS participants based on similar genetic profiles that differed on self-report health outcomes, clinical phenotypes, and severity. These findings are interesting given that the subtypes were distinguished by symptom severity as well as specific clinical patterns.
Considering that fatigue is the primary symptom of ME/CFS, some investigators have also explored the idea that fatigue is made up of a few underlying dimensions or states. Indeed, a number of recent studies have supported this finding19–22 and these studies have also observed that persons with ME/CFS are acutely sensitive to the different fatigue states as compared to healthy individuals (i.e. individuals who experience mild, benign fatigue that is associated with normal daily activities). This conceptualization of fatigue as a multi-faceted entity has been reflected in several of the fatigue measurement scales used in ME/CFS research over the last two decades.15, 22–24 However, it has been suggested that some of these commonly-used instruments lack accuracy in profiling the severe fatigue experienced by persons with ME/CFS25 and differentiating persons with ME/CFS from other psychiatric and somatoform disorders.26
Recently, Jason et al.27 developed a 22-item fatigue scale—the ME/CFS Fatigue Types Questionnaire (MFTQ)—that is designed to measure the different types of fatigue found in ME/CFS. The MFTQ, in addition to displaying sound psychometric properties, revealed a five-factor solution for a group of ME/CFS respondents when subjected to an exploratory factor analysis. The five-factors were extracted as follows: Post-Exertional Fatigue, Wired Fatigue, Brain Fog, Energy Fatigue, and Flu-Like Fatigue (see below in the “Methods” section for a detailed description of these factors). Additionally, a separate exploratory factor analysis was conducted for a group of healthy controls, from which only one factor emerged. This factor was labeled Global Fatigue and denotes a generally feeling of physical and/or mental tiredness associated with everyday activities (as noted earlier). Clearly, efforts aimed at understanding the latent structure of chronic fatigue and fatigue-related symptoms are accumulating and have important implications for deriving ME/CFS subtypes.
The aim of the present study is to build on prior work to determine whether ME/CFS can be appropriately represented as a general illness category that consists of distinct patient subgroups. The current study used a sample of persons with ME/CFS as opposed to a sample of individuals with chronic fatigue. Departing from previous classification studies, a fatigue typology measure was used to classify individuals as opposed to a symptom inventory. Although there is undoubtedly overlap between ME/CFS symptom factors and fatigue states (e.g. cognitive symptoms and mental fatigue), it is still of interest to use slightly different (though highly-related) metrics to validate solutions from prior investigations. We hypothesized that severity of symptoms would best differentiate the patients into clusters, as has been found in other studies.
Method
Participants & Procedure
The present study was approved by an Institutional Review Board (IRB) at the primary authors’ institution. A total of 130 participants were enrolled in the study. The participants were recruited through announcements sent to several ME/CFS support groups, academic and clinical conferences, and newsletters. Participants that responded to the announcement were told about the study and sent the test battery if they indicated an interest in participating, with a return envelope included. Only participants that had received a diagnosis of ME/CFS (determined by self-report) from a physician were included in the study.
Measures
ME/CFS Fatigue Types Questionnaire
The MFTQ contains 22 items that assess five different states of fatigue including: Post-Exertional, Wired, Brain Fog, Energy, and Flu-Like fatigue. The Post-Exertional fatigue factor is defined as severe exhaustion attributable to physical or mental effort. Wired fatigue is characterized by an aroused state of the mind or body concomitant with feelings of tiredness. Brain Fog fatigue refers to cognitive exhaustion that affects various mental capacities such as memory, speech, and information processing. Energy fatigue is defined as a depletion of energy resources needed for daily activities. Finally, Flu-Like fatigue is associated with physical symptoms commonly seen in cases of influenza, such as nausea, dizziness, and an elevated temperature.
For each item, respondents were asked to report the onset, frequency, and severity of each fatigue-related sensation as described in the corresponding statement. Onset was identified by the month/season and year in which the sensation emerged, frequency was reported on a Likert-scaled question (i.e. Never, Seldom, Often, Usually, Always), and severity was rated on a scale of 1 to 100 with higher scores denoting more fatigue experienced. A composite score for each item was calculated by multiplying the symptom rating score by the frequency score, resulting in an item score between 0 and 400. The MFTQ demonstrated acceptable reliability (alpha coefficients between .76 and .89 for each factor) and construct validity.27
Statistical Analysis
The statistical software package used for data analysis was SPSS for Windows, version 16.0. Study participants were classified according to their MFTQ mean factor scores using an agglomerative hierarchical cluster analysis. This technique treats each individual as its own cluster and iteratively combines similar individuals until a single cluster remains that contains every case.28 Similarity between cases—commonly referred to as distance—can be measured in a number of ways. In the present study, we used the squared Euclidean distance, which is the sum of squared differences on each variable between any two given cases (or clusters). The method of clustering used was the average within-groups linkage, which maximizes within-cluster homogeneity.29
Results
Participants
Of the 130 participants that were enrolled in the study, 8 were excluded from the analysis because they indicated they were never officially diagnosed with ME/CFS. Of the remaining 122 participants, 22 were excluded because they didn’t provide information on one or more of the MFTQ subscales (i.e. they had missing data on all items for that subscale). The final sample thus consisted of 100 individuals who reported being diagnosed by a physician with ME/CFS and had at least partial information for each of the MFTQ subscale scores. Of these 100 respondents, 11 (11%) were males and 88 (88%) were females (1 participant did not report their biological sex). The mean age of participants was 58.9 years with a standard deviation of 9.3 years. In regards to race, the overwhelming majority of participants were Caucasian (98%) with one other participant that reported being African-American and the remaining participant not identifying their racial background. Exactly half of the sample was married at the time of data collection (50) with 48 individuals reporting not currently being married (e.g. never married, divorced, widowed) and 2 participants not reporting their marital status. A large percentage of the sample had received a college education (87%) as opposed to a standard high school degree or GED (10%). Only 26 individuals reported that they were currently working, with the remaining participants reporting that they were receiving disability compensation (62%) were unemployed/retired (8%) or did not respond (4%).
A three-cluster solution was examined in order to replicate previous findings.7, 14 This three cluster solution was also supported by the dendrogram and the agglomeration schedule which showed a sharp jump in the cluster coefficient from the 4 cluster to the 3 cluster solution (from 45329.6 to 50321.9). The cluster coefficient is the distance that the clustering algorithm must ‘travel’ in order to join the two nearest neighboring clusters. A dendrogram presents this information graphically and shows how far apart cases are, and, eventually, how far apart clusters are as cases are linked together by the procedure.
3-Cluster Solution
Using a one-way Analysis of Variance (ANOVA) test, a significant difference was found among the mean ages of participants in each cluster; 57.5 years (SD = 6.8) for Cluster 1; 57.6 years (SD = 4.2) for Cluster 2; and 52.7 years (SD = 10.6) for Cluster 3 [F(2, 96) = 3.56; p < .05]. Using Scheffé’s30 post-hoc test, participants in Cluster 1 were found to be significantly older than those in Cluster 3. Chi-square tests were conducted on all remaining demographic variables and no significant differences were found for race [χ2(2, N = 100) = 1.52, p > .05]; sex [χ2(2, N = 100) = 0.24, p > .05]; marital status [χ2(2, N = 100) = 3.50, p > .05]; education level [χ2(2, N = 100) = 1.89, p > .05] and work status [χ2(4, N = 100) = 5.86, p > .05].
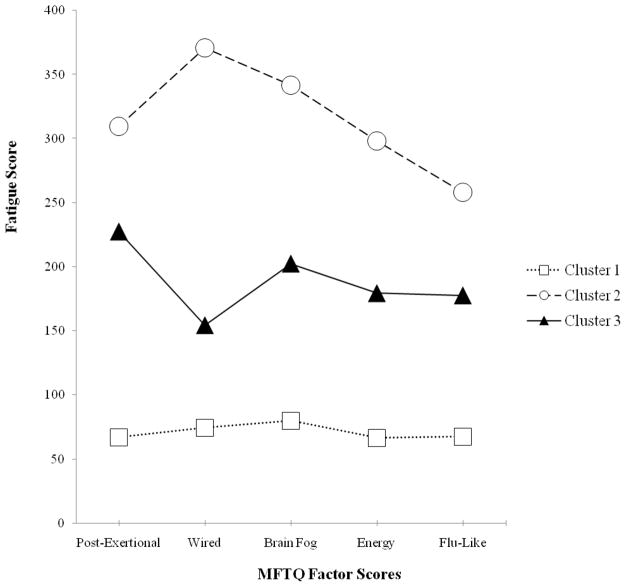
Means and standard deviations for the MFTQ are given in Table 1 and are presented graphically in Figure 1. Cluster 1 contained 40 individuals from the original sample of 100 and was characterized by low symptom scores across all of the fatigue dimensions. A small group (n=6) of consistently high-scoring respondents (severe symptom group) combined to form Cluster 2. Finally, Cluster 3 contained the majority the sample (n=54) who recorded moderate severity levels of the 5 fatigue states. Mean comparisons were conducted using Analysis of Covariance (ANCOVA) tests with age entered as a covariate. There were significant differences for all MFTQ factors: Post Exertional [F(2, 95) = 68.92, p < .01], Wired [F(2, 95) = 37.25, p < .01], Brain Fog [F(2, 95) = 43.83, p < .01], Energy [F(2, 97) = 35.63, p < .01], and Flu-Like [F(2, 97) = 34.64, p < .01]. This cluster analysis classified individuals into subgroups according to their similarity on their factor scores on the MFTQ. We identified a meaningful typology that was characterized by a small amount of within-cluster variation and a large amount of between-cluster variation. In other words, those individuals with ME/CFS who were in Cluster 2 had extremely high Post-Exertional, Wired, Brain Fog, Energy and Flu-like MFTQ factors scores. Those in Cluster 2 had the most severe different forms of fatigue. In contrast, those individuals in Cluster 1 have significantly lower scores on the MFTQ factors. Therefore, those in this cluster had the lowest levels of different types of fatigue. Finally, those in Cluster 3 had MFTQ factor scores that were significantly lower than Cluster 2 and significantly higher than Cluster 1.
Table 1.
MFTQ Factor Scores for the 3 Cluster Solution (N=100)
| Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|
|
| |||
| n = 40 | n = 6 | n = 54 | |
|
| |||
| M (S.D.) | M (S.D.) | M (S.D.) | |
| Post-Exertional | 67.0 (45.8) a | 309.5 (69.1) a | 227.6 (84.0) a |
| Wired | 74.6 (82.3) a | 371.0 (25.0) a | 154.5 (76.0) a |
| Brain Fog | 79.9 (74.7) a | 342.0 (58.1) a | 202.6 (77.5) a |
| Energy | 66.4 (63.8) a | 298.3 (80.3) a | 179.5 (80.9) a |
| Flu-Like | 67.4 (52.0) a | 258.1 (101.3) a | 177.8 (75.5) a |
Clusters across rows with a similar superscript are significantly different from each other at the p < .05 level. All post-hoc tests were conducted using Scheffe’s method.
Figure 1.
Mean factor scores on MFTQ dimensions for 3-cluster solution. Group size: Cluster 1 (n=40); Cluster 2 (n=6); Cluster 3 (n=54). All between-group mean comparisons were significantly different at the p < .05 level.
Using Bonferroni-adjusted post hoc comparisons, significant differences were found between the three clusters for all of the fatigue dimensions. In Table 1, for each of the 5 MFTQ Factor Scores (Post-Exertional, Wired, etc.), if two clusters were significantly different, they would have a similar letter superscript. Because in Table 1, all cluster scores were significantly different from each other, so all numbers have a superscript a next to them. However, for Table 2, there is more variety, and one can see for example, for Post-Exertional, Clusters 1 and 3 are significantly different because they have a similar superscript a, but Clusters 1 and 2 are not significantly different because they do not have a similar superscript.
Table 2.
MFTQ Factor Scores for the 5 Cluster Solution (N=100)
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | |
|---|---|---|---|---|---|
|
| |||||
| n = 40 | n = 6 | n = 9 | n = 14 | n = 31 | |
|
| |||||
| M (S.D.) | M (S.D.) | M (S.D.) | M (S.D.) | M (S.D.) | |
| Post-Exertional | 67.0 (45.8) a,b | 309.5 (69.1) b, c | 324.9 (70.1) a | 131.8 (38.7) a,c | 242.6 (56.0) a |
| Wired | 74.6 (82.3) a,b | 371.0 (25.0) a,c,d | 139.6 (70.1) c | 187.7 (70.1) a | 143.9 (77.8) b,d |
| Brain Fog | 79.9 (74.7) a,b | 342.0 (58.1) a,c | 295.2 (65.9) b,d | 186.3 (71.6) a,d | 183.1 (64.4) b,c |
| Energy | 66.4 (63.8) a,b | 298.3 (80.3) a,c | 268.3 (70.8) b,d | 172.0 (80.8) a,d | 157.2 (67.1) b,c |
| Flu-Like | 67.4 (52.0) a,b | 258.1 (101.3) b,c | 261.9 (58.8) a,d | 122.2 (41.5) c,d | 178.5 (69.4) a |
Groups across rows with a similar superscript are significantly different at the p < .05 level. All post-hoc tests were conducted using Scheffe’s method.
5-Cluster Solution
Upon closer examination of the dendrogram and the agglomeration schedule, it was also determined that a 5 cluster solution was tenable. Specifically, the large moderate cluster (Cluster 3) seemed to break off into three smaller clusters (Clusters 3, 4, and 5) according to the dendrogram, and once the cluster coefficient leveled off at the 6 cluster stage, there was again a sharp spike in the cluster coefficient for the 5 cluster solution (from 50954.2 to 62155.4).
There were no significant sociodemographic differences for age [F(4, 94) = 2.29, p > .05], race [χ2(4, N = 100) = 1.52, p > .05]; sex [χ2(4, N = 100) = 5.29, p > .05]; marital status [χ2(4, N = 100) = 5.21, p > .05]; education level [χ2(4, N = 100) = 2.89, p > .05]; and work status [χ2(8, N = 100) = 15.42, p > .05].
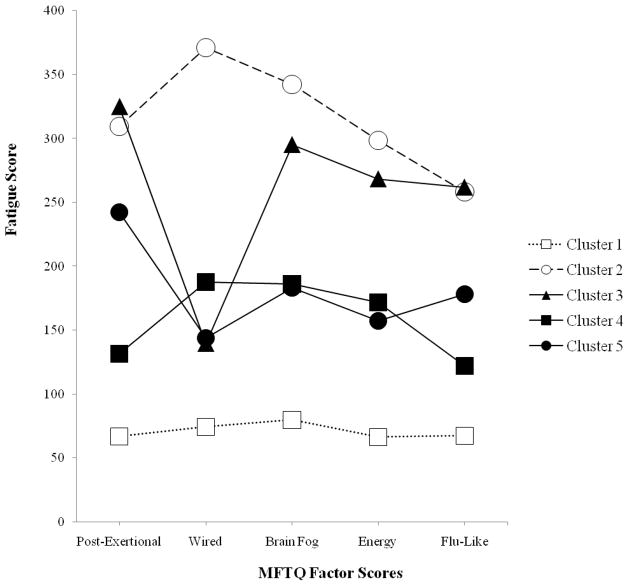
The resulting solution contained Clusters 1 and 2 from the previous 3 Cluster solution described above; but in addition, persons in Cluster 3 of the original 3 Cluster solution were further classified into Cluster 3 (n=9), Cluster 4 (n=14), and Cluster 5 (n=31) for this 5 Cluster solution. MTFQ factor scores are listed in Table 2 and presented graphically in Figure 2. There were significant group differences for each of the fatigue dimensions: Post Exertional [F(4, 95) = 86.38, p < .01], Wired [F(4, 95) = 22.43, p < .01], Brain Fog [F(4, 95) = 33.33, p < .01], Energy [F(4, 95) = 28.35, p < .01] and Flu-Like [F(4, 95) = 32.35, p < .01]. Post-hoc tests were again conducted using the Scheffé method. The results can be found in Table 2 and are described below. As with Table 1, for each of the 5 MFTQ Factor Scores (Post-Exertional, Wired, etc.), if two clusters were significantly different, they would have a similar letter superscript. For Table 2, as an example, for Post-Exertional, Clusters 1 and 3 are significantly different because they have a similar superscript a, but Clusters 1 and 2 are not significantly different because they do not have a similar superscript.
Figure 2.
Mean factor scores on MFTQ dimensions for 5-cluster solution. Group size: Cluster 1 (n=40); Cluster 2 (n=6); Cluster 3 (n=9); Cluster 4 (n=14); Cluster 5 (n=31). Between-group mean comparisons can be found in Table 2.
In the 5 Cluster solution, Cluster 3 was very similar to Cluster 2, as there were no significant differences for any of the factors except for the Wired factor, where those in Cluster 3 had significantly lower scores than those in Cluster 2. Those in Cluster 3 also had significantly higher scores than Clusters 1, 4, and 5 on almost every dimension (with the exception of this Wired factor, for which it was not significantly different from Clusters 1, 4, and 5). Thus, Cluster 3 appears to be comprised of severely fatigued individuals but who do not experience high levels of Wired fatigue.
Cluster 4 included individuals who experienced generally moderate levels of the different fatigue states. Those in Cluster 4 were significantly less impaired than those in Cluster 2 on all of the fatigue dimensions. In addition, those in Cluster 4 were significantly more impaired than Cluster 1 on every dimension except for the Flu-Like factor. This cluster was very similar to Cluster 5 except for the Post-Exertional factor, for which Cluster 4 individuals were significantly less impaired. Consequently, this cluster may represent a transition point between the more severe Clusters 2 and 3 and the less severe Cluster 1.
The fifth cluster was also comprised of individuals with generally moderate levels of fatigue across all dimensions. Participants in Cluster 5 had significantly higher scores compared to Cluster 1 on all of the fatigue dimensions. Cluster 5 also had significantly higher scores for the Post-Exertional factor as compared to the other moderately fatigued Cluster 4. For the Post-Exertional and Flu-Like factor, Cluster 5 was not significantly different than Cluster 2. Therefore, it is possible that these respondents are moderate on most dimensions except for the Flu-Like symptoms and Post-Exertional fatigue for which they are more similar to the severely fatigued participants in Cluster 2.
Discussion
Investigations that use data reduction and/or clustering techniques can help identify sub-types through empirical methods. In the present study, a three-cluster solution was identified that contained significantly different subgroups for all aspects of fatigue found in ME/CFS, with one group having a high level of symptoms on all five fatigue categories, another group having moderate symptoms on all fatigue categories, and a final group having the lowest symptoms on the categories. Therefore, this finding replicates prior findings7, 14 that suggest persons with ME/CFS can be differentiated into subgroups based on severity.
As mentioned in the introduction, one study that used clustering methods16 found distinct illness subgroups that represented more than just illness severity. Based upon the agglomeration schedule which showed a sharp increase in the cluster coefficient between the 6 and 5 cluster solutions, we examined a five-cluster solution as well. Although the present study did not replicate Hickie et al.’s16 two-cluster solution, the five-cluster solution was considered interpretable and classified individuals with ME/CFS according to different symptom patterns as well as differences in fatigue severity. The most highly symptomatic group (Cluster 2) and the least symptomatic group (Cluster 1) again emerged; however, in this 5 Cluster solution, the moderate group from the 3 Cluster solution broke into 3 distinct groups.
Within the 5 Cluster solution, Cluster 3 mirrored the fatigue scores of Cluster 2 with the exception of the Wired fatigue factor. This is an interesting finding given the irregularities in autonomic and nervous system functioning reported for some patients with ME/CFS.1 As mentioned earlier, Wired fatigue refers to a state of overstimulation or arousal despite a lack of energy resources. Some researchers have noted that chronic overactivation of the sympathetic nervous system can disrupt sleep, deplete cortisol levels and cause a shift towards an overproduction of Th2 anti-inflammatory cytokines.1 Other evidence has emerged that identified increased cortical excitability for individuals with ME/CFS that could lead to a hypersensitivity to immunogenic stimuli.31 Such abnormalities can lead to the ‘wired but tired’ feeling for some individuals with this illness. It is possible then that persons in Cluster 3 experience less feeling wired, and consequently less autonomic and neurological deregulation, though other biological abnormalities are present that explain their high levels of fatigue along other dimensions.
It is apparent within the 5 Cluster solution that Cluster 3 does differ on important dimensions from Clusters 4 and 5, and yet in the 3 cluster solution (Figure 1), these three groups were all placed within the moderate category. Combining patients with such different profiles could lead to more heterogeneity. In addition, Clusters 4 and 5 were also combined in the 3 Cluster solution in Figure 1. Yet, there are also potentially important differences between Clusters 4 and 5, as seen in Figure 2. Specifically, those in Cluster 5 reported significantly higher scores on the Post-Exertional factor than those in Cluster 4. This could be indicative of any number of distinct biological process that are present in one group of individuals but not the other.32 In sum, Clusters 3, 4 and 5 may be differentiated by important variations among their fatigue type patterns. Such an explanation is more compatible with the findings of Hickie et al.16 and supports a different perspective of ME/CFS subtypes outside of only severity found in the 3 Cluster solution.
There were several limitations to the present study. First, the large percentage of female and Caucasian participants precludes the generalization of the results to larger ME/CFS population. This imbalance is likely due to the sampling methods that were used, and although difficult to achieve with this patient population, community-based random-sampling designs are always preferred.33 The sample size was relatively small, and future studies should attempt to include larger samples so that there are more individuals within each of the clusters. Finally, we did not include more biological measures, and future studies might attempt to see if biological or genetic findings also differentiated the clusters reported in this study.
Acknowledgments
The authors appreciate the financial assistance provided by the National Institute of Allergy and Infectious Diseases (grant numbers AI36295 and AI49720).
References
- 1.Jason LA, Corradi K, Torres-Harding S, Taylor RR, King C. Chronic fatigue syndrome: the need for subtypes. Neuropsychology Review. 2005;15(1):29–58. doi: 10.1007/s11065-005-3588-2. [DOI] [PubMed] [Google Scholar]
- 2.Natelson BH, Weaver SA, Tseng C-L, Ottenweller JE. Spinal fluid abnormalities in patients with chronic fatigue syndrome. Clin Diagn Lab Immunol. 2005 Jan 1;12(1):52–55. doi: 10.1128/CDLI.12.1.52-55.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJF. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radical Biology and Medicine. 2005;39(5):584–589. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto S, Ouchi Y, Onoe H, et al. Reduction of serotonin transporters of patients with chronic fatigue syndrome. Neuroreport. 2004;15(17):2571. doi: 10.1097/00001756-200412030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Nisenbaum R, Reyes M, Mawle AC, Reeves WC. Factor analysis of unexplained severe fatigue and interrelated symptoms: overlap with criteria for chronic fatigue syndrome. Am J Epidemiol. 1998 Jul 1;148(1):72–77. doi: 10.1093/oxfordjournals.aje.a009562. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994 Dec 15;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Nisenbaum R, Reyes M, Unger ER, Reeves WC. Factor analysis of symptoms among subjects with unexplained chronic fatigue What can we learn about chronic fatigue syndrome? Journal of Psychosomatic Research. 2004;56(2):171–178. doi: 10.1016/S0022-3999(03)00039-4. [DOI] [PubMed] [Google Scholar]
- 8.Friedberg F, Dechene L, McKenzie MJ, Fontanetta R. Symptom patterns in long-duration chronic fatigue syndrome. Journal of Psychosomatic Research. 2000;48(1):59–68. doi: 10.1016/s0022-3999(99)00077-x. [DOI] [PubMed] [Google Scholar]
- 9.Jason LA, Taylor RR, Kennedy CL, et al. A factor analysis of chronic fatigue symptoms in a community-based sample. Soc Psychiatry Psychiatr Epidemiol. 2002 Apr;37(4):183–189. doi: 10.1007/s001270200013. [DOI] [PubMed] [Google Scholar]
- 10.Arroll MA, Senior V. Symptom typology and sub-grouping in chronic fatigue syndrome. Bulletin of the IACFS/ME. 2009;17(2):39–52. [Google Scholar]
- 11.Hickie I, Davenport T, Vernon SD, et al. Are chronic fatigue and chronic fatigue syndrome valid clinical entities across countries and health-care settings? Australian and New Zealand Journal of Psychiatry. 2009;43(1):25– 35. doi: 10.1080/00048670802534432. [DOI] [PubMed] [Google Scholar]
- 12.Brown TA. Confirmatory factor analysis for applied research. New York: Guilford Press; 2006. [Google Scholar]
- 13.Bailey KD. Typologies and taxonomies: An introduction to classification techniques. Thousand Oaks, CA: Sage Publications, Inc; 1994. [Google Scholar]
- 14.Jason L, Taylor R. Applying cluster analysis to define a typology of chronic fatigue syndrome in a medically-evaluated, random community sample. Psychology & Health. 2002;17(3):323–337. [Google Scholar]
- 15.Ray C, Weir WRC, Phillips S, Cullen S. Development of a measure of symptoms in chronic fatigue syndrome: The profile of fatigue-related symptoms (PFRS) Psychology & Health. 1992;7(1):27– 43. [Google Scholar]
- 16.Hickie I, Lloyd A, Hadzi-Pavlovic D, Parker G, Bird K, Wakefield D. Can the chronic fatigue syndrome be defined by distinct clinical features? Psychological Medicine. 1995;25(5):925. doi: 10.1017/s0033291700037417. [DOI] [PubMed] [Google Scholar]
- 17.Wilson A, Hickie I, Hadzi-Pavlovic D, et al. What is chronic fatigue syndrome? Heterogeneity within an international multicentre study. Australian and New Zealand Journal of Psychiatry. 2001;35(4):520–527. doi: 10.1046/j.1440-1614.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 18.Kerr JR, Petty R, Burke B, et al. Gene expression subtypes in patients with chronic fatigue syndrome/myalgic encephalomyelitis. The Journal of Infectious Diseases. 2008;197(8):1171–1184. doi: 10.1086/533453. [DOI] [PubMed] [Google Scholar]
- 19.Dechene L, Friedberg F, McKenzie MJ, II, Fontanetta R. A new fatigue typology for the chronic fatigue syndrome. 1994:29. doi: 10.1016/s0022-3999(99)00077-x. [DOI] [PubMed] [Google Scholar]
- 20.Gielissen MF, Knoop H, Servaes P, et al. Differences in the experience of fatigue in patients and healthy controls: patients’ descriptions. Health Qual Life Outcomes. 2007;5:36. doi: 10.1186/1477-7525-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libman E, Creti L, Rizzo D, Jastremski M, Bailes S, Fichten CS. Descriptors of Fatigue in Chronic Fatigue Syndrome. Journal of Chronic Fatigue Syndrome. 2008;14(3):37–45. [Google Scholar]
- 22.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995 Apr;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda S, Takashima S, Iwase M, Yamaguti K, Kuratsune H, Watanabe Y. Development and validation of a new fatigue scale for fatigued subjects with and without chronic fatigue syndrome. In: Watanabe Y, Evengard B, Natelson BH, Jason LA, editors. Fatigue Science for Human Health. Tokyo; New York: Springer; 2008. pp. 89–102. [Google Scholar]
- 24.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 25.Stouten B. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Services Research. 2005;5(1):37. doi: 10.1186/1472-6963-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedberg F, Jason L. Understanding chronic fatigue syndrome : an empirical guide to assessment and treatment. 1. Washington, DC: American Psychological Association; 1998. [Google Scholar]
- 27.Jason LA, Jessen T, Porter NS, Boulton A, Njoku M-G, Friedberg F. Examining types of fatigue in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Disability Studies Quarterly. 2009;29 available at http://www.dsq-sds.org/article/view/938/1113. [Google Scholar]
- 28.Clatworthy J, Buick D, Hankins M, Weinman J, Horne R. The use and reporting of cluster analysis in health psychology: A review. British journal of health psychology. 2005;10:329–358. doi: 10.1348/135910705X25697. [DOI] [PubMed] [Google Scholar]
- 29.Sokal RR, Michener CD. A statistical method for evaluating systematic relationships. Multivariate Statistical Methods, Among-groups Covariation. 1975:269. [Google Scholar]
- 30.Scheffe H. A method for judging all contrasts in the analysis of variance. Biometrika. 1953;40(1/2):87–104. [Google Scholar]
- 31.Brouwer B, Packer T. Corticospinal excitability in patients diagnosed with chronic fatigue syndrome. Muscle & nerve. 1994;17(10) doi: 10.1002/mus.880171012. [DOI] [PubMed] [Google Scholar]
- 32.Bellinger AM, Reiken S, Dura M, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proceedings of the National Academy of Sciences. 2008;105(6):2198. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159:2129– 2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]